Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival
Abstract
1. Introduction
2. Antiangiogenic Strategies
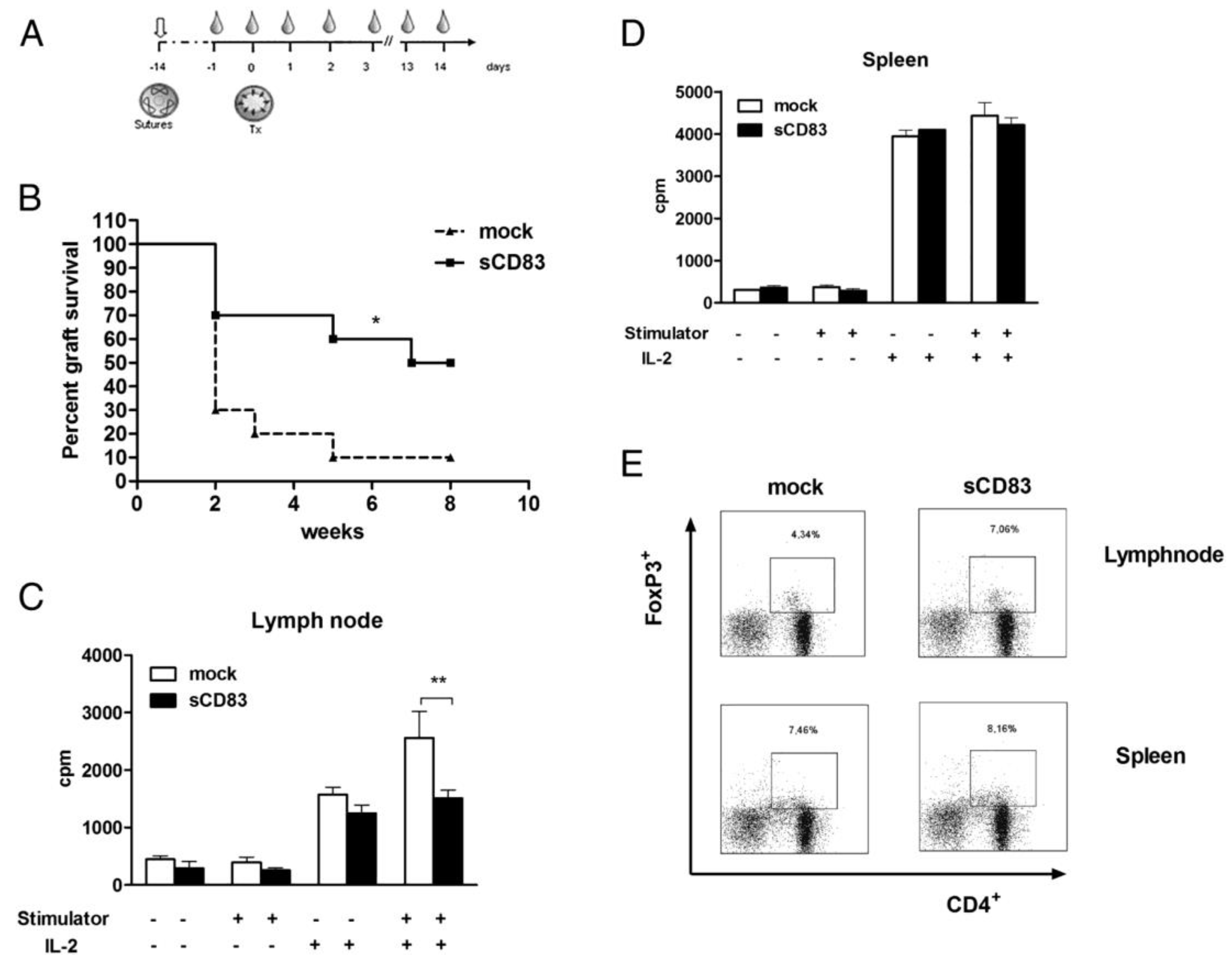
3. Soluble CD83 (sCD83)
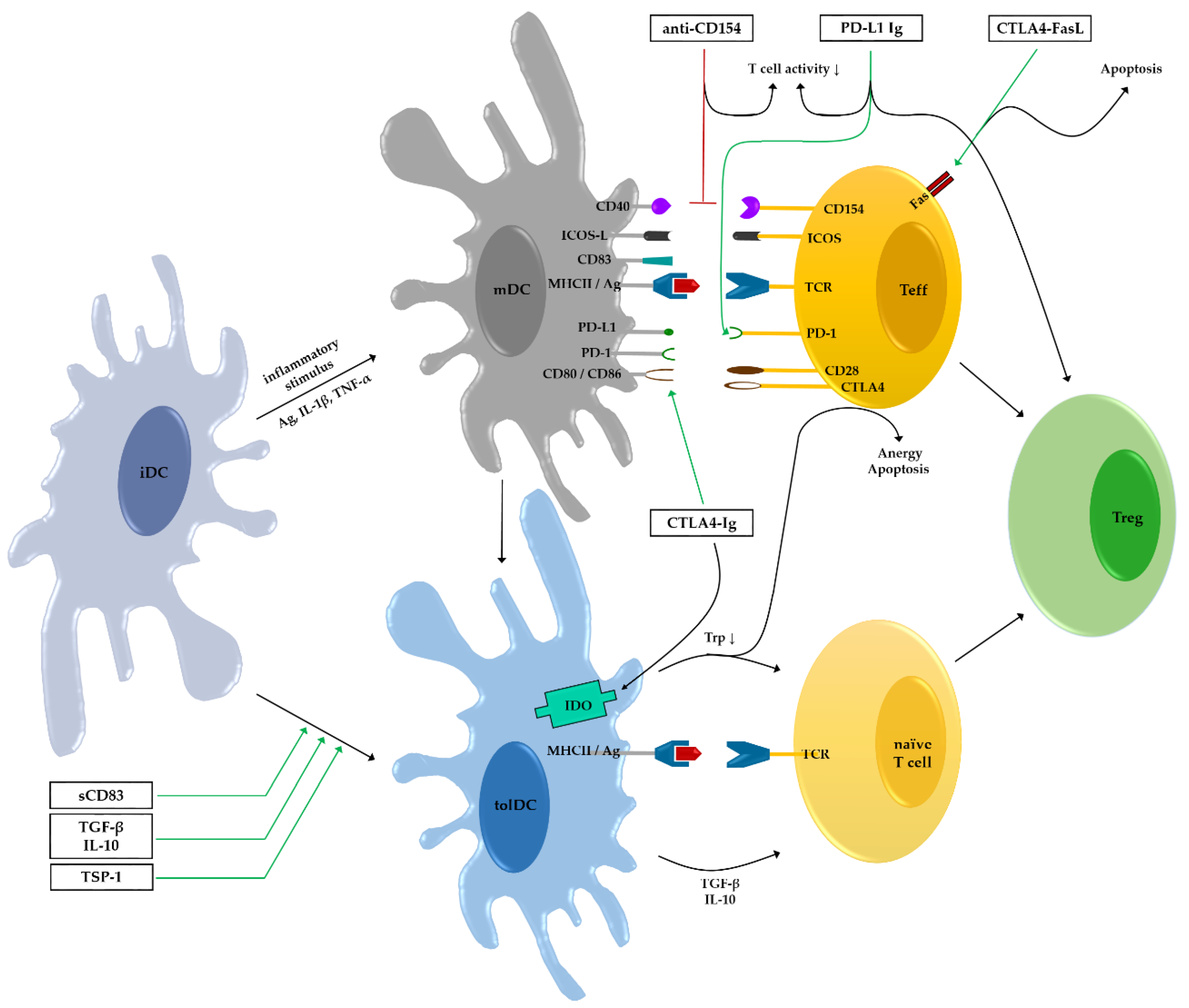
4. Co-Stimulatory Molecules
4.1. Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA4)
4.2. CD40–CD154
4.3. Inducible T Cell Co-Stimulator (ICOS)
4.4. Programmed Death Protein 1 (PD-1)–PD-L1
5. Thrombospondin-1 (TSP-1)
6. Interleukin-10 (IL-10)
7. Activated Leukocyte Cell Adhesion Molecule (ALCAM)
8. Adoptive Cell Transfer
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cursiefen, C. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy 2007, 92, 50–57. [Google Scholar] [PubMed]
- Fannon, M.; Forsten-Williams, K.; Dowd, C.J.; Freedman, D.A.; Folkman, J.; Nugent, M.A. Binding inhibition of angiogenic factors by heparan sulfate proteoglycans in aqueous humor: Potential mechanism for maintenance of an avascular environment. FASEB J. 2003, 17, 1–20. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Braun, G.; Schneider, A.-C.; Hos, D.; Bi, Y.; Bachmann, B.O.; Cursiefen, C. Identification of novel endogenous anti(lymph)angiogenic factors in the aqueous humor. Investig. Opthalmol. Vis. Sci. 2016, 57, 6554. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, P.; Seitz, B.; Schlötzer-Schrehardt, U.; Naumann, G.O.H. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res. 1997, 289, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Wada, M.; Otsuji, T.; Jo, N.; Tombran-Tink, J.; Matsumura, M. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1168–1175. [Google Scholar] [PubMed]
- Tan, Y.; Cruz-Guilloty, F.; Medina-Mendez, C.A.; Cutrufello, N.J.; Martinez, R.E.; Urbieta, M.; Wilson, D.; Li, Y.; Perez, V.L. Immunological disruption of antiangiogenic signals by recruited allospecific t cells leads to corneal allograft rejection. J. Immunol. 2012, 188, 5962–5969. [Google Scholar] [CrossRef]
- Cursiefen, C.; Masli, S.; Ng, T.F.; Dana, M.R.; Bornstein, P.; Lawler, J.; Streilein, J.W. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1117–1124. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The immune privilege of corneal grafts. J. Leukoc. Biol. 2003, 74, 167–171. [Google Scholar] [CrossRef]
- Carnahan, M.C.; Goldstein, D.A. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr. Opin. Ophthalmol. 2000, 11, 478–483. [Google Scholar] [CrossRef]
- Lechler, R.I.; Sykes, M.; Thomson, A.W.; Turka, L.A. Organ transplantation—How much of the promise has been realized? Nat. Med. 2005, 11, 605–613. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Saban, D.R.; Emami-Naeini, P.; Chauhan, S.K.; Funaki, T.; Ueno, H.; Dana, R. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J. Leukoc. Biol. 2012, 91, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Hackstein, H.; Thomson, A.W. Dendritic cells: Emerging pharmacological targets of immunosuppressive drugs. Nat. Rev. Immunol. 2004, 4, 24–34. [Google Scholar] [CrossRef]
- Amouzegar, A.; Chauhan, S.K.; Dana, R. Alloimmunity and tolerance in corneal transplantation. J. Immunol. 2016, 196, 3983–3991. [Google Scholar] [CrossRef]
- Novak, N.; Siepmann, K.; Zierhut, M.; Bieber, T. The good, the bad and the ugly—APCs of the eye. Trends Immunol. 2003, 24, 570–574. [Google Scholar] [CrossRef]
- Chen, L.; Hamrah, P.; Cursiefen, C.; Zhang, Q.; Pytowski, B.; Streilein, J.W.; Dana, M.R. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat. Med. 2004, 10, 813–815. [Google Scholar] [CrossRef]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef]
- Lan, Y.Y.; Wang, Z.; Raimondi, G.; Wu, W.; Colvin, B.L.; de Creus, A.; Thomson, A.W. “Alternatively activated” dendritic cells preferentially secrete il-10, expand foxp3+cd4+ t cells, and induce long-term organ allograft survival in combination with ctla4-ig. J. Immunol. 2006, 177, 5868–5877. [Google Scholar] [CrossRef]
- Thomson, A.W.; Ezzelarab, M.B. Regulatory dendritic cells: Profiling, targeting, and therapeutic application. Curr. Opin. Organ. Transplant. 2018, 23, 538–545. [Google Scholar] [CrossRef]
- Lafferty, K.J.; Cunningham, A.J. A new analysis of allogeneic interactions. Aust. J. Exp. Biol. Med. Sci. 1975, 53, 27–42. [Google Scholar] [CrossRef]
- Pilat, N.; Sayegh, M.H.; Wekerle, T. Costimulatory pathways in transplantation. Semin. Immunol. 2011, 23, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Coyle, A.J.; Gutierrez-Ramos, J.C. The expanding b7 superfamily: Increasing complexity in costimulatory signals regulating t cell function. Nat. Immunol. 2001, 2, 203–209. [Google Scholar] [CrossRef]
- Horton, C.; Shanmugarajah, K.; Fairchild, P.J. Harnessing the properties of dendritic cells in the pursuit of immunological tolerance. Biomed. J. 2017, 40, 80–93. [Google Scholar] [CrossRef]
- Morelli, A.E.; Thomson, A.W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007, 7, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Tang, Q. How do cd4+cd25+ regulatory t cells control autoimmunity? Curr. Opin. Immunol. 2005, 17, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Ordikhani, F.; Jordan, S.; Boros, P.; Thomson, A.W. Tolerogenic dendritic cells in organ transplantation. Transpl. Int. 2020, 33, 113–127. [Google Scholar] [CrossRef]
- Gonzalez-Nolasco, B.; Wang, M.; Prunevieille, A.; Benichou, G. Emerging role of exosomes in allorecognition and allograft rejection. Curr. Opin. Organ. Transplant. 2018, 23, 22–27. [Google Scholar] [CrossRef]
- Teijeira, A.; Russo, E.; Halin, C. Taking the lymphatic route: Dendritic cell migration to draining lymph nodes. Semin. Immunopathol. 2014, 36, 261–274. [Google Scholar] [CrossRef]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.O.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting edge: Lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J. Immunol. 2010, 184, 535–539. [Google Scholar] [CrossRef]
- Hamrah, Y.Q.a.P. Corneal allograft rejection: Immunopathogenesis to therapeutics. J. Clin. Cell. Immunol. 2013. [Google Scholar] [CrossRef]
- Sene, A.; Chin-Yee, D.; Apte, R.S. Seeing through vegf: Innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol. Med. 2015, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Salabarria, A.C.; Braun, G.; Heykants, M.; Koch, M.; Reuten, R.; Mahabir, E.; Cursiefen, C.; Bock, F. Local vegf-a blockade modulates the microenvironment of the corneal graft bed. Am. J. Transplant. 2019, 19, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Bucher, F.; Regenfuss, B.; Dreisow, M.L.; Bock, F.; Heindl, L.M.; Eming, S.A.; Cursiefen, C. Il-10 indirectly regulates corneal lymphangiogenesis and resolution of inflammation via macrophages. Am. J. Pathol. 2016, 186, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Dorrie, J.; Schaft, N.; Bock, F.; Notara, M.; Kruse, F.E.; Krautwald, S.; Cursiefen, C.; Bachmann, B.O. Blockade of ccr7 leads to decreased dendritic cell migration to draining lymph nodes and promotes graft survival in low-risk corneal transplantation. Exp. Eye Res. 2016, 146, 1–6. [Google Scholar] [CrossRef]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.E.; Wiegand, S.J.; Dana, M.R.; Streilein, J.W. Inhibition of hemangiogenesis and lymphangiogenesisafternormal-risk corneal transplantation by neutralizing vegf promotes graft survival. Investig. Opthalmol. Vis. Sci. 2004, 45, 2666. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Pytowski, B.; Cursiefen, C. Blockade of vegfr3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 115–119. [Google Scholar] [CrossRef]
- Dohlman, T.H.; Omoto, M.; Hua, J.; Stevenson, W.; Lee, S.M.; Chauhan, S.K.; Dana, R. Vegf-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 2015, 99, 678–686. [Google Scholar] [CrossRef]
- Hos, D.; Regenfuss, B.; Bock, F.; Onderka, J.; Cursiefen, C. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5778–5785. [Google Scholar] [CrossRef]
- Cursiefen, C.; Viaud, E.; Bock, F.; Geudelin, B.; Ferry, A.; Kadlecova, P.; Levy, M.; Al Mahmood, S.; Colin, S.; Thorin, E.; et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: The i-can study. Ophthalmology 2014, 121, 1683–1692. [Google Scholar] [CrossRef]
- Heishi, T.; Hosaka, T.; Suzuki, Y.; Miyashita, H.; Oike, Y.; Takahashi, T.; Nakamura, T.; Arioka, S.; Mitsuda, Y.; Takakura, T.; et al. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am. J. Pathol. 2010, 176, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, W.; Luo, C.; Wang, X.; Song, X.; Fu, Y.; Luo, Y. Endostatin inhibits tumour lymphangiogenesis and lymphatic metastasis via cell surface nucleolin on lymphangiogenic endothelial cells. J. Pathol. 2010, 222, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Reuer, T.; Schneider, A.C.; Cakir, B.; Buhler, A.D.; Walz, J.M.; Lapp, T.; Lange, C.; Agostini, H.; Schlunck, G.; Cursiefen, C.; et al. Semaphorin 3f modulates corneal lymphangiogenesis and promotes corneal graft survival. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5277–5284. [Google Scholar] [CrossRef]
- Buttner, C.; Clahsen, T.; Regenfuss, B.; Dreisow, M.L.; Steiber, Z.; Bock, F.; Reis, A.; Cursiefen, C. Tyrosinase is a novel endogenous regulator of developmental and inflammatory lymphangiogenesis. Am. J. Pathol. 2019, 189, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Schwarting, R.; Smith, H.M.; Tedder, T.F. A novel cell-surface molecule expressed by human interdigitating reticulum cells, langerhans cells, and activated lymphocytes is a new member of the ig superfamily. J. Immunol. 1992, 149, 735–742. [Google Scholar] [PubMed]
- Kruse, M.; Rosorius, O.; Kratzer, F.; Bevec, D.; Kuhnt, C.; Steinkasserer, A.; Schuler, G.; Hauber, J. Inhibition of cd83 cell surface expression during dendritic cell maturation by interference with nuclear export of cd83 mrna. J. Exp. Med. 2000, 191, 1581–1590. [Google Scholar] [CrossRef]
- Zhou, L.J.; Tedder, T.F. Cd14+ blood monocytes can differentiate into functionally mature cd83+ dendritic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2588–2592. [Google Scholar] [CrossRef]
- Lechmann, M.; Krooshoop, D.J.; Dudziak, D.; Kremmer, E.; Kuhnt, C.; Figdor, C.G.; Schuler, G.; Steinkasserer, A. The extracellular domain of cd83 inhibits dendritic cell-mediated t cell stimulation and binds to a ligand on dendritic cells. J. Exp. Med. 2001, 194, 1813–1821. [Google Scholar] [CrossRef]
- Hock, B.D.; Kato, M.; McKenzie, J.L.; Hart, D.N. A soluble form of cd83 is released from activated dendritic cells and b lymphocytes, and is detectable in normal human sera. Int. Immunol. 2001, 13, 959–967. [Google Scholar] [CrossRef]
- Zinser, E.; Lechmann, M.; Golka, A.; Lutz, M.B.; Steinkasserer, A. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble cd83. J. Exp. Med. 2004, 200, 345–351. [Google Scholar] [CrossRef]
- Lin, W.; Buscher, K.; Wang, B.; Fan, Z.; Song, N.; Li, P.; Yue, Y.; Li, B.; Li, C.; Bi, H. Soluble cd83 alleviates experimental autoimmune uveitis by inhibiting filamentous actin-dependent calcium release in dendritic cells. Front. Immunol. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Eckhardt, J.; Kreiser, S.; Dobbeler, M.; Nicolette, C.; DeBenedette, M.A.; Tcherepanova, I.Y.; Ostalecki, C.; Pommer, A.J.; Becker, C.; Gunther, C.; et al. Soluble cd83 ameliorates experimental colitis in mice. Mucosal. Immunol. 2014, 7, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.; Rossner, S.; Onderka, J.; Lechmann, M.; Pallotta, M.T.; Fallarino, F.; Boon, L.; Nicolette, C.; DeBenedette, M.A.; Tcherepanova, I.Y.; et al. Topical application of soluble cd83 induces ido-mediated immune modulation, increases foxp3+ t cells, and prolongs allogeneic corneal graft survival. J. Immunol. 2013, 191, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Munn, D.H. Ido expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, M.T.; Orabona, C.; Volpi, C.; Vacca, C.; Belladonna, M.L.; Bianchi, R.; Servillo, G.; Brunacci, C.; Calvitti, M.; Bicciato, S.; et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011, 12, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef]
- Beutelspacher, S.C.; Pillai, R.; Watson, M.P.; Tan, P.H.; Tsang, J.; McClure, M.O.; George, A.J.; Larkin, D.F. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur. J. Immunol. 2006, 36, 690–700. [Google Scholar] [CrossRef]
- Belladonna, M.L.; Orabona, C.; Grohmann, U.; Puccetti, P. Tgf-beta and kynurenines as the key to infectious tolerance. Trends Mol. Med. 2009, 15, 41–49. [Google Scholar] [CrossRef]
- Romani, L.; Fallarino, F.; De Luca, A.; Montagnoli, C.; D’Angelo, C.; Zelante, T.; Vacca, C.; Bistoni, F.; Fioretti, M.C.; Grohmann, U.; et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 2008, 451, 211–215. [Google Scholar] [CrossRef]
- Puccetti, P.; Fallarino, F. Generation of t cell regulatory activity by plasmacytoid dendritic cells and tryptophan catabolism. Blood Cells Mol. Dis. 2008, 40, 101–105. [Google Scholar] [CrossRef]
- Serbecic, N.; Lahdou, I.; Scheuerle, A.; Hoftberger, R.; Aboul-Enein, F. Function of the tryptophan metabolite, l-kynurenine, in human corneal endothelial cells. Mol. Vis. 2009, 15, 1312–1324. [Google Scholar]
- Qin, S.; Cobbold, S.P.; Pope, H.; Elliott, J.; Kioussis, D.; Davies, J.; Waldmann, H. “Infectious” transplantation tolerance. Science 1993, 259, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Zaher, S.S.; Germain, C.; Fu, H.; Larkin, D.F.; George, A.J. 3-hydroxykynurenine suppresses cd4+ t-cell proliferation, induces t-regulatory-cell development, and prolongs corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Zaher, S.S.; Coe, D.; Chai, J.G.; Larkin, D.F.; George, A.J. Suppression of the allogeneic response by the anti-allergy drug n-(3,4-dimethoxycinnamonyl) anthranilic acid results from t-cell cycle arrest. Immunology 2013, 138, 157–164. [Google Scholar] [CrossRef]
- Poirier, N.; Azimzadeh, A.M.; Zhang, T.; Dilek, N.; Mary, C.; Nguyen, B.; Tillou, X.; Wu, G.; Reneaudin, K.; Hervouet, J.; et al. Inducing ctla-4-dependent immune regulation by selective cd28 blockade promotes regulatory t cells in organ transplantation. Sci. Transl. Med. 2010, 2, 17ra10. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.P.; George, A.J.; Larkin, D.F. Differential effects of costimulatory pathway modulation on corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3417–3422. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-endocytosis of cd80 and cd86: A molecular basis for the cell-extrinsic function of ctla-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Freeman, G.J. The b7-cd28 superfamily. Nat. Rev. Immunol. 2002, 2, 116–126. [Google Scholar] [CrossRef]
- Hoffmann, F.; Zhang, E.P.; Pohl, T.; Kunzendorf, U.; Wachtlin, J.; Bulfone-Paus, S. Inhibition of corneal allograft reaction by ctla4-ig. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 535–540. [Google Scholar] [CrossRef]
- Gebhardt, B.M.; Hodkin, M.; Varnell, E.D.; Kaufman, H.E. Protection of corneal allografts by ctla4-ig. Cornea 1999, 18, 314–320. [Google Scholar] [CrossRef]
- Comer, R.M.; King, W.J.; Ardjomand, N.; Theoharis, S.; George, A.J.; Larkin, D.F. Effect of administration of ctla4-ig as protein or cdna on corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1095–1103. [Google Scholar]
- Gong, N.; Pleyer, U.; Yang, J.; Vogt, K.; Hill, M.; Anegon, I.; Volk, H.D.; Ritter, T. Influence of local and systemic ctla4ig gene transfer on corneal allograft survival. J. Gene Med. 2006, 8, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bolling, S.F.; Linsley, P.S.; Wei, R.Q.; Gordon, D.; Thompson, C.B.; Turka, L.A. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by ctla4ig plus donor-specific transfusion. J. Exp. Med. 1993, 178, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Baliga, P.; Chavin, K.D.; Qin, L.; Woodward, J.; Lin, J.; Linsley, P.S.; Bromberg, J.S. Ctla4ig prolongs allograft survival while suppressing cell-mediated immunity. Transplantation 1994, 58, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Turka, L.A.; Linsley, P.S.; Lin, H.; Brady, W.; Leiden, J.M.; Wei, R.Q.; Gibson, M.L.; Zheng, X.G.; Myrdal, S.; Gordon, D.; et al. T-cell activation by the cd28 ligand b7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 11102–11105. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Zeng, Y.; Thistlethwaite, J.R.; Montag, A.; Brady, W.; Gibson, M.G.; Linsley, P.S.; Bluestone, J.A. Long-term survival of xenogeneic pancreatic islet grafts induced by ctla4lg. Science 1992, 257, 789–792. [Google Scholar] [CrossRef]
- Azuma, H.; Chandraker, A.; Nadeau, K.; Hancock, W.W.; Carpenter, C.B.; Tilney, N.L.; Sayegh, M.H. Blockade of t-cell costimulation prevents development of experimental chronic renal allograft rejection. Proc. Natl. Acad. Sci. USA 1996, 93, 12439–12444. [Google Scholar] [CrossRef]
- Chandraker, A.; Azuma, H.; Nadeau, K.; Carpenter, C.B.; Tilney, N.L.; Hancock, W.W.; Sayegh, M.H. Late blockade of t cell costimulation interrupts progression of experimental chronic allograft rejection. J. Clin. Investig. 1998, 101, 2309–2318. [Google Scholar] [CrossRef]
- Yamagami, S.; Kawashima, H.; Tsuru, T.; Yamagami, H.; Kayagaki, N.; Yagita, H.; Okumura, K.; Gregerson, D.S. Role of fas-fas ligand interactions in the immunorejection of allogeneic mouse corneal transplants. Transplantation 1997, 64, 1107–1111. [Google Scholar] [CrossRef]
- Huang, J.H.; Tykocinski, M.L. Ctla-4-fas ligand functions as a trans signal converter protein in bridging antigen-presenting cells and t cells. Int. Immunol. 2001, 13, 529–539. [Google Scholar] [CrossRef][Green Version]
- Stuart, P.M.; Griffith, T.S.; Usui, N.; Pepose, J.; Yu, X.; Ferguson, T.A. Cd95 ligand (fasl)-induced apoptosis is necessary for corneal allograft survival. J. Clin. Investig. 1997, 99, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Boise, L.H.; Minn, A.J.; Noel, P.J.; June, C.H.; Accavitti, M.A.; Lindsten, T.; Thompson, C.B. Cd28 costimulation can promote t cell survival by enhancing the expression of bcl-xl. Immunity. J. Immunol. 2010, 185, 3788–3799. [Google Scholar]
- Noel, P.J.; Boise, L.H.; Green, J.M.; Thompson, C.B. Cd28 costimulation prevents cell death during primary t cell activation. J. Immunol. 1996, 157, 636–642. [Google Scholar]
- Lu, L.; Qian, S.; Hershberger, P.A.; Rudert, W.A.; Lynch, D.H.; Thomson, A.W. Fas ligand (cd95l) and b7 expression on dendritic cells provide counter-regulatory signals for t cell survival and proliferation. J. Immunol. 1997, 158, 5676–5684. [Google Scholar] [PubMed]
- Li, Y.; Li, X.C.; Zheng, X.X.; Wells, A.D.; Turka, L.A.; Strom, T.B. Blocking both signal 1 and signal 2 of t-cell activation prevents apoptosis of alloreactive t cells and induction of peripheral allograft tolerance. Nat. Med. 1999, 5, 1298–1302. [Google Scholar] [CrossRef]
- Shi, W.; Chen, M.; Xie, L. Prolongation of corneal allograft survival by ctla4-fasl in a murine model. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Kurimoto, I.; Streilein, J.W. Role of cd4+ t cells in immunobiology of orthotopic corneal transplants in mice. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2614–2621. [Google Scholar] [PubMed]
- Zheng, L.; Fisher, G.; Miller, R.E.; Peschon, J.; Lynch, D.H.; Lenardo, M.J. Induction of apoptosis in mature t cells by tumour necrosis factor. Nature 1995, 377, 348–351. [Google Scholar] [CrossRef]
- Ehl, S.; Hoffmann-Rohrer, U.; Nagata, S.; Hengartner, H.; Zinkernagel, R. Different susceptibility of cytotoxic t cells to cd95 (fas/apo-1) ligand-mediated cell death after activation in vitro versus in vivo. J. Immunol. 1996, 156, 2357–2360. [Google Scholar]
- Zhang, T.; Pierson, R.N., 3rd; Azimzadeh, A.M. Update on cd40 and cd154 blockade in transplant models. Immunotherapy 2015, 7, 899–911. [Google Scholar] [CrossRef]
- Frleta, D.; Lin, J.T.; Quezada, S.A.; Wade, T.K.; Barth, R.J.; Noelle, R.J.; Wade, W.F. Distinctive maturation of in vitro versus in vivo anti-cd40 mab-matured dendritic cells in mice. J. Immunother. 2003, 26, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Pearson, T.C. The cd40 pathway in allograft rejection, acceptance, and tolerance. Curr. Opin. Immunol. 1997, 9, 641–647. [Google Scholar] [CrossRef]
- Hancock, W.W.; Sayegh, M.H.; Zheng, X.G.; Peach, R.; Linsley, P.S.; Turka, L.A. Costimulatory function and expression of cd40 ligand, cd80, and cd86 in vascularized murine cardiac allograft rejection. Proc. Natl. Acad. Sci. USA 1996, 93, 13967–13972. [Google Scholar] [CrossRef]
- Parker, D.C.; Greiner, D.L.; Phillips, N.E.; Appel, M.C.; Steele, A.W.; Durie, F.H.; Noelle, R.J.; Mordes, J.P.; Rossini, A.A. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to cd40 ligand. Proc. Natl. Acad. Sci. USA 1995, 92, 9560–9564. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Alexander, D.Z.; Hollenbaugh, D.; Elwood, E.T.; Ritchie, S.C.; Aruffo, A.; Hendrix, R.; Pearson, T.C. Cd40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the cd40-gp39 pathway. Transplantation 1996, 61, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sho, M.; Sandner, S.E.; Najafian, N.; Salama, A.D.; Dong, V.; Yamada, A.; Kishimoto, K.; Harada, H.; Schmitt, I.; Sayegh, M.H. New insights into the interactions between t-cell costimulatory blockade and conventional immunosuppressive drugs. Ann. Surg. 2002, 236, 667–675. [Google Scholar] [CrossRef]
- Quezada, S.A.; Jarvinen, L.Z.; Lind, E.F.; Noelle, R.J. Cd40/cd154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004, 22, 307–328. [Google Scholar] [CrossRef]
- Qian, Y.; Boisgerault, F.; Benichou, G.; Dana, M.R. Blockade of cd40-cd154 costimulatory pathway promotes survival of allogeneic corneal transplants. Investig. Ophthalmol. Vis. Sci. 2001, 42, 987–994. [Google Scholar]
- Tan, X.; Zeng, H.; Jie, Y.; Zhang, Y.; Xu, Q.; Pan, Z. Cd154 blockade modulates the ratio of treg to th1 cells and prolongs the survival of allogeneic corneal grafts in mice. Exp. Ther. Med. 2014, 7, 827–834. [Google Scholar] [CrossRef][Green Version]
- Qian, Y.; Dana, M.R. Effect of locally administered anti-cd154 (cd40 ligand) monoclonal antibody on survival of allogeneic corneal transplants. Cornea 2002, 21, 592–597. [Google Scholar] [CrossRef]
- Qian, Y.; Hamrah, P.; Boisgerault, F.; Yamagami, S.; Vora, S.; Benichou, G.; Dana, M.R. Mechanisms of immunotherapeutic intervention by anti-cd154 (cd40l) antibody in high-risk corneal transplantation. J. Interferon. Cytokine Res. 2002, 22, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, E.; Gao, W.; Shemmeri, N.; Wang, C.; Gutierrez-Ramos, J.C.; Amaral, J.; Qin, S.; Rottman, J.B.; Coyle, A.J.; Hancock, W.W. Importance of icos-b7rp-1 costimulation in acute and chronic allograft rejection. Nat. Immunol. 2001, 2, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, X.K.; Funeshima, N.; Fujino, M.; Nagata, Y.; Kimura, H.; Amemiya, H.; Enosawa, S.; Tsuji, T.; Harihara, Y.; et al. Prolonged survival in rat liver transplantation with mouse monoclonal antibody against an inducible costimulator (icos). Transplantation 2002, 73, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Panoskaltsis-Mortari, A.; Freeman, G.J.; Sharpe, A.H.; Noelle, R.J.; Rudensky, A.Y.; Mak, T.W.; Serody, J.S.; Blazar, B.R. Targeting of inducible costimulator (ICOS) expressed on alloreactive t cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM). Blood 2005, 105, 3372–3380. [Google Scholar] [CrossRef] [PubMed]
- Hutloff, A.; Dittrich, A.M.; Beier, K.C.; Eljaschewitsch, B.; Kraft, R.; Anagnostopoulos, I.; Kroczek, R.A. Icos is an inducible t-cell co-stimulator structurally and functionally related to cd28. Nature 1999, 397, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Salama, A.D.; Sho, M.; Izawa, A.; Sandner, S.E.; Ito, T.; Akiba, H.; Yagita, H.; Sharpe, A.H.; Freeman, G.J.; et al. The role of the icos-b7h t cell costimulatory pathway in transplantation immunity. J. Clin. Investig. 2003, 112, 234–243. [Google Scholar] [CrossRef]
- Tang, G.; Qin, Q.; Zhang, P.; Wang, G.; Liu, M.; Ding, Q.; Qin, Y.; Shen, Q. Reverse signaling using an inducible costimulator to enhance immunogenic function of dendritic cells. Cell Mol. Life Sci. 2009, 66, 3067–3080. [Google Scholar] [CrossRef]
- Occhipinti, S.; Dianzani, C.; Chiocchetti, A.; Boggio, E.; Clemente, N.; Gigliotti, C.L.; Soluri, M.F.; Minelli, R.; Fantozzi, R.; Yagi, J.; et al. Triggering of b7h by the icos modulates maturation and migration of monocyte-derived dendritic cells. J. Immunol. 2013, 190, 1125–1134. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yasunami, Y.; Satoh, M.; Hirakawa, E.; Katsuta, H.; Ono, J.; Kamada, M.; Todo, S.; Nakayama, T.; Taniguchi, M.; et al. Acceptance of islet allografts in the liver of mice by blockade of an inducible costimulator. Transplantation 2003, 75, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Kingsley, C.I.; Zhang, X.; Jabs, C.; Izikson, L.; Sobel, R.A.; Weiner, H.L.; Kuchroo, V.K.; Sharpe, A.H. The icos molecule plays a crucial role in the development of mucosal tolerance. J. Immunol. 2005, 175, 7341–7347. [Google Scholar] [CrossRef] [PubMed]
- Akbari, O.; Freeman, G.J.; Meyer, E.H.; Greenfield, E.A.; Chang, T.T.; Sharpe, A.H.; Berry, G.; DeKruyff, R.H.; Umetsu, D.T. Antigen-specific regulatory t cells develop via the icos-icos-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002, 8, 1024–1032. [Google Scholar] [CrossRef]
- Burmeister, Y.; Lischke, T.; Dahler, A.C.; Mages, H.W.; Lam, K.P.; Coyle, A.J.; Kroczek, R.A.; Hutloff, A. Icos controls the pool size of effector-memory and regulatory t cells. J. Immunol. 2008, 180, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Iclozan, C.; Suh, W.K.; Anasetti, C.; Yu, X.Z. Cd28 controls differentiation of regulatory t cells from naive cd4 t cells. J. Immunol. 2008, 181, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, S.; Gorentla, B.K.; Gao, J.; Zhong, X.P. Murine regulatory t cells contain hyperproliferative and death-prone subsets with differential icos expression. J. Immunol. 2012, 188, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Kunishige, T.; Taniguchi, H.; Terada, M.; Akiba, H.; Yagita, H.; Abe, R.; Hori, J. Protective role of icos and icos ligand in corneal transplantation and in maintenance of immune privilege. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6815–6823. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Gong, N.; Vogt, K.; Volk, H.D.; Pleyer, U.; Ritter, T. The influence of inducible costimulator fusion protein (ICOSIG) gene transfer on corneal allograft survival. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the pd-1 immunoinhibitory receptor by a novel b7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the b7-1 costimulatory molecule to inhibit t cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. Pd-l1 regulates the development, maintenance, and function of induced regulatory t cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Versteven, M.; Van den Bergh, J.M.J.; Marcq, E.; Smits, E.L.J.; Van Tendeloo, V.F.I.; Hobo, W.; Lion, E. Dendritic cells and programmed death-1 blockade: A joint venture to combat cancer. Front. Immunol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. Pd-l2 is a second ligand for pd-1 and inhibits t cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; Muskens, F.; Willart, M.; Hijdra, D.; van Assema, F.B.; Coyle, A.J.; Hoogsteden, H.C.; Lambrecht, B.N. Contribution of the pd-1 ligands/pd-1 signaling pathway to dendritic cell-mediated cd4+ t cell activation. Eur. J. Immunol. 2006, 36, 2472–2482. [Google Scholar] [CrossRef] [PubMed]
- Hori, J.; Wang, M.; Miyashita, M.; Tanemoto, K.; Takahashi, H.; Takemori, T.; Okumura, K.; Yagita, H.; Azuma, M. B7-h1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J. Immunol. 2006, 177, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jin, Y.; Freeman, G.J.; Sharpe, A.H.; Dana, M.R. The function of donor versus recipient programmed death-ligand 1 in corneal allograft survival. J. Immunol. 2007, 179, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chauhan, S.K.; El Annan, J.; Sage, P.T.; Sharpe, A.H.; Dana, R. A novel function for programmed death ligand-1 regulation of angiogenesis. Am. J. Pathol. 2011, 178, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Nosov, M.; Wilk, M.; Morcos, M.; Cregg, M.; O’Flynn, L.; Treacy, O.; Ritter, T. Role of lentivirus-mediated overexpression of programmed death-ligand 1 on corneal allograft survival. Am. J. Transplant. 2012, 12, 1313–1322. [Google Scholar] [CrossRef]
- Gao, W.; Demirci, G.; Strom, T.B.; Li, X.C. Stimulating pd-1-negative signals concurrent with blocking cd154 co-stimulation induces long-term islet allograft survival. Transplantation 2003, 76, 994–999. [Google Scholar] [CrossRef]
- Kosuge, H.; Suzuki, J.; Gotoh, R.; Koga, N.; Ito, H.; Isobe, M.; Inobe, M.; Uede, T. Induction of immunologic tolerance to cardiac allograft by simultaneous blockade of inducible co-stimulator and cytotoxic t-lymphocyte antigen 4 pathway. Transplantation 2003, 75, 1374–1379. [Google Scholar] [CrossRef]
- Doyen, V.; Rubio, M.; Braun, D.; Nakajima, T.; Abe, J.; Saito, H.; Delespesse, G.; Sarfati, M. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 2003, 198, 1277–1283. [Google Scholar] [CrossRef]
- Grimbert, P.; Bouguermouh, S.; Baba, N.; Nakajima, T.; Allakhverdi, Z.; Braun, D.; Saito, H.; Rubio, M.; Delespesse, G.; Sarfati, M. Thrombospondin/cd47 interaction: A pathway to generate regulatory t cells from human cd4+ cd25- t cells in response to inflammation. J. Immunol. 2006, 177, 3534–3541. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.; Sunday, M.; Thibert, V.; Duquette, M.; George, E.L.; Rayburn, H.; Hynes, R.O. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J. Clin. Investig. 1998, 101, 982–992. [Google Scholar] [CrossRef]
- Crawford, S.E.; Stellmach, V.; Murphy-Ullrich, J.E.; Ribeiro, S.M.; Lawler, J.; Hynes, R.O.; Boivin, G.P.; Bouck, N. Thrombospondin-1 is a major activator of tgf-beta1 in vivo. Cell 1998, 93, 1159–1170. [Google Scholar] [CrossRef]
- Krispin, A.; Bledi, Y.; Atallah, M.; Trahtemberg, U.; Verbovetski, I.; Nahari, E.; Zelig, O.; Linial, M.; Mevorach, D. Apoptotic cell thrombospondin-1 and heparin-binding domain lead to dendritic-cell phagocytic and tolerizing states. Blood 2006, 108, 3580–3589. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. ‘Yin-yang’ functions of transforming growth factor-beta and t regulatory cells in immune regulation. Immunol. Rev. 2007, 220, 199–213. [Google Scholar] [CrossRef]
- Yehualaeshet, T.; O’Connor, R.; Green-Johnson, J.; Mai, S.; Silverstein, R.; Murphy-Ullrich, J.E.; Khalil, N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am. J. Pathol. 1999, 155, 841–851. [Google Scholar] [CrossRef]
- Demeure, C.E.; Tanaka, H.; Mateo, V.; Rubio, M.; Delespesse, G.; Sarfati, M. Cd47 engagement inhibits cytokine production and maturation of human dendritic cells. J. Immunol. 2000, 164, 2193–2199. [Google Scholar] [CrossRef]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by cd36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Saban, D.R.; Bock, F.; Chauhan, S.K.; Masli, S.; Dana, R. Thrombospondin-1 derived from apcs regulates their capacity for allosensitization. J. Immunol. 2010, 185, 4691–4697. [Google Scholar] [CrossRef]
- Soriano-Romani, L.; Contreras-Ruiz, L.; Lopez-Garcia, A.; Diebold, Y.; Masli, S. Topical application of TGF-β-activating peptide, KRFK, prevents inflammatory manifestations in the TSP-1-deficient mouse model of chronic ocular inflammation. Int. J. Mol. Sci. 2018, 20, 9. [Google Scholar] [CrossRef]
- Bromberg, J.S. Il-10 immunosuppression in transplantation. Curr. Opin. Immunol. 1995, 7, 639–643. [Google Scholar] [CrossRef]
- Sato, K.; Yamashita, N.; Matsuyama, T. Human peripheral blood monocyte-derived interleukin-10-induced semi-mature dendritic cells induce anergic CD4(+) and CD8(+) t cells via presentation of the internalized soluble antigen and cross-presentation of the phagocytosed necrotic cellular fragments. Cell. Immunol. 2002, 215, 186–194. [Google Scholar] [CrossRef]
- Muller, G.; Muller, A.; Tuting, T.; Steinbrink, K.; Saloga, J.; Szalma, C.; Knop, J.; Enk, A.H. Interleukin-10-treated dendritic cells modulate immune responses of naive and sensitized t cells in vivo. J. Investig. Dermatol. 2002, 119, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Klebe, S.; Sykes, P.J.; Coster, D.J.; Krishnan, R.; Williams, K.A. Prolongation of sheep corneal allograft survival by ex vivo transfer of the gene encoding interleukin-10. Transplantation 2001, 71, 1214–1220. [Google Scholar] [CrossRef]
- Gong, N.; Pleyer, U.; Volk, H.D.; Ritter, T. Effects of local and systemic viral interleukin-10 gene transfer on corneal allograft survival. Gene Ther. 2007, 14, 484–490. [Google Scholar] [CrossRef]
- Tahvildari, M.; Emami-Naeini, P.; Omoto, M.; Mashaghi, A.; Chauhan, S.K.; Dana, R. Treatment of donor corneal tissue with immunomodulatory cytokines: A novel strategy to promote graft survival in high-risk corneal transplantation. Sci. Rep. 2017, 7, 971. [Google Scholar] [CrossRef]
- Wai Wong, C.; Dye, D.E.; Coombe, D.R. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int. J. Cell Biol. 2012, 2012, 340296. [Google Scholar] [CrossRef]
- Ikeda, K.; Quertermous, T. Molecular isolation and characterization of a soluble isoform of activated leukocyte cell adhesion molecule that modulates endothelial cell function. J. Biol. Chem. 2004, 279, 55315–55323. [Google Scholar] [CrossRef]
- Van Kempen, L.C.; Nelissen, J.M.; Degen, W.G.; Torensma, R.; Weidle, U.H.; Bloemers, H.P.; Figdor, C.G.; Swart, G.W. Molecular basis for the homophilic activated leukocyte cell adhesion molecule (ALCAM)-ALCAM interaction. J. Biol. Chem. 2001, 276, 25783–25790. [Google Scholar] [CrossRef]
- Bowen, M.A.; Patel, D.D.; Li, X.; Modrell, B.; Malacko, A.R.; Wang, W.C.; Marquardt, H.; Neubauer, M.; Pesando, J.M.; Francke, U.; et al. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J. Exp. Med. 1995, 181, 2213–2220. [Google Scholar] [CrossRef]
- Kim, M.N.; Hong, J.Y.; Shim, D.H.; Sol, I.S.; Kim, Y.S.; Lee, J.H.; Kim, K.W.; Lee, J.M.; Sohn, M.H. Activated leukocyte cell adhesion molecule stimulates the t-cell response in allergic asthma. Am. J. Respir. Crit. Care Med. 2018, 197, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Iolyeva, M.; Karaman, S.; Willrodt, A.H.; Weingartner, S.; Vigl, B.; Halin, C. Novel role for ALCAM in lymphatic network formation and function. FASEB J. 2013, 27, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, A.H.; Beffinger, M.; Vranova, M.; Protsyuk, D.; Schuler, K.; Jadhav, M.; Heikenwalder, M.; van den Broek, M.; Borsig, L.; Halin, C. Stromal expression of activated leukocyte cell adhesion molecule promotes lung tumor growth and metastasis. Am. J. Pathol. 2017, 187, 2558–2569. [Google Scholar] [CrossRef]
- Lecuyer, M.A.; Saint-Laurent, O.; Bourbonniere, L.; Larouche, S.; Larochelle, C.; Michel, L.; Charabati, M.; Abadier, M.; Zandee, S.; Haghayegh Jahromi, N.; et al. Dual role of alcam in neuroinflammation and blood-brain barrier homeostasis. Proc. Natl. Acad. Sci. USA 2017, 114, E524–E533. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, A.H.; Salabarria, A.C.; Schineis, P.; Ignatova, D.; Hunter, M.C.; Vranova, M.; Golding-Ochsenbein, A.M.; Sigmund, E.; Romagna, A.; Strassberger, V.; et al. Alcam mediates DC migration through afferent lymphatics and promotes allospecific immune reactions. Front. Immunol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shan, J.; Guo, Y.; Li, S.; Long, D.; Li, Y.; Feng, L. Effects of adoptive transfer of tolerogenic dendritic cells on allograft survival in organ transplantation models: An overview of systematic reviews. J. Immunol. Res. 2016, 2016, 5730674. [Google Scholar] [CrossRef]
- Moreau, A.; Varey, E.; Beriou, G.; Hill, M.; Bouchet-Delbos, L.; Segovia, M.; Cuturi, M.C. Tolerogenic dendritic cells and negative vaccination in transplantation: From rodents to clinical trials. Front. Immunol. 2012, 3, 218. [Google Scholar] [CrossRef]
- Yan, F.; Cai, L.; Hui, Y.; Chen, S.; Meng, H.; Huang, Z. Tolerogenic dendritic cells suppress murine corneal allograft rejection by modulating CD28/CTLA-4 expression on regulatory t cells. Cell. Biol. Int. 2014, 38, 835–848. [Google Scholar] [CrossRef]
- Wang, Z.; Divito, S.J.; Shufesky, W.J.; Sumpter, T.; Wang, H.; Tkacheva, O.A.; Wang, W.; Liu, C.; Larregina, A.T.; Morelli, A.E. Dendritic cell therapies in transplantation revisited: Deletion of recipient dcs deters the effect of therapeutic DCS. Am. J. Transplant. 2012, 12, 1398–1408. [Google Scholar] [CrossRef]
| Compound | Function/Effect | Treatment | Results | Ref. |
|---|---|---|---|---|
| sCD83 | tolerogenic | systemic | prolongation of corneal graft survival* | [52] |
| eye drops | prolongation of corneal graft survival* | [52] | ||
| CTLA4Ig | mimics CTLA4-CD80/CD86 interaction | systemic | moderate effect on corneal graft survival*† | [71,73] |
| subconjunctival | no effect on corneal graft survival* | [71] | ||
| pre-incubation | prolongation of corneal graft survival†‡ | [72,73] | ||
| ex vivo gene transfer (viral) | moderate effect on corneal graft survival† | [73,74] | ||
| systemic gene transfer (viral) | prolongation of corneal graft survival† | [73,74] | ||
| CTLA4-FasL | mimics CTLA-CD80/CD86 interaction facilitating Fas-mediated apoptosis | pre-incubation | prolongation of corneal graft survival* | [87] |
| anti-CD154 | blocks CD40-CD154 interaction | systemic | prolongation of corneal graft survival*1 | [97,98] |
| subconjunctival | prolongation of corneal graft survival*1 | [99] | ||
| anti-ICOS | blocks ICOS-ICOSL interaction | systemic | no effect on corneal graft survival* | [69] |
| ICOS-Ig | mimics ICOS-ICOSL interaction | ex vivo and systemic gene transfer (viral) | no effect on corneal graft survival† | [113] |
| PD-L1 | stimulates PD-1 | ex vivo gene transfer (viral) | prolongation of corneal graft survival† | [123] |
| PD-L1-Ig | mimics PD-L1-PD-1 interaction | systemic | prolongation of corneal graft survival* | [69] |
| IL-10 | inhibits Th1 immune response, induces tolDCs | subconjunctival | no effect on corneal graft survival† | [139] |
| systemic | no effect on corneal graft survival† | [139] | ||
| ex vivo gene transfer (viral) | prolongation of corneal graft survivalΦ | [140] | ||
| ex vivo gene transfer (plasmid/liposome) | moderate effect on corneal graft survival† | [141] | ||
| systemic gene transfer (viral) | prolongation of corneal graft survival† | [141] | ||
| IL-10, TGF-β | inhibits Th1 immune response, induces tolDCs | local treatment of the donor | prolongation of corneal graft survival* | [142] |
| anti-ALCAM | blocks ALCAM | systemic | prolongation of corneal graft survival* | [151] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönberg, A.; Hamdorf, M.; Bock, F. Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 1280. https://doi.org/10.3390/jcm9051280
Schönberg A, Hamdorf M, Bock F. Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival. Journal of Clinical Medicine. 2020; 9(5):1280. https://doi.org/10.3390/jcm9051280
Chicago/Turabian StyleSchönberg, Alfrun, Matthias Hamdorf, and Felix Bock. 2020. "Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival" Journal of Clinical Medicine 9, no. 5: 1280. https://doi.org/10.3390/jcm9051280
APA StyleSchönberg, A., Hamdorf, M., & Bock, F. (2020). Immunomodulatory Strategies Targeting Dendritic Cells to Improve Corneal Graft Survival. Journal of Clinical Medicine, 9(5), 1280. https://doi.org/10.3390/jcm9051280





