Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Treatment
2.2. Participants
2.3. Patient Information and Follow-Up
2.4. Study End Points
2.5. Safety
2.6. Sample Size Calculation
2.7. Randomization
2.8. Statistical Methods
2.9. Missing Data
3. Results
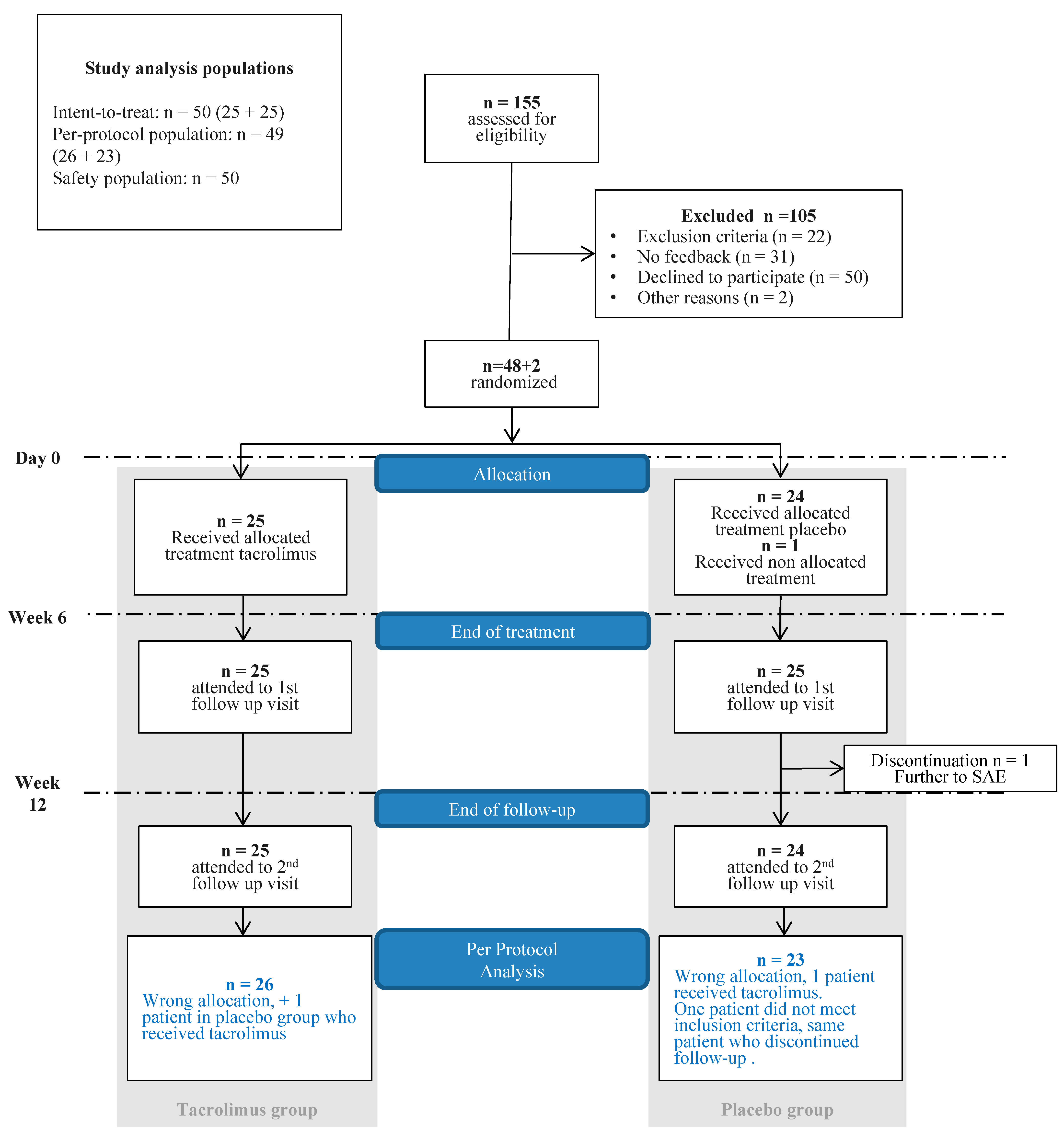
3.1. Trial Population
3.2. Response to Treatment
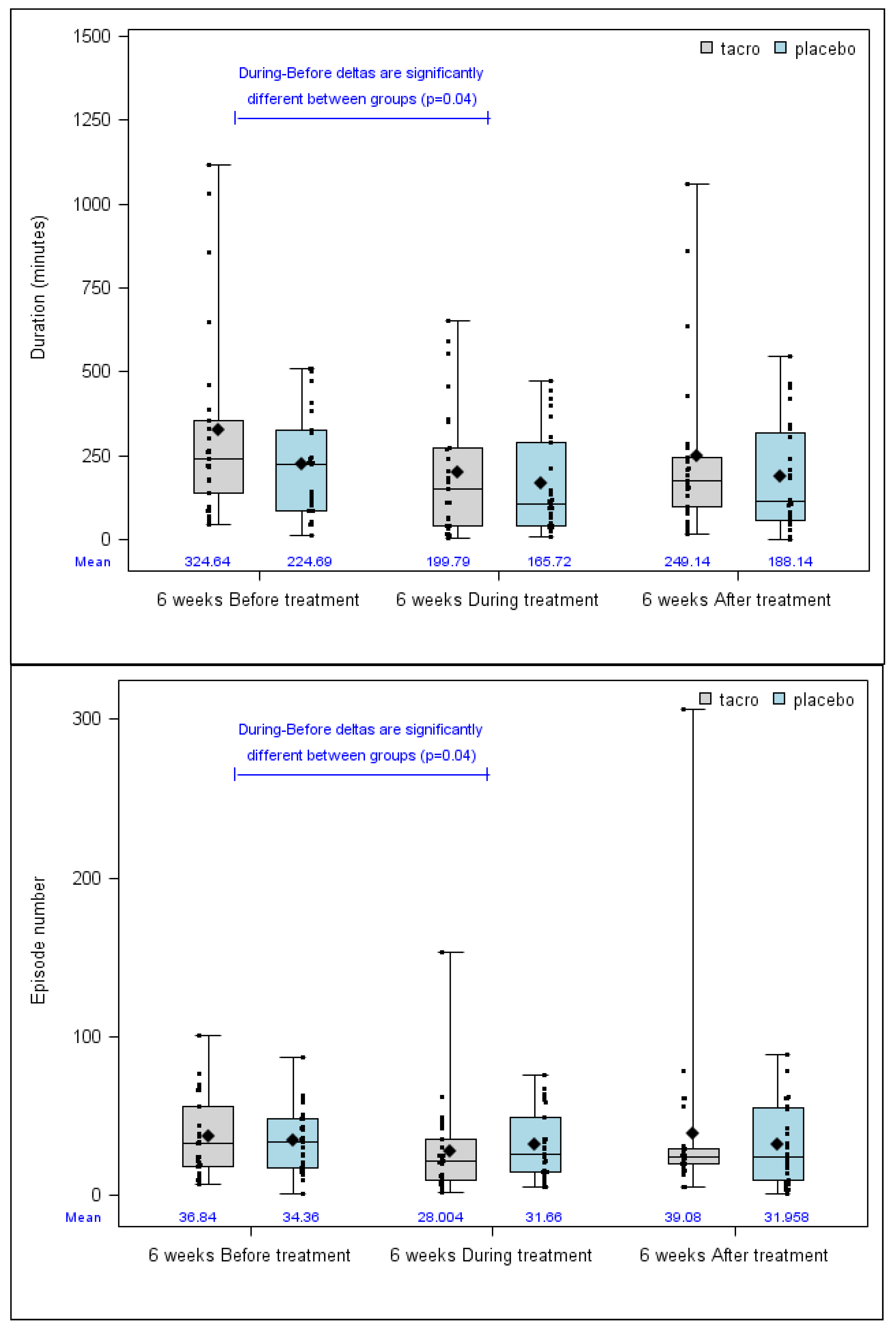
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.3. Safety Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Trial Registration
References
- Lesca, G.; Olivieri, C.; Burnichon, N.; Pagella, F.; Carette, M.F.; Gilbert-Dussardier, B.; Goizet, C.; Roume, J.; Rabilloud, M.; Saurin, J.C.; et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: Data from the French-Italian HHT network. Genet. Med. 2007, 9, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Tillet, E.; Bailly, S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front. Genet. 2014, 5, 456. [Google Scholar] [CrossRef] [PubMed]
- Guttmacher, A.E.; Marchuk, D.A.; White, R.I., Jr. Hereditary hemorrhagic telangiectasia. N. Engl. J. Med. 1995, 333, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Girod, S.; Bailly, S.; Plauchu, H. Hereditary hemorrhagic telangiectasia: From molecular biology to patient care. J. Thromb. Haemost. 2010, 8, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Geisthoff, U.W.; Heckmann, K.; D’Amelio, R.; Grunewald, S.; Knobber, D.; Falkai, P.; Konig, J. Health-related quality of life in hereditary hemorrhagic telangiectasia. Otolaryngol. Head Neck Surg. 2007, 136, 726-e1. [Google Scholar] [CrossRef]
- Robert, F.; Desroches-Castan, A.; Bailly, S.; Dupuis-Girod, S.; Feige, J.J. Future treatments for hereditary hemorrhagic telangiectasia. Orphanet J. Rare Dis. 2020, 15, 4. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Ginon, I.; Saurin, J.C.; Marion, D.; Guillot, E.; Decullier, E.; Roux, A.; Carette, M.F.; Gilbert-Dussardier, B.; Hatron, P.Y.; et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA 2012, 307, 948–955. [Google Scholar] [CrossRef]
- Lebrin, F.; Srun, S.; Raymond, K.; Martin, S.; van den Brink, S.; Freitas, C.; Breant, C.; Mathivet, T.; Larrivee, B.; Thomas, J.L.; et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat. Med. 2010, 16, 420–428. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Ambrun, A.; Decullier, E.; Fargeton, A.E.; Roux, A.; Breant, V.; Colombet, B.; Riviere, S.; Cartier, C.; Lacombe, P.; et al. Effect of bevacizumab nasal spray on epistaxis duration in hereditary hemorrhagic telangectasia: a randomized clinical trial. JAMA 2016, 316, 934–942. [Google Scholar] [CrossRef]
- Whitehead, K.J.; Sautter, N.B.; McWilliams, J.P.; Chakinala, M.M.; Merlo, C.A.; Johnson, M.H.; James, M.; Everett, E.M.; Clancy, M.S.; Faughnan, M.E.; et al. Effect of topical intranasal therapy on epistaxis frequency in patients with hereditary hemorrhagic telangiectasia: A randomized clinical trial. JAMA 2016, 316, 943–951. [Google Scholar] [CrossRef]
- Ruiz, S.; Chandakkar, P.; Zhao, H.; Papoin, J.; Chatterjee, P.K.; Christen, E.; Metz, C.N.; Blanc, L.; Campagne, F.; Marambaud, P. Tacrolimus rescues the signaling and gene expression signature of endothelial ALK1 loss-of-function and improves HHT vascular pathology. Hum. Mol. Genet. 2017, 26, 4786–4798. [Google Scholar] [CrossRef]
- Spiekerkoetter, E.; Tian, X.; Cai, J.; Hopper, R.K.; Sudheendra, D.; Li, C.G.; El-Bizri, N.; Sawada, H.; Haghighat, R.; Chan, R.; et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Investig. 2013, 123, 3600–3613. [Google Scholar] [CrossRef]
- Albinana, V.; Sanz-Rodriguez, F.; Recio-Poveda, L.; Bernabeu, C.; Botella, L.M. Immunosuppressor FK506 increases endoglin and activin receptor-like kinase 1 expression and modulates transforming growth factor-beta1 signaling in endothelial cells. Mol. Pharmacol. 2011, 79, 833–843. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Chesnais, A.L.; Ginon, I.; Dumortier, J.; Saurin, J.C.; Finet, G.; Decullier, E.; Marion, D.; Plauchu, H.; Boillot, O. Long-term outcome of patients with hereditary hemorrhagic telangiectasia and severe hepatic involvement after orthotopic liver transplantation: A single-center study. Liver Transplant. 2010, 16, 340–347. [Google Scholar]
- Liao, Y.H.; Chiu, H.C.; Tseng, Y.S.; Tsai, T.F. Comparison of cutaneous tolerance and efficacy of calcitriol 3 microg g(-1) ointment and tacrolimus 0.3 mg g(-1) ointment in chronic plaque psoriasis involving facial or genitofemoral areas: A double-blind, randomized controlled trial. Br. J. Dermatol. 2007, 157, 1005–1012. [Google Scholar] [CrossRef]
- Corrocher, G.; Di Lorenzo, G.; Martinelli, N.; Mansueto, P.; Biasi, D.; Nocini, P.F.; Lombardo, G.; Fior, A.; Corrocher, R.; Bambara, L.M.; et al. Comparative effect of tacrolimus 0.1% ointment and clobetasol 0.05% ointment in patients with oral lichen planus. J. Clin. Periodontol. 2008, 35, 244–249. [Google Scholar] [CrossRef]
- Vohra, S.; Singal, A.; Sharma, S.B. Clinical and serological efficacy of topical calcineurin inhibitors in oral lichen planus: A prospective randomized controlled trial. Int. J. Dermatol. 2016, 55, 101–105. [Google Scholar] [CrossRef]
- Arduino, P.G.; Carbone, M.; Della Ferrera, F.; Elia, A.; Conrotto, D.; Gambino, A.; Comba, A.; Calogiuri, P.L.; Broccoletti, R. Pimecrolimus vs. tacrolimus for the topical treatment of unresponsive oral erosive lichen planus: A 8 week randomized double-blind controlled study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 475–482. [Google Scholar] [CrossRef]
- Reitamo, S.; Mandelin, J.; Rubins, A.; Remitz, A.; Makela, M.; Cirule, K.; Rubins, S.; Zigure, S.; Ho, V.; Dickinson, J.; et al. The pharmacokinetics of tacrolimus after first and repeated dosing with 0.03% ointment in infants with atopic dermatitis. Int. J. Dermatol. 2009, 48, 348–355. [Google Scholar] [CrossRef]
- Harper, J.; Smith, C.; Rubins, A.; Green, A.; Jackson, K.; Zigure, S.; Bourke, J.; Alomar, A.; Stevenson, P.; Foster, C.; et al. A multicenter study of the pharmacokinetics of tacrolimus ointment after first and repeated application to children with atopic dermatitis. J. Investig. Dermatol. 2005, 124, 695–699. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Droege, F.; Gamen, K.E.; Geisthoff, U.; Gall, H.; Tello, K.; Richter, M.J.; Deubner, L.M.; Schmiedel, R.; Hecker, M.; et al. Treatment with low-dose tacrolimus inhibits bleeding complications in a patient with hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension. Pulm. Circ. 2019, 9, 2045894018805406. [Google Scholar] [CrossRef] [PubMed]
- Hoag, J.B.; Terry, P.; Mitchell, S.; Reh, D.; Merlo, C.A. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 2010, 120, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.X.; Reh, D.D.; Hoag, J.B.; Mitchell, S.E.; Mathai, S.C.; Robinson, G.M.; Merlo, C.A. The minimal important difference of the epistaxis severity score in hereditary hemorrhagic telangiectasia. Laryngoscope 2016, 126, 1029–1032. [Google Scholar] [CrossRef]
- Hrobjartsson, A.; Forfang, E.; Haahr, M.T.; Als-Nielsen, B.; Brorson, S. Blinded trials taken to the test: An analysis of randomized clinical trials that report tests for the success of blinding. Int. J. Epidemiol. 2007, 36, 654–663. [Google Scholar] [CrossRef]
| Variable | Modality | All | Tacro Group n (%) | Placebo Group n (%) |
|---|---|---|---|---|
| Number | n | 50 | 25 | 25 |
| Age (years) | Median (min–max) | 62 (27–85) | 60 (27–81) | 64 (39–85) |
| Mean (SD) | 60.92 (11.26) | 59.04 (12.26) | 62.8 (10.06) | |
| Females | n (%) | 23 (46) | 9 (36) | 14 (56) |
| Mutated gene | n (%) | |||
| ALK1 | 36 (72) | 20 (80) | 16 (64) | |
| ENG | 10 (20) | 5 (20) | 5 (20) | |
| On-going | 2 (4) | 2 (8) | ||
| Not known | 2 (4) | 2 (8) | ||
| Blood transfusions in the last 6 weeks before inclusion | n (%) | 2 (4) | 0 (0) | 2 (8) |
| Parameters on inclusion (D0) | ||||
| Nasal surgery | n (%) | 35 (70) | 21 (84) | 14 (56) |
| Nasal septum perforation | n (%) | 7 (14.3) | 3 (12.5) | 4 (16) |
| Hemoglobin level | Mean ± SD | 126.62 (22.66) | 127.6 (20.82) | 125.64 (24.75) |
| (g/dL) | Median (Min–Max) | 130 (66–163) | 129 (90–163) | 130 (66–158) |
| Ferritin level (ng/mL) | Mean ± SD | 50.12 (73.7) | 51.28 (94.79) | 48.96 (45.83) |
| Median (Min–Max) | 28 (4–458) | 23 (4–458) | 33 (6–174) | |
| Systolic blood pressure (mmHg) | Mean ± SD | 130.7 (20.98) | 133.16 (16.5) | 128.24 (24.78) |
| Median (Min–Max) | 126.5 (100–181) | 130 (110–163) | 124 (100–181) | |
| Diastolic blood pressure (mmHg) | Mean ± SD | 80.24 (16.32) | 82.2 (11.42) | 78.28 (20.13) |
| Median (Min–Max) | 80 (28–129) | 80 (60–105) | 80 (28–129) |
| Variable | Modality | All | Tacro Group n (%) | Placebo Group n (%) | p-Value | Effect Size * |
|---|---|---|---|---|---|---|
| Epistaxis duration decrease > 30% (ITT) | No | 31 (62) | 15 (60) | 16 (64) | 0.77 | RD 4.0 (−23.4–31.2) RR 1.11 (0.55–2.26) |
| Yes | 19 (38) | 10 (40) | 9 (36) | |||
| Epistaxis duration decrease > 30% (PP) | No | 30 (61.2) | 16 (61.5) | 14 (60.9) | 0.96 | RD −1.0 (–28.2–28.0) RR 0.98 (0.49–1.99) |
| Yes | 19 (38.8) | 10 (38.5) | 9 (39.1) | |||
| Other data related to primary outcome | ||||||
| Epistaxis total duration 6 weeks before treatment (min) | n | 50 | 25 | 25 | 0.34 | −0.42 (–1.00–0.15) |
| median (min–max) | 226.5 (11–1116) | 240 (46–1116) | 226 (11–510.8) | |||
| Mean (SD) | 274.67 (239.24) | 324.64 (292.06) | 224.69 (162.35) | |||
| Epistaxis total duration 6 weeks immediately after the end of the treatment (min) | n | 49 | 25 | 24 | 0.42 | −0.29 (−0.86–0.29) |
| median (min–max) | 170 (1–1058) | 177 (16–1058) | 114.5 (1–547) | |||
| Mean (SD) | 219.26 (213.72) | 249.14 (252.7) | 188.14 (163.43) | |||
| Variable | All | Tacro Group | Placebo Group | p-Value * | Effect Size ** | ||
|---|---|---|---|---|---|---|---|
| Physical functioning | B/D | n | 46 | 22 | 24 | 0.89 | 0.04 (−0.55–0.63) |
| median (min–max) | 0 (−25–40) | 0 (−15–20) | 0 (−25–40) | ||||
| Mean (SD) | 0.43 (11.44) | 0.68 (9.55) | 0.21 (13.14) | ||||
| B/A | n | 49 | 25 | 24 | 0.38• | 0.07 (−0.5–0.64) | |
| median (min–max) | 0 (−35–55) | 0 (−35–20) | 0 (−30–55) | ||||
| Mean (SD) | −0.51 (14.62) | 0 (10.99) | −1.04 (17.88) | ||||
| Physical role | B/D | n | 45 | 22 | 23 | 0.28• | 0.39 (−0.21–0.99) |
| median (min–max) | 0 (−50–125) | 0 (−50–125) | 0 (−50–75) | ||||
| Mean (SD) | 5 (37.91) | 12.5 (42.08) | −2.17 (32.78) | ||||
| B/A | n | 46 | 23 | 23 | 0.78• | 0.3 (−0.29–0.9) | |
| median (min–max) | 0 (−75–125) | 0 (−50–125) | 0 (−75–50) | ||||
| Mean (SD) | 2.17 (35.68) | 7.61 (40.19) | −3.26 (30.44) | ||||
| Bodily pain | B/D | n | 46 | 22 | 24 | 0.53 | 0.19 (−0.41–0.78) |
| median (min–max) | 0 (−59–38) | 0 (−59–38) | 0 (−29–22) | ||||
| Mean (SD) | −0.02 (17.36) | 1.68 (20.24) | −1.58 (14.51) | ||||
| B/A | n | 49 | 25 | 24 | 0.88• | −0.12 (−0.69–0.45) | |
| median (min–max) | 0 (−43–22) | 0 (−38–22) | 0 (−43–22) | ||||
| Mean (SD) | −0.49 (15.66) | −1.4 (17.14) | 0.46 (14.26) | ||||
| General health | B/D | n | 46 | 22 | 24 | 0.36 | 0.27 (−0.32–0.87) |
| median (min–max) | 0 (−20–25) | 2.75 (−20–23.75) | 0 (−20–25) | ||||
| Mean (SD) | 2.06 (10.06) | 3.5 (11.35) | 0.74 (8.75) | ||||
| B/A | n | 49 | 25 | 24 | 0.59 | 0.15 (−0.42–0.73) | |
| median (min–max) | 0 (−17–28.25) | 0 (−15–28.25) | 0 (−17–25) | ||||
| Mean (SD) | 0.14 (10.01) | 0.9 (10.23) | −0.65 (9.93) | ||||
| Vitality | B/D | n | 46 | 22 | 24 | 0.45 | 0.23 (−0.37–0.82) |
| median (min–max) | 0 (−25–30) | 3.34 (−10–25) | 0 (−25–30) | ||||
| Mean (SD) | 2.32 (11.81) | 3.71 (9.64) | 1.04 (13.59) | ||||
| B/A | n | 49 | 25 | 24 | 0.64 | −0.13 (−0.71–0.44) | |
| median (min–max) | −3.33 (−35–35) | −3.33 (−35–35) | −2.5 (−20–20) | ||||
| Mean (SD) | −2.11 (12.54) | −2.93 (14.06) | −1.25 (10.96) | ||||
| Social functioning | B/D | n | 46 | 22 | 24 | 0.67 | 0.13 (−0.47–0.72) |
| median (min–max) | 0 (−37.5–37.5) | 12.5 (−25–37.5) | 0 (−37.5–37.5) | ||||
| Mean (SD) | 5.16 (16.37) | 6.25 (15.79) | 4.17 (17.16) | ||||
| B/A | n | 49 | 25 | 24 | 0.93 | 0.02 (−0.55–0.6) | |
| median (min–max) | 0 (−25–37.5) | 0 (−25–37.5) | 0 (−25–37.5) | ||||
| Mean (SD) | 2.81 (16.39) | 3 (16.65) | 2.6 (16.48) | ||||
| Emotional role | B/D | n | 46 | 22 | 24 | 0.69• | 0.21 (−0.38–0.8) |
| median (min–max) | 0 (−100–100) | 0 (−66.67–100) | 0 (−100–100) | ||||
| Mean (SD) | 5.8 (43.49) | 10.61 (44.11) | 1.39 (43.38) | ||||
| B/A | n | 49 | 25 | 24 | 0.42• | 0.18 (−0.39–0.75) | |
| median (min–max) | 0 (−66.67–100) | 0 (−66.67–100) | 0 (−33.33–100) | ||||
| Mean (SD) | 7.48 (35.53) | 10.67 (41.63) | 4.17 (28.34) | ||||
| Mental health | B/D | n | 46 | 22 | 24 | 0.1 | −0.5 (−1.1–0.1) |
| median (min–max) | 1 (−19–28) | 0 (−19–24) | 4 (−16–28) | ||||
| Mean (SD) | 2.59 (9.9) | 0.05 (9.54) | 4.92 (9.83) | ||||
| B/A | n | 49 | 25 | 24 | 0.03• | −0.65 (−1.24–0.06) | |
| median (min–max) | 0 (−44–24) | 0 (−44–24) | 8 (−12–24) | ||||
| 1.33 (11.68) | −2.24 (13.27) | 5.04 (8.52) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuis-Girod, S.; Fargeton, A.-E.; Grobost, V.; Rivière, S.; Beaudoin, M.; Decullier, E.; Bernard, L.; Bréant, V.; Colombet, B.; Philouze, P.; et al. Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. J. Clin. Med. 2020, 9, 1262. https://doi.org/10.3390/jcm9051262
Dupuis-Girod S, Fargeton A-E, Grobost V, Rivière S, Beaudoin M, Decullier E, Bernard L, Bréant V, Colombet B, Philouze P, et al. Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. Journal of Clinical Medicine. 2020; 9(5):1262. https://doi.org/10.3390/jcm9051262
Chicago/Turabian StyleDupuis-Girod, Sophie, Anne-Emmanuelle Fargeton, Vincent Grobost, Sophie Rivière, Marjolaine Beaudoin, Evelyne Decullier, Lorraine Bernard, Valentine Bréant, Bettina Colombet, Pierre Philouze, and et al. 2020. "Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial" Journal of Clinical Medicine 9, no. 5: 1262. https://doi.org/10.3390/jcm9051262
APA StyleDupuis-Girod, S., Fargeton, A.-E., Grobost, V., Rivière, S., Beaudoin, M., Decullier, E., Bernard, L., Bréant, V., Colombet, B., Philouze, P., Bailly, S., Faure, F., & Hermann, R. (2020). Efficacy and Safety of a 0.1% Tacrolimus Nasal Ointment as a Treatment for Epistaxis in Hereditary Hemorrhagic Telangiectasia: A Double-Blind, Randomized, Placebo-Controlled, Multicenter Trial. Journal of Clinical Medicine, 9(5), 1262. https://doi.org/10.3390/jcm9051262





