Anemia and Systemic Inflammation Rather than Arterial Circulatory Dysfunction Predict Decompensation of Liver Cirrhosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Hemodynamic Assessment
2.3. Quantification of IL-6 Serum Levels
2.4. Statistical Analyses
3. Results
3.1. Patient Characteristics
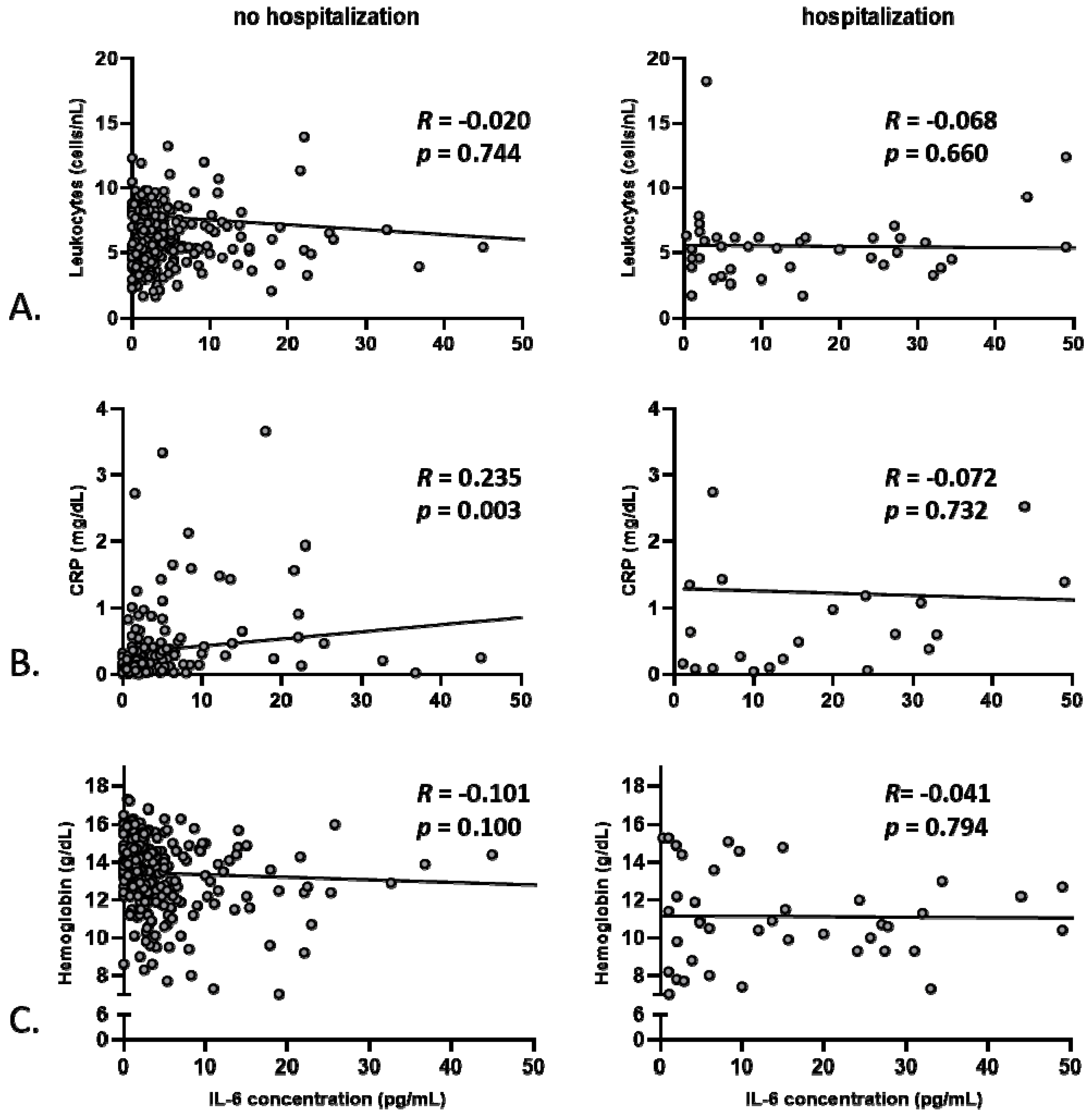
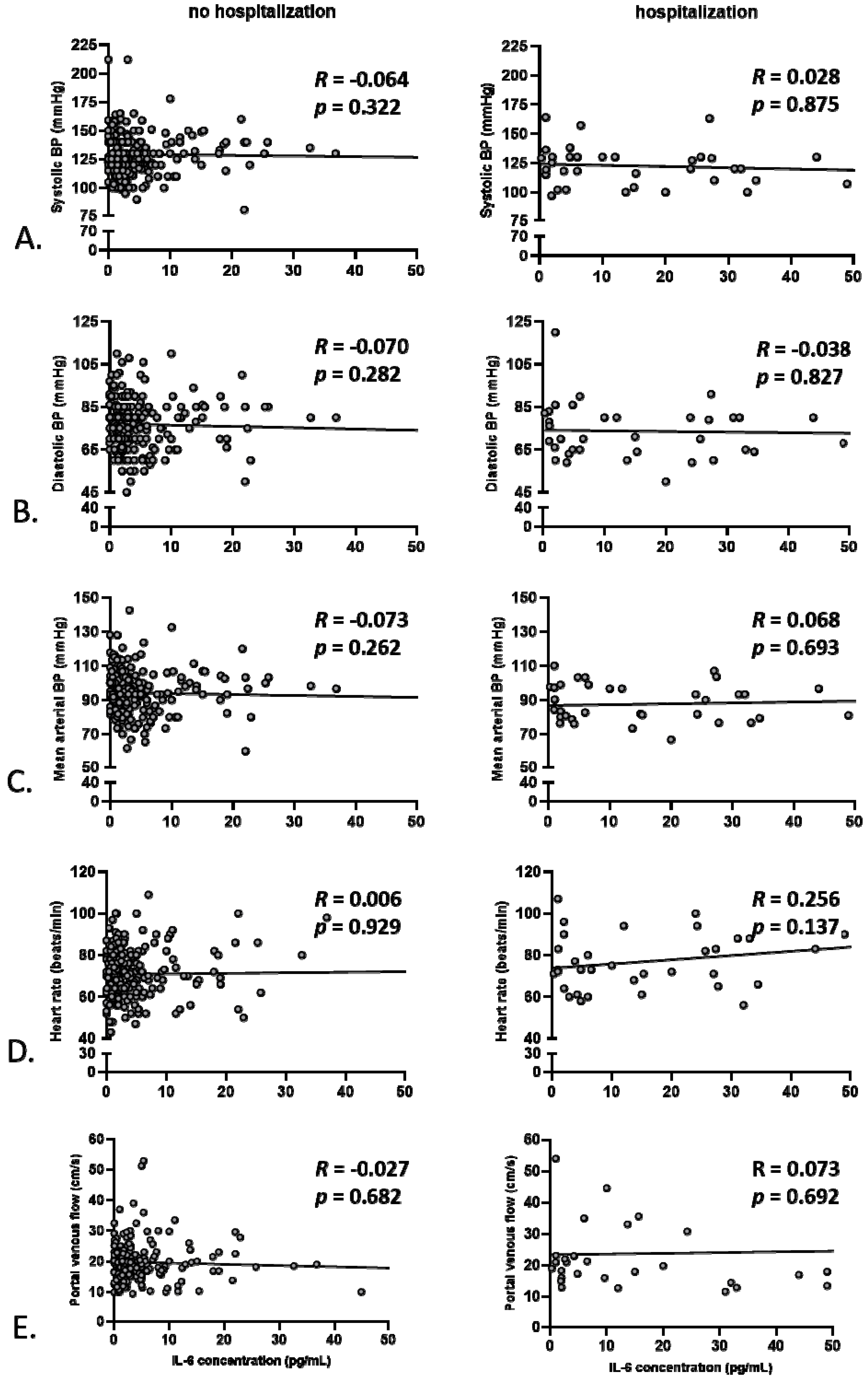
3.2. Anemia, but Not Markers of Systemic Inflammation, Correlates with Baseline Hemodynamics
3.3. Predictors of Hospitalization Due to Hepatic Decompensation
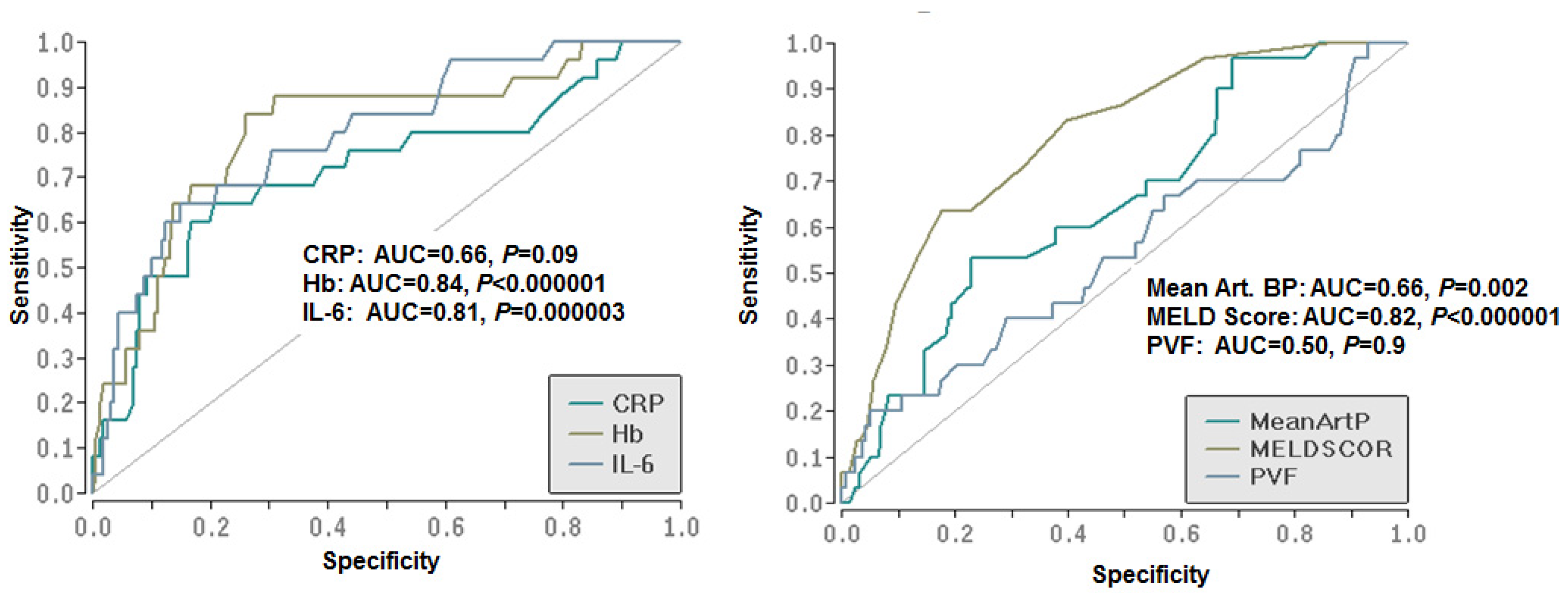
3.4. Diagnostic Capacity of the Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Everhart, J.E.; Ruhl, C.E. Burden of Digestive Diseases in the United States Part III: Liver, Biliary Tract, and Pancreas. Gastroenterology 2009, 136, 1134–1144. [Google Scholar] [CrossRef]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.; Roudot-Thoraval, F. The burden of liver disease in Europe: A review of available epidemiological data. J. Hepatol. 2013, 58, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Talwalkar, J.A. Prophylaxis with beta blockers as a performance measure of quality health care in cirrhosis. Gastroenterology 2006, 130, 1005–1007. [Google Scholar] [CrossRef]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Friedman, S.L.; Iredale, J.; Pinzani, M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010, 51, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Quintero, E.; Arroyo, V.; Teres, J.; Bruguera, M.; Rimola, A.; Caballería, J.; Rodés, J.; Rozman, C. Compensated cirrhosis: Natural history and prognostic factors. Hepatology 1987, 7, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Bosch, J.; Groszmann, R.J. Portal hypertension and variceal bleeding-Unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology 2008, 47, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, C.; Groszmann, R.; Garcia–Tsao, G.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, À.; García-Pagán, J.C.; Makuch, R.; Patch, D.; et al. Hepatic Venous Pressure Gradient Predicts Clinical Decompensation in Patients With Compensated Cirrhosis. Gastroenterology 2007, 133, 481–488. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Bernardi, M.; Moreau, R.; Angeli, P.; Schnabl, B.; Arroyo, V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 2015, 63, 1272–1284. [Google Scholar] [CrossRef]
- Clària, J.; Stauber, R.E.; Coenraad, M.J.; Moreau, R.; Jalan, R.; Pavesi, M.; Amorós, À.; Titos, E.; Alcaraz-Quiles, J.; Oettl, K.; et al. Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology 2016, 64, 1249–1264. [Google Scholar] [CrossRef]
- Finkelmeier, F.; Bettinger, D.; Koberle, V.; Schultheiss, M.; Zeuzem, S.; Kronenberger, B.; Piiper, A.; Waidmann, O. Single measurement of hemoglobin predicts outcome of HCC patients. Med. Oncol. 2014, 31, 806. [Google Scholar] [PubMed]
- Piano, S.; Tonon, M.; Vettore, E.; Stanco, M.; Pilutti, C.; Romano, A.; Mareso, S.; Gambino, C.; Brocca, A.; Sticca, A.; et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J. Hepatol. 2017, 67, 1177–1184. [Google Scholar] [CrossRef]
- Kubesch, A.; Quenstedt, L.; Saleh, M.; Rüschenbaum, S.; Schwarzkopf, K.; Martinez, Y.; Welsch, C.; Zeuzem, S.; Welzel, T.M.; Lange, C.M. Vitamin D deficiency is associated with hepatic decompensation and inflammation in patients with liver cirrhosis: A prospective cohort study. PLoS ONE 2018, 13, e0207162. [Google Scholar] [CrossRef]
- American Association for the Study of Liver Diseases and European Association for the Study of the Liver. Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J. Hepatol. 2014, 61, 642–659. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2016, 65, 310–335. [Google Scholar] [CrossRef] [PubMed]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.; Trebicka, J.; Krag, A.; Laleman, W.; Ginès, P. European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Angeli, P.; Ginès, P.; Wong, F.; Bernardi, M.; Boyer, T.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015, 62, 968–974. [Google Scholar] [CrossRef]
- Iyriboz, Y. Indirect Blood-Pressure Measurement Using the Riva-Rocci-Korotkoff Method—Reply. J. Clin. Monit. 1995, 11, 149–150. [Google Scholar]
- Lin, L.-W.; Duan, X.-J.; Wang, X.-Y.; Xue, E.-S.; He, Y.-M.; Gao, S.-D.; Yu, L.-Y. Color Doppler velocity profile and contrast-enhanced ultrasonography in assessment of liver cirrhosis. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 34–39. [Google Scholar] [PubMed]
- Remmler, J.; Schneider, C.; Treuner-Kaueroff, T.; Bartels, M.; Seehofer, D.; Scholz, M.; Berg, T.; Kaiser, T. Increased Level of Interleukin 6 Associates with Increased 90-Day and 1-Year Mortality in Patients with End-Stage Liver Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 730–737. [Google Scholar] [CrossRef]
- Hernaez, R.; Solà, E.; Moreau, R.; Ginès, P. Acute-on-chronic liver failure: An update. Gut 2017, 66, 541–553. [Google Scholar] [CrossRef]
- Gustot, T. Multiple organ failure in sepsis: Prognosis and role of systemic inflammatory response. Curr. Opin. Crit. Care 2011, 17, 153–159. [Google Scholar] [CrossRef]
- Pettilä, V.; Hynninen, M.; Takkunen, O.; Kuusela, P.; Valtonen, M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensiv. Care Med. 2002, 28, 1220–1225. [Google Scholar] [CrossRef]
- Gonzalez-Casas, R.; Jones, E.A.; Moreno-Otero, R. Spectrum of anemia associated with chronic liver disease. World J. Gastroenterol. 2009, 15, 4653–4658. [Google Scholar] [CrossRef]
- Qamar, A.A.; Grace, N.D.; Groszmann, R.J.; Garcia–Tsao, G.; Bosch, J.; Burroughs, A.K.; Ripoll, C.; Maurer, R.; Planas, R.; Escorsell, A.; et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin. Gastroenterol. Hepatol. 2009, 7, 689–695. [Google Scholar] [CrossRef]

| All Patients (N = 338) | No Hospitalization Due to Decompensation at Follow-Up (N = 287) | Hospitalization at Follow-Up Due to Decompensation (N = 51) | p-Value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 59.7 ± 11.9 | 59.4 ± 12.1 | 61.5 ± 10.9 | 0.3 |
| Male gender, N (%) | 194 (57) | 163 (57) | 31 (60) | 0.7 |
| BMI (kg/m2), mean ± SD | 27 ± 5 | 27 ± 5 | 26 ± 6 | 0.1 |
| Death during follow-up, N (%) | 16 (5) | 1 (0.3) | 15 (30) | <0.0001 |
| Underlying liver disease | ||||
| NASH | 33 (10) | 26 (9) | 7 (14) | 0.4 |
| Alcohol | 103 (30) | 88 (31) | 15 (29) | 0.9 |
| HCV | 117 (35) | 102 (36) | 15 (29) | 0.5 |
| Other or unclear | 85 (25) | 71 (25) | 14 (27) | 0.7 |
| MELD score, mean ± SD | 10.2 ± 4.2 | 9.7 ± 3.8 | 13.8 ± 4.4 | <0.0001 |
| Decompensation at baseline, N (%) | 131 (39) | 105 (37) | 26 (51) | 0.05 |
| Prior decompensation, N (%) | 172 (51) | 130 (45) | 42 (82) | <0.0001 |
| CRP (mg/L), mean ± SD | 0.53 ± 0.89 | 0.4 ± 0.6 | 1.4 ± 1.6 | <0.0001 |
| Leukocytes/nL, mean ± SD | 5.98 ± 2.3 | 6.0 ± 2.2 | 5.7 ± 2.7 | 0.1 |
| Platelets/nL, mean ± SD | 138 ± 72 | 141 ± 71 | 123 ± 79 | 0.02 |
| Hemoglobin (mg/dL), mean ± SD | 13.08 ± 2.08 | 13.4 ± 1.9 | 11.2 ± 2.3 | <0.0001 |
| Creatinine (mg/dL), mean ± SD | 0.9 ± 0.43 | 0.9 ± 0.3 | 1.1 ± 0.8 | 0.02 |
| Albumin (g/dL), mean ± SD | 3.9 ± 0.7 | 4.1 ± 0.6 | 3.3 ± 0.6 | <0.0001 |
| Bilirubin (mg/dL), mean ± SD | 1.47 ± 1.52 | 1.3 ± 1.5 | 2.3 ± 1.7 | <0.0001 |
| INR, mean ± SD | 1.22 ± 0.34 | 1.2 ± 0.3 | 1.4 ± 0.3 | <0.0001 |
| IL-6 (pg/mL), mean ± SD | 7.8 ± 14 | 7.3 ± 16 | 24.9 ± 24 | <0.0001 |
| Systolic BP (mmHg), mean ± SD | 134 ± 44 | 129 ± 18 | 119 ± 23 | 0.004 |
| Diastolic BP (mmHg), mean ± SD | 80 ± 49 | 77 ± 11 | 72 ± 13 | 0.02 |
| Heart rate (beats/min), mean ± SD | 71 ± 11 | 71 ± 11 | 75 ± 15 | 0.09 |
| Max. Portal Venous Flow (cm/s), mean ± SD | 19.9 ± 8.5 | 20 ± 7.5 | 23 ± 13 | 0.4 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 * | |||||
| p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | |
| Male gender | 0.6 | 1.18 (0.63–2.24) | ||||
| Age | 0.3 | 1.01 (0.98–1.04) | ||||
| Active alcoholism | 0.002 | 2.70 (1.41–5.15) | 0.05 | 2.17 (1.00–4.77) | ||
| Hepatic decompensation at baseline | <0.00001 | 9.87 (4.34–22.3) | 0.01 | 3.4 (1.31–8.84) | ||
| Mean arterial blood pressure (mmHg) | 0.008 | 0.96 (0.93–0.99) | ||||
| Heart rate (beats/min) | 0.03 | 1.03 (1.00–1.07) | ||||
| Max. Portal Venous Flow (m/s) | 0.008 | 1.87 (1.18–2.97) | ||||
| MELD score | 0.00002 | 1.19 (1.09–1.29) | 0.02 | 1.11 (1.01–1.22) | 0.02 | 1.12 (1.02–1.23) |
| Platelets cells/nL | 0.15 | 0.99 (0.98–1.00) | ||||
| Hemoglobin (g/dL) | <0.00001 | 0.57 (0.47–0.70) | 0.0001 | 0.62 (0.51–0.78) | 0.0005 | 0.68 (0.55–0.84) |
| IL-6 (pg/mL) | 0.006 | 2.44 (1.22–4.76) | 0.03 | 1.02 (1.01–1.04) | 0.03 | 1.02 (1.01–1.04) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bothou, C.; Rüschenbaum, S.; Kubesch, A.; Quenstedt, L.; Schwarzkopf, K.; Welsch, C.; Zeuzem, S.; Welzel, T.M.; Lange, C.M. Anemia and Systemic Inflammation Rather than Arterial Circulatory Dysfunction Predict Decompensation of Liver Cirrhosis. J. Clin. Med. 2020, 9, 1263. https://doi.org/10.3390/jcm9051263
Bothou C, Rüschenbaum S, Kubesch A, Quenstedt L, Schwarzkopf K, Welsch C, Zeuzem S, Welzel TM, Lange CM. Anemia and Systemic Inflammation Rather than Arterial Circulatory Dysfunction Predict Decompensation of Liver Cirrhosis. Journal of Clinical Medicine. 2020; 9(5):1263. https://doi.org/10.3390/jcm9051263
Chicago/Turabian StyleBothou, Christina, Sabrina Rüschenbaum, Alica Kubesch, Leonie Quenstedt, Katharina Schwarzkopf, Christoph Welsch, Stefan Zeuzem, Tania Mara Welzel, and Christian Markus Lange. 2020. "Anemia and Systemic Inflammation Rather than Arterial Circulatory Dysfunction Predict Decompensation of Liver Cirrhosis" Journal of Clinical Medicine 9, no. 5: 1263. https://doi.org/10.3390/jcm9051263
APA StyleBothou, C., Rüschenbaum, S., Kubesch, A., Quenstedt, L., Schwarzkopf, K., Welsch, C., Zeuzem, S., Welzel, T. M., & Lange, C. M. (2020). Anemia and Systemic Inflammation Rather than Arterial Circulatory Dysfunction Predict Decompensation of Liver Cirrhosis. Journal of Clinical Medicine, 9(5), 1263. https://doi.org/10.3390/jcm9051263





