Enhanced Serum Levels of sFlt1: Impact on Materno–Fetal CMV Transmission
Abstract
1. Introduction
2. Material and Methods
3. Results
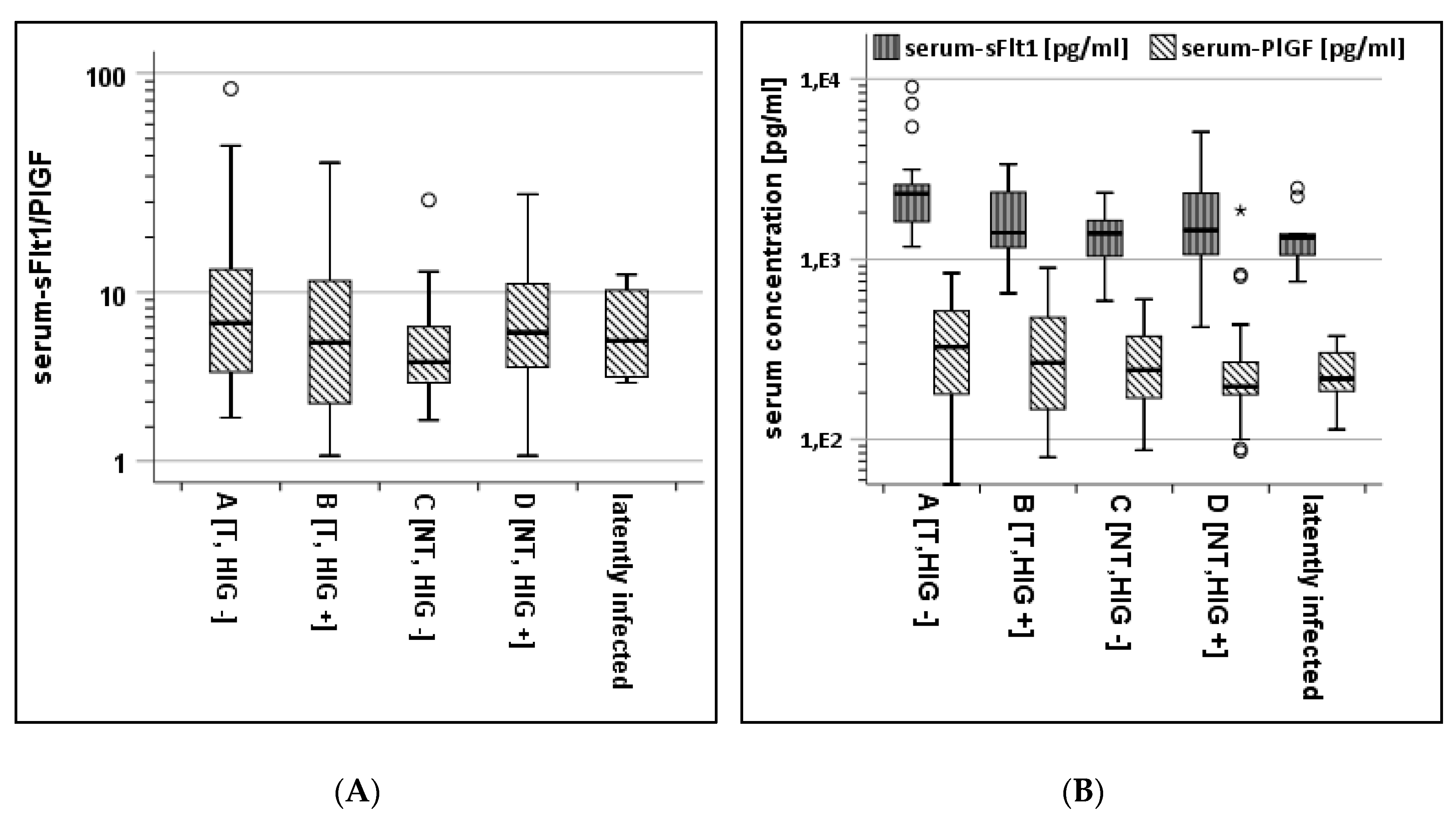
3.1. Serum-Analysis of Subcohorts
3.2. AF-Analysis of Subcohorts
3.3. Protein-Levels and Ultrasound Examinations
3.4. ROC Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penka, L.; Kagan, K.-O.; Goelz, R.; Hamprecht, K. Comparison of quantitative real-timePCR and short-term (18h-) microculture in diagnosis of fetal cytomegalovirus infection: Impact of hyperimmunoglobulin treatment. Prenat Diagn 2018. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.; Sass, N.; Boute, T.; Moron, A.F. sFlt−1 and PlGF levels in a patient with mirror syndrome related to cytomegalovirus infection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Maidji, E.; Nigro, G.; Tabata, T.; McDonagh, S.; Nozawa, N.; Shiboski, S.; Muci, S.; Anceschi, M.M.; Aziz, N.; Adler, S.P.; et al. Antibody Treatment Promotes Compensation for Human Cytomegalovirus-Induced Pathogenesis and a Hypoxia-Like Condition in Placentas with Congenital Infection. Am. J. Pathol. 2010, 177, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennstrom, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Sacks, G.P.; Sargent, I.L. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 499–506. [Google Scholar] [CrossRef]
- Cornelius, D.C. Preeclampsia: From Inflammation to Immunoregulation. Clin. Med. Insights Blood Disord. 2017, 11, 1179545X17752325. [Google Scholar] [CrossRef]
- Armaly, Z.; Jadaon, J.E.; Jabbour, A.; Abassi, Z.A. Preeclampsia: Novel Mechanisms and Potential Therapeutic Approaches. Front. Physiol. 2018, 9, 973. [Google Scholar] [CrossRef]
- Kagan, K.O.; Enders, M.; Schampera, M.S.; Baeumel, E.; Hoopmann, M.; Geipel, A.; Berg, C.; Goelz, R.; de Catte, L.; Wallwiener, D.; et al. Prevention of maternal-fetal transmission of CMV by hyperimmunoglobulin (HIG) administered after a primary maternal CMV infectionin early gestation. Ultrasound Obstet Gynecol. 2018. [Google Scholar] [CrossRef]
- Kagan, K.O.; Hamprecht, K. Cytomegalovirus infection in pregnancy. Arch. Gynecol. Obstet. 2017, 296, 15–26. [Google Scholar] [CrossRef]
- Rawlinson, W.; Hamilton, S.; van Zuylen, W. Update on treatment of cytomegalovirus infection in pregnancy and of the newborn with congenital cytomegalovirus. Curr. Opin. Infect. Dis. 2016, 29, 615–624. [Google Scholar] [CrossRef]
- Revello, M.G.; Lazzarotto, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Stirnemann, J.; Sellier, Y.; Guilleminot, T.; Dejean, A.; Magny, J.-F.; Couderc, S.; Jacquemard, F.; Ville, Y. Feasibility of predicting the outcome of fetal infection with cytomegalovirus at the time of prenatal diagnosis. Am. J. Obstet. Gynecol. 2016, 215, 342.e1-9. [Google Scholar] [CrossRef] [PubMed]
- Klages, K.; Kundu, S.; Erlenwein, J.; Elsaesser, M.; Hillemanns, P.; Scharf, A.; Staboulidou, I. Maternal anxiety and its correlation with pain experience during chorion villus sampling and amniocentesis. J. Pain Res. 2017, 10, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Benoist, G.; Salomon, L.J.; Jacquemard, F.; Daffos, F.; Ville, Y. The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 2008, 115, 823–829. [Google Scholar] [CrossRef]
- Fabbri, E.; Revello, M.G.; Furione, M.; Zavattoni, M.; Lilleri, D.; Tassis, B.; Quarenghi, A.; Rustico, M.; Nicolini, U.; Ferrazzi, E.; et al. Prognostic markers of symptomatic congenital human cytomegalovirus infection in fetal blood. BJOG 2011, 118, 448–456. [Google Scholar] [CrossRef]
- Guerra, B.; Simonazzi, G.; Puccetti, C.; Lanari, M.; Farina, A.; Lazzarotto, T.; Rizzo, N. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am. J. Obs. Gynecol. 2008, 198, 380.e1-7. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Dolegowska, B.; Kwiatkowska, E.; Rzepka, R.; Marczuk, N.; Loj, B.; Torbe, A. Maternal endothelial damage as a disorder shared by early preeclampsia, late preeclampsia and intrauterine growth restriction. J. Perinat. Med. 2017, 45, 793–802. [Google Scholar] [CrossRef]
- Pereira, L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef]
- Rana, S.; Venkatesha, S.; DePaepe, M.; Chien, E.K.; Paglia, M.; Karumanchi, S.A. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obs. Gynecol. 2007, 109, 549–552. [Google Scholar] [CrossRef]
- Chiaie, L.D.; Neuberger, P.; Vochem, M.; Lihs, A.; Karck, U.; Enders, M. No evidence of obstetrical adverse events after hyperimmune globulin application for primary cytomegalovirus infection in pregnancy: Experience from a single centre. Arch. Gynecol. Obs. 2018, 297, 1389–1395. [Google Scholar] [CrossRef]
- Nigro, G. Hyperimmune globulin to prevent congenital CMV infection. N. Engl. J. Med. 2014, 370, 2544. [Google Scholar] [CrossRef]

| Characteristics | T, HIG− | T, HIG+ | NT, HIG− | NT, HIG+ | |
|---|---|---|---|---|---|
| sample size | 18 | 16 | 21 | 49 | |
| maternal age at PI, years, mean (SD) | 31 (6) | 32 (4) | 32 (4) | 30 (4) | n.s.¶ |
| gravidity, median (IQR) | 2 (2) | 2 (1) | 2 (1) | 2 (2) | n.s.§ |
| parity, median (IQR) | 1 (1) | 1 (0) | 1 (0) | 1 (1) | n.s.§ |
| GA at amniocentesis, median (IQR) | 22 (5) | 21 (2) | 22 (5) | 20 (1) | n.s.§ |
| HIG applications, mean (SD) | 3 (2) | 4 (1) | n.s.# | ||
| bodyweight, kg, median (IQR) | 65 (23) | 63 (13) | n.s.& |
| Characteristics | T, HIG− (A) | T, HIG+ (B) | NT, HIG− (C) | NT, HIG+ (D) | Latent |
|---|---|---|---|---|---|
| Sample size | 18 | 16 | 21 | 49 | 10 |
| Median sFlt1 in ng/mL (IQR) | 2.3 (1.1) | 1.4 (1.2) | 1.4 (0.7) | 1.5 (1.3) | 1.3 (0.6) |
| Median PlGF in pg/mL (IQR) | 326 (363) | 268 (345) | 243 (211) | 197 (104) | 218 (130) |
| Median sFlt1/PlGF (IQR) | 7.1 (9.2) | 5.6 (9.4) | 4.4 (3.7) | 6.3 (7.2) | 5.8 (6.8) |
| Characteristics | T, HIG− (A) | T, HIG+ (B) | NT, HIG− (C) | NT, HIG+ (D) | Latent |
|---|---|---|---|---|---|
| Sample size | 13 | 11 | 9 | 31 | 8 |
| Median sFlt1 in ng/mL (IQR) | 99 (139) | 63 (117) | 67 (109) | 54 (49) | 39 (47) |
| Median PlGF in pg/mL (IQR) | 239 (201) | 172 (190) | 214 (158) | 173 (92) | 125 (96) |
| Median sFlt1/PlGF (IQR) | 423 (265) | 438 (316) | 492 (245) | 355 (245) | 291 (183) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penka, L.; Kagan, K.-O.; Hamprecht, K. Enhanced Serum Levels of sFlt1: Impact on Materno–Fetal CMV Transmission. J. Clin. Med. 2020, 9, 1258. https://doi.org/10.3390/jcm9051258
Penka L, Kagan K-O, Hamprecht K. Enhanced Serum Levels of sFlt1: Impact on Materno–Fetal CMV Transmission. Journal of Clinical Medicine. 2020; 9(5):1258. https://doi.org/10.3390/jcm9051258
Chicago/Turabian StylePenka, Lukas, Karl-Oliver Kagan, and Klaus Hamprecht. 2020. "Enhanced Serum Levels of sFlt1: Impact on Materno–Fetal CMV Transmission" Journal of Clinical Medicine 9, no. 5: 1258. https://doi.org/10.3390/jcm9051258
APA StylePenka, L., Kagan, K.-O., & Hamprecht, K. (2020). Enhanced Serum Levels of sFlt1: Impact on Materno–Fetal CMV Transmission. Journal of Clinical Medicine, 9(5), 1258. https://doi.org/10.3390/jcm9051258





