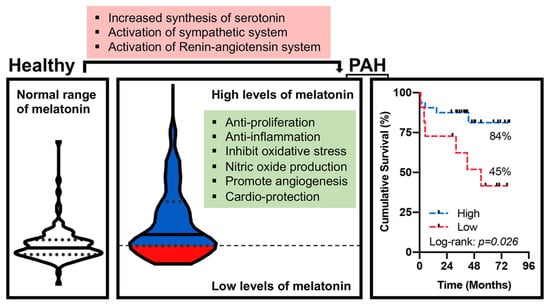
Lower Plasma Melatonin Levels Predict Worse Long-Term Survival in Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Methods
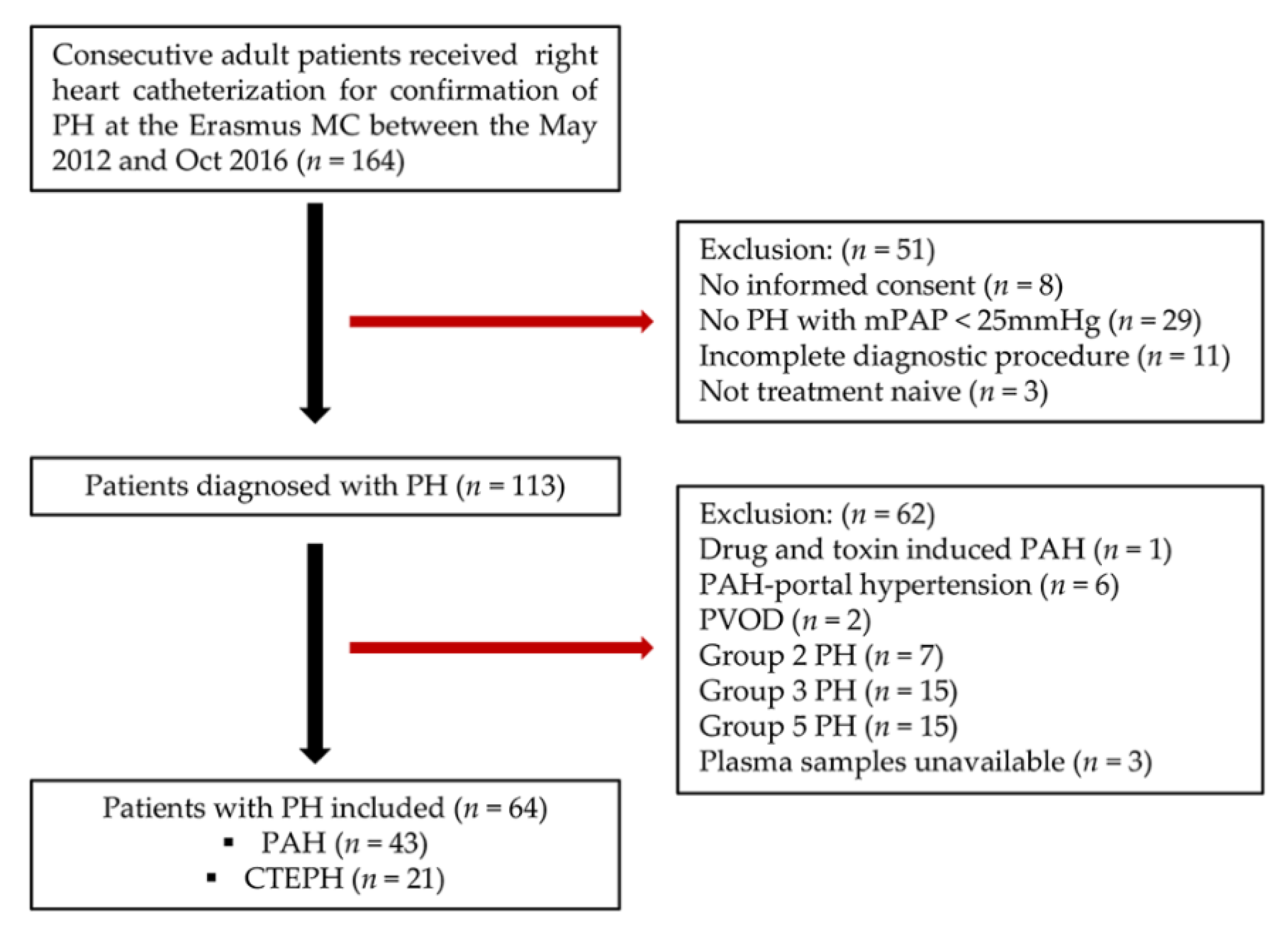
2.1. Study Population
2.2. Follow-Up of PH Patients
2.3. Animal Models of PH
2.4. Blood Sampling and Measurement of Melatonin
3. Statistical Analysis
4. Results
4.1. Baseline Characteristics
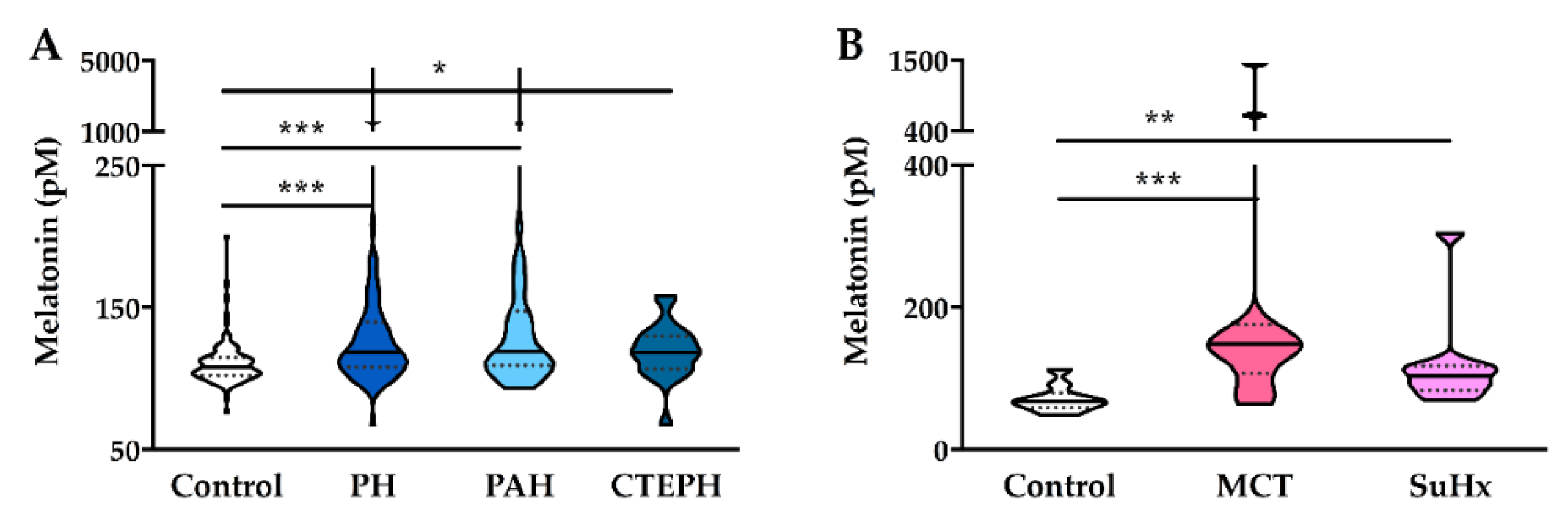
4.2. Levels of Plasma Melatonin
4.3. Correlation Analysis
4.4. Logistic Regression Analyses
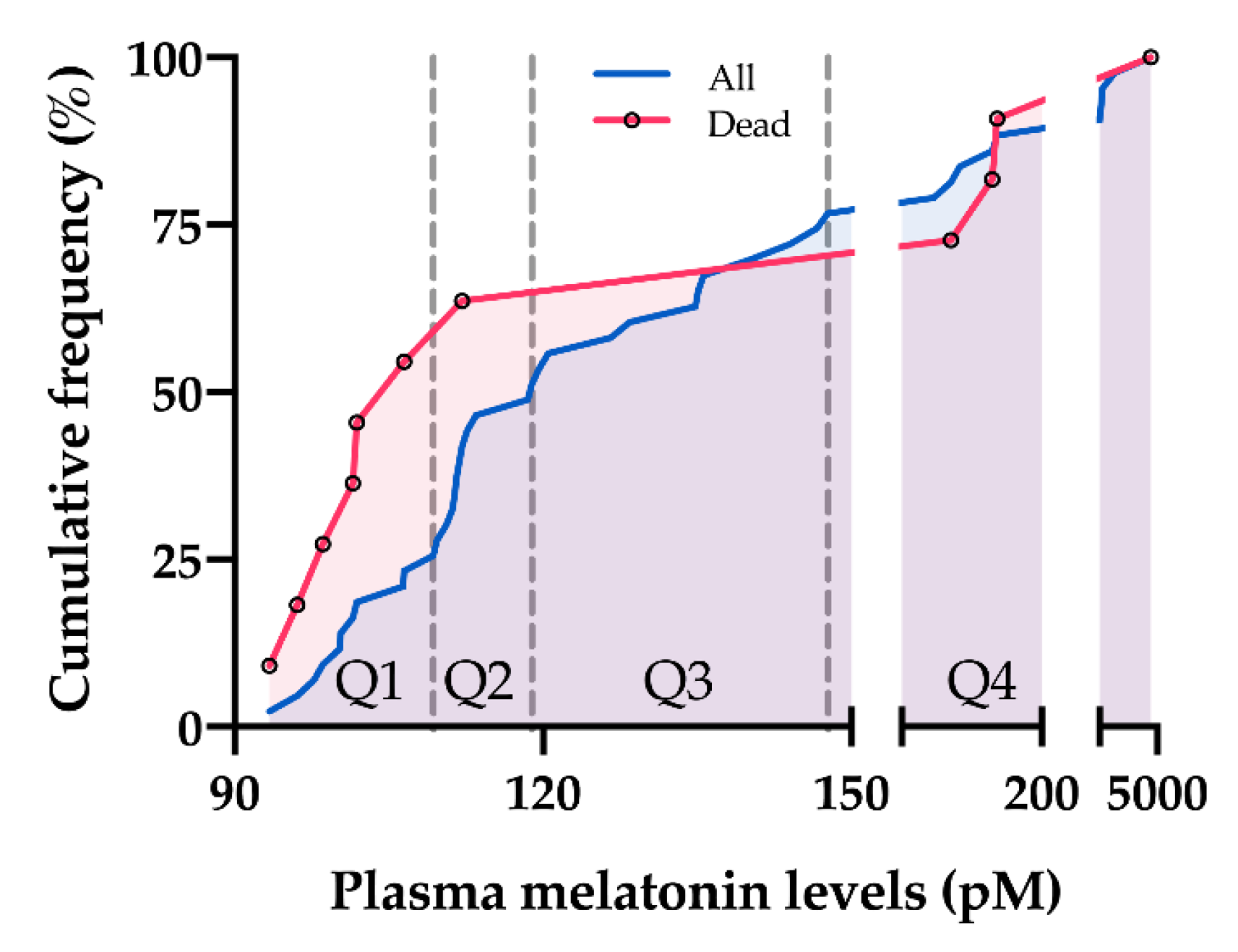
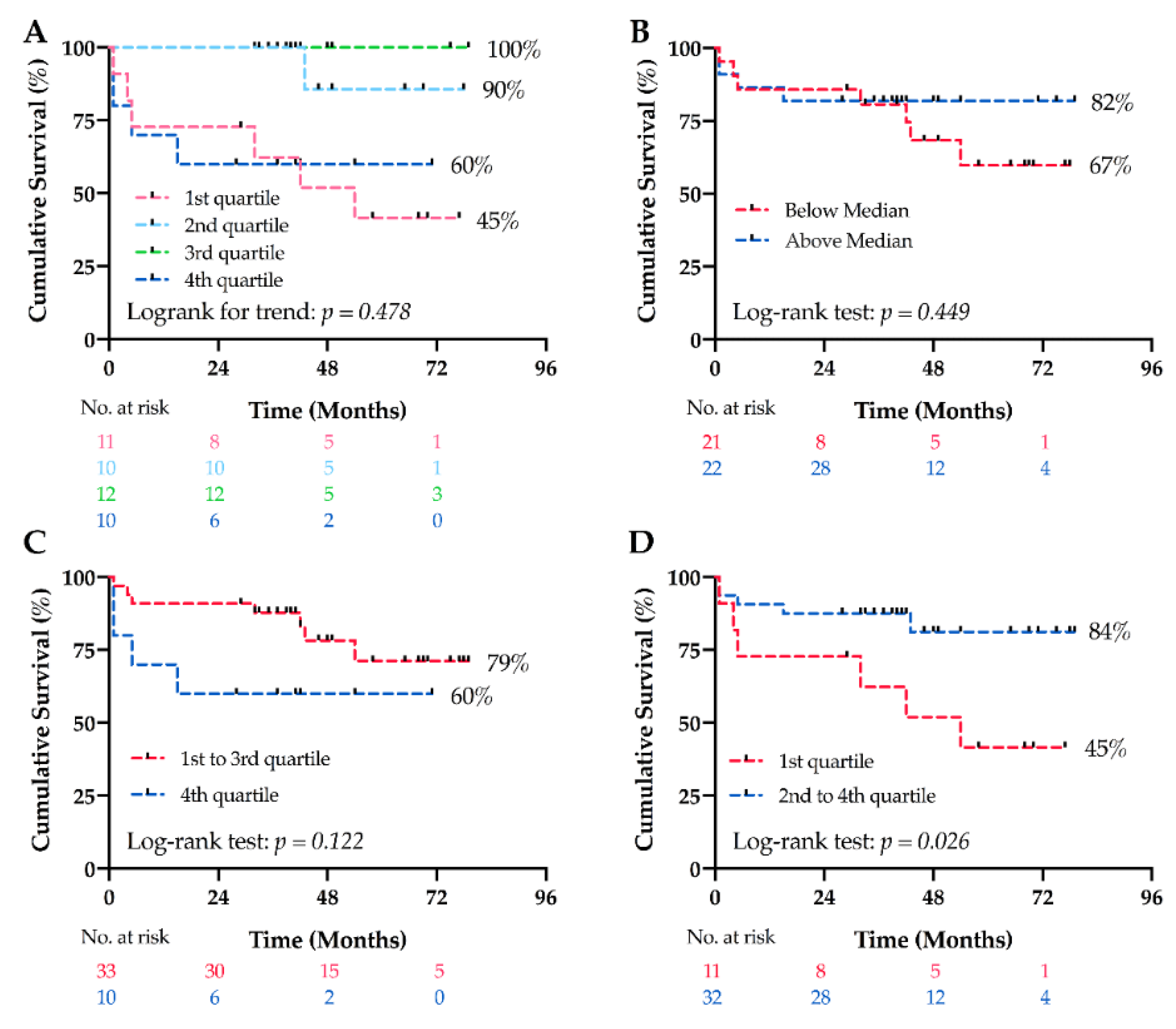
4.5. Long-Term Survival Analyses
5. Discussion
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Pathophysiology. Eur. Respir. Rev. 2010, 19, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmuller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Xu, L.; Su, Y.; Zhao, Y.; Sheng, X.; Tong, R.; Ying, X.; Gao, L.; Ji, Q.; Gao, Y.; Yan, Y.; et al. Melatonin differentially regulates pathological and physiological cardiac hypertrophy: Crucial role of circadian nuclear receptor RORalpha signaling. J. Pineal Res. 2019, 67, e12579. [Google Scholar] [CrossRef]
- Misaka, T.; Yoshihisa, A.; Yokokawa, T.; Sato, T.; Oikawa, M.; Kobayashi, A.; Yamaki, T.; Sugimoto, K.; Kunii, H.; Nakazato, K.; et al. Plasma levels of melatonin in dilated cardiomyopathy. J. Pineal Res 2019, 66, e12564. [Google Scholar] [CrossRef]
- Sun, H.; Gusdon, A.M.; Qu, S. Effects of melatonin on cardiovascular diseases: Progress in the past year. Curr. Opin. Lipidol. 2016, 27, 408–413. [Google Scholar] [CrossRef]
- Habtemariam, S.; Daglia, M.; Sureda, A.; Selamoglu, Z.; Gulhan, M.F.; Nabavi, S.M. Melatonin and Respiratory Diseases: A Review. Curr. Top. Med. Chem. 2017, 17, 467–488. [Google Scholar] [CrossRef]
- Moradkhani, F.; Moloudizargari, M.; Fallah, M.; Asghari, N.; Heidari Khoei, H.; Asghari, M.H. Immunoregulatory role of melatonin in cancer. J. Cell. Physiol. 2020, 235, 745–757. [Google Scholar] [CrossRef]
- Das, R.; Balonan, L.; Ballard, H.J.; Ho, S. Chronic hypoxia inhibits the antihypertensive effect of melatonin on pulmonary artery. Int. J. Cardiol. 2008, 126, 340–345. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Y.; Zhou, L.; Liu, L.; Zhang, P.; Deng, W.; Yuan, Y. Melatonin attenuates hypoxic pulmonary hypertension by inhibiting the inflammation and the proliferation of pulmonary arterial smooth muscle cells. J. Pineal Res. 2014, 57, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Liu, M.; Fan, H.; Zheng, H.; Zhang, S.; Rahman, N.; Wolczynski, S.; Kretowski, A.; Li, X. Melatonin inhibits inflammasome-associated activation of endothelium and macrophages attenuating pulmonary arterial hypertension. Cardiovasc. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.W.; Yeung, H.M.; Lau, C.F.; Poon, A.M.S.; Tipoe, G.L.; Fung, M.L. Melatonin Attenuates Pulmonary Hypertension in Chronically Hypoxic Rats. Int. J. Mol. Sci. 2017, 18, 1125. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Gonzalez-Candia, A.; Montt, C.; Ebensperger, G.; Chubretovic, M.; Seron-Ferre, M.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J. Pineal Res. 2015, 58, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Candia, A.; Candia, A.A.; Figueroa, E.G.; Feixes, E.; Gonzalez-Candia, C.; Aguilar, S.A.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin long-lasting beneficial effects on pulmonary vascular reactivity and redox balance in chronic hypoxic ovine neonates. J. Pineal Res. 2020, 68, e12613. [Google Scholar] [CrossRef] [PubMed]
- Astorga, C.R.; Gonzalez-Candia, A.; Candia, A.A.; Figueroa, E.G.; Canas, D.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin Decreases Pulmonary Vascular Remodeling and Oxygen Sensitivity in Pulmonary Hypertensive Newborn Lambs. Front. Physiol. 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.; Blackhurst, D.; Thienemann, F.; Blauwet, L.; Butrous, G.; Davies, N.; Sliwa, K.; Lecour, S. Melatonin as a preventive and curative therapy against pulmonary hypertension. J. Pineal Res. 2015, 59, 343–353. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, S.; Wu, P.; Li, M.; Ding, X.; Sun, L.; Xu, X.; Zhou, X.; Zhou, L.; Cao, C.; et al. Identifying Involvement of H19-miR-675-3p-IGF1R and H19-miR-200a-PDCD4 in Treating Pulmonary Hypertension with Melatonin. Mol. Ther. Nucleic Acids 2018, 13, 44–54. [Google Scholar] [CrossRef]
- Galie, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.L.; Barbera, J.A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.S.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62 (Suppl. 25), D42–D50. [Google Scholar] [CrossRef]
- Geenen, L.W.; Baggen, V.J.M.; Koudstaal, T.; Boomars, K.A.; Eindhoven, J.A.; Boersma, E.; Roos-Hesselink, J.W.; van den Bosch, A.E. The prognostic value of various biomarkers in adults with pulmonary hypertension; a multi-biomarker approach. Am. Heart J. 2019, 208, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Menting, M.E.; McGhie, J.S.; Koopman, L.P.; Vletter, W.B.; Helbing, W.A.; van den Bosch, A.E.; Roos-Hesselink, J.W. Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography 2016, 33, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Tu, L.; Desroches-Castan, A.; Mallet, C.; Guyon, L.; Cumont, A.; Phan, C.; Robert, F.; Thuillet, R.; Bordenave, J.; Sekine, A.; et al. Selective BMP-9 Inhibition Partially Protects Against Experimental Pulmonary Hypertension. Circ. Res. 2019, 124, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, L.; Lischko, M.M.; Rollag, M.D.; Niswender, G.D. Melatonin: Daily cycle in plasma and cerebrospinal fluid of calves. Science 1977, 195, 686–687. [Google Scholar] [CrossRef]
- Sack, R.L.; Lewy, A.J.; Erb, D.L.; Vollmer, W.M.; Singer, C.M. Human melatonin production decreases with age. J. Pineal Res. 1986, 3, 379–388. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Cajochen, C.; Krauchi, K.; Wirz-Justice, A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocrinol. 2003, 15, 432–437. [Google Scholar] [CrossRef]
- Wehr, T.A. Melatonin and seasonal rhythms. J. Biol. Rhythms 1997, 12, 518–527. [Google Scholar] [CrossRef]
- Backlund, P.S.; Urbanski, H.F.; Doll, M.A.; Hein, D.W.; Bozinoski, M.; Mason, C.E.; Coon, S.L.; Klein, D.C. Daily Rhythm in Plasma N-acetyltryptamine. J. Biol. Rhythms 2017, 32, 195–211. [Google Scholar] [CrossRef]
- Fukuda, T.; Akiyama, N.; Ikegami, M.; Takahashi, H.; Sasaki, A.; Oka, H.; Komori, T.; Tanaka, Y.; Nakazato, Y.; Akimoto, J.; et al. Expression of hydroxyindole-O-methyltransferase enzyme in the human central nervous system and in pineal parenchymal cell tumors. J. Neuropathol. Exp. Neurol. 2010, 69, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Abrahao, M.V.; Dos Santos, N.F.T.; Kuwabara, W.M.T.; do Amaral, F.G.; do Carmo Buonfiglio, D.; Peres, R.; Vendrame, R.F.A.; Flavio da Silveira, P.; Cipolla-Neto, J.; Baltatu, O.C.; et al. Identification of insulin-regulated aminopeptidase (IRAP) in the rat pineal gland and the modulation of melatonin synthesis by angiotensin IV. Brain Res. 2019, 1704, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, J.; Zhou, Y.; Li, X.; Li, H.; Liu, J.; Gou, K.; Zhao, J.; Cui, S. MicroRNA-7 inhibits melatonin synthesis by acting as a linking molecule between leptin and norepinephrine signaling pathways in pig pineal gland. J. Pineal Res. 2019, 66, e12552. [Google Scholar] [CrossRef] [PubMed]
- Drijfhout, W.J.; van der Linde, A.G.; de Vries, J.B.; Grol, C.J.; Westerink, B.H. Microdialysis reveals dynamics of coupling between noradrenaline release and melatonin secretion in conscious rats. Neurosci. Lett. 1996, 202, 185–188. [Google Scholar] [CrossRef]
- Schomerus, C.; Korf, H.W. Mechanisms regulating melatonin synthesis in the mammalian pineal organ. Ann. N. Y. Acad. Sci. 2005, 1057, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Velez-Roa, S.; Ciarka, A.; Najem, B.; Vachiery, J.L.; Naeije, R.; van de Borne, P. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 2004, 110, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- De Man, F.S.; Tu, L.; Handoko, M.L.; Rain, S.; Ruiter, G.; Francois, C.; Schalij, I.; Dorfmuller, P.; Simonneau, G.; Fadel, E.; et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 780–789. [Google Scholar] [CrossRef]
- Maron, B.A.; Leopold, J.A. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series). Pulm. Circ. 2014, 4, 200–210. [Google Scholar] [CrossRef]
- Sharma, R.K.; Oliveira, A.C.; Kim, S.; Rigatto, K.; Zubcevic, J.; Rathinasabapathy, A.; Kumar, A.; Lebowitz, J.J.; Khoshbouei, H.; Lobaton, G.; et al. Involvement of Neuroinflammation in the Pathogenesis of Monocrotaline-Induced Pulmonary Hypertension. Hypertension 2018, 71, 1156–1163. [Google Scholar] [CrossRef]
- De Man, F.S.; Handoko, M.L.; Guignabert, C.; Bogaard, H.J.; Vonk-Noordegraaf, A. Neurohormonal axis in patients with pulmonary arterial hypertension: Friend or foe? Am. J. Respir. Crit. Care Med. 2013, 187, 14–19. [Google Scholar] [CrossRef]
- MacLean, M.M.R. The serotonin hypothesis in pulmonary hypertension revisited: Targets for novel therapies (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045894018759125. [Google Scholar] [CrossRef] [PubMed]
- Jiki, Z.; Lecour, S.; Nduhirabandi, F. Cardiovascular Benefits of Dietary Melatonin: A Myth or a Reality? Front. Physiol. 2018, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Candia, A.; Veliz, M.; Carrasco-Pozo, C.; Castillo, R.L.; Cardenas, J.C.; Ebensperger, G.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Antenatal melatonin modulates an enhanced antioxidant/pro-oxidant ratio in pulmonary hypertensive newborn sheep. Redox Biol. 2019, 22, 101128. [Google Scholar] [CrossRef]
- Ciarka, A.; Doan, V.; Velez-Roa, S.; Naeije, R.; van de Borne, P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2010, 181, 1269–1275. [Google Scholar] [CrossRef]
- Forfia, P.R.; Mathai, S.C.; Fisher, M.R.; Housten-Harris, T.; Hemnes, A.R.; Champion, H.C.; Girgis, R.E.; Hassoun, P.M. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1364–1369. [Google Scholar] [CrossRef]
| Control | PH | |||
|---|---|---|---|---|
| Total | PAH | CTEPH | ||
| n | 111 | 64 | 43 | 21 |
| Aetiology | ||||
| iPAH, n (%) | 15 (35) | |||
| CTD-PAH, n (%) | 17 (40) | |||
| CHD-PAH, n (%) | 11 (25) | |||
| Age, years old | 43 ± 13 | 55 ± 17 *** | 53 ± 17 ** | 58 ± 18 *** |
| Sex, women n (%) | 59 (53) | 41 (64) | 29 (67) | 12 (57) |
| sBP, mmHg | 123 (115–128) | 127 (115–136) | 122 (114–132) | 133 (124–141) **,† |
| HR, beats·min −1 | 68 (62–76) | 78 (65–90) ** | 78 (67–90) ** | 71 (61–88) |
| BMI, kg·m −2 | 23.8 ± 2.9 | 28.4 ± 6.3 *** | 27.0 ± 6.1 *** | 31.4 ± 5.7 *** |
| mPAP, mmHg | - | 46.8 ± 15.7 | 50.5 ± 16.1 | 39.3 ± 12.3 †† |
| PAWP, mmHg | - | 12.4 ± 5.1 | 11.8 ± 5.6 | 13.7 ± 3.3 |
| PVR, WU | - | 5.8 (3.3–9.8) | 7.1 (5.1–11.8) | 3.4 (3.0–5.3) †† |
| CO, L·min −1 | - | 5.0 (4.1–5.9) | 4.7 (3.9–5.5) | 5.4 (4.7–6.4) † |
| CI, L·min −1·m −2 | - | 2.6 (2.3–3.2) | 2.5 (2.2–3.3) | 2.7 (2.3–3.0) |
| 6MWD, m | - | 353 ± 146 | 337 ± 153 | 385 ± 130 |
| NYHA, 1:2:3:4 | - | 1:25:31:7 | 1:13:23:6 | 0:12:8:1 |
| Plasma Levels of Melatonin | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | PH | PAH | CTEPH | |||||
| r | p Value | r | p Value | r | p Value | r | p Value | |
| Baseline characteristics | ||||||||
| Age | −0.119 | 0.212 | −0.368 | 0.003 | −0.334 | 0.029 | −0.363 | 0.106 |
| Sex | −0.070 | 0.466 | 0.103 | 0.417 | 0.112 | 0.475 | 0.159 | 0.491 |
| sBP | −0.178 | 0.063 | −0.251 | 0.046 | −0.279 | 0.070 | −0.015 | 0.949 |
| HR | −0.229 | 0.016 | 0.088 | 0.488 | 0.155 | 0.321 | −0.182 | 0.430 |
| BMI | −0.025 | 0.796 | −0.162 | 0.201 | −0.140 | 0.372 | −0.018 | 0.938 |
| mPAP | 0.166 | 0.191 | 0.061 | 0.699 | 0.403 | 0.070 | ||
| PAWP | −0.028 | 0.841 | −0.039 | 0.820 | 0.178 | 0.509 | ||
| PVR | 0.094 | 0.518 | 0.097 | 0.584 | 0.091 | 0.737 | ||
| CO | −0.184 | 0.160 | −0.154 | 0.351 | −0.302 | 0.184 | ||
| CI | −0.185 | 0.158 | −0.170 | 0.301 | −0.339 | 0.133 | ||
| 6MWD | 0.103 | 0.459 | 0.164 | 0.340 | −0.057 | 0.823 | ||
| NYHA | 0.029 | 0.821 | 0.033 | 0.832 | −0.084 | 0.717 | ||
| Univariate | Multivariate # | |||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| PH | Odds Ratio (95% CI) | 1.035 (1.016–1.055) | 1.048 (1.022–1.074) | 1.047 (1.021–1.073) |
| p value | <0.001 | <0.001 | <0.001 | |
| PAH | Odds Ratio (95% CI) | 1.036 (1.016–1.057) | 1.049 (1.022–1.076) | 1.047 (1.020–1.074) |
| p value | <0.001 | <0.001 | <0.001 | |
| CTEPH | Odds Ratio (95% CI) | 1.029 (1.002–1.056) | 1.025 (0.989–1.062) | 1.025 (0.988–1.062) |
| p value | 0.033 | 0.175 | 0.184 | |
| Analyses | Hazard Ratio (95% CI) | p Value |
|---|---|---|
| Univariate | 0.995 (0.981–1.010) | 0.546 |
| Multivariate # | ||
| Model 1 | 0.999 (0.992–1.005) | 0.653 |
| Model 2 | 0.998 (0.992–1.005) | 0.645 |
| PAH | |||
|---|---|---|---|
| 1st Quartile (<109.3 pM) | Quartile 2–4 (≥109.3 pM) | p Value | |
| n | 11 | 32 | |
| Aetiology | |||
| iPAH, n (%) | 2 (18) | 13 (41) | |
| CTD-PAH, n (%) | 6 (55) | 11 (34) | |
| CHD-PAH, n (%) | 3 (27) | 8 (25) | |
| Age, years old | 66 ± 13 | 48 ± 15 | 0.001 |
| Sex, women n (%) | 9 (82) | 20 (63) | 0.213 |
| sBP, mmHg | 127 ± 14 | 123 ± 15 | 0.466 |
| HR, beats·min −1 | 79 ± 14 | 80 ± 18 | 0.965 |
| BMI, kg·m −2 | 27.1 ± 3.9 | 26.9 ± 6.8 | 0.931 |
| mPAP, mmHg | 47.0 (38.0–65.0) | 45.0 (38.8–65.3) | 0.880 |
| PAWP, mmHg | 13.0 ± 5.1 | 11.3 ± 5.8 | 0.450 |
| PVR, WU | 5.7 (3.9–11.4) | 8.8 (5.6–11.9) | 0.316 |
| CO, L·min −1 | 5.1 ± 1.5 | 4.8 ± 1.4 | 0.522 |
| CI, L·min −1·m −2 | 2.9 ± 0.8 | 2.6 ± 0.7 | 0.312 |
| 6MWD, m | 271 ± 148 | 356 ± 152 | 0.172 |
| NYHA, 1:2:3:4 | 0:4:4:3 | 1:9:19:3 | 0.359 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Klein, T.; Geenen, L.W.; Tu, L.; Tian, S.; van den Bosch, A.E.; de Rijke, Y.B.; Reiss, I.K.M.; Boersma, E.; Duncker, D.J.; et al. Lower Plasma Melatonin Levels Predict Worse Long-Term Survival in Pulmonary Arterial Hypertension. J. Clin. Med. 2020, 9, 1248. https://doi.org/10.3390/jcm9051248
Cai Z, Klein T, Geenen LW, Tu L, Tian S, van den Bosch AE, de Rijke YB, Reiss IKM, Boersma E, Duncker DJ, et al. Lower Plasma Melatonin Levels Predict Worse Long-Term Survival in Pulmonary Arterial Hypertension. Journal of Clinical Medicine. 2020; 9(5):1248. https://doi.org/10.3390/jcm9051248
Chicago/Turabian StyleCai, Zongye, Theo Klein, Laurie W. Geenen, Ly Tu, Siyu Tian, Annemien E. van den Bosch, Yolanda B. de Rijke, Irwin K. M. Reiss, Eric Boersma, Dirk J. Duncker, and et al. 2020. "Lower Plasma Melatonin Levels Predict Worse Long-Term Survival in Pulmonary Arterial Hypertension" Journal of Clinical Medicine 9, no. 5: 1248. https://doi.org/10.3390/jcm9051248
APA StyleCai, Z., Klein, T., Geenen, L. W., Tu, L., Tian, S., van den Bosch, A. E., de Rijke, Y. B., Reiss, I. K. M., Boersma, E., Duncker, D. J., Boomars, K. A., Guignabert, C., & Merkus, D. (2020). Lower Plasma Melatonin Levels Predict Worse Long-Term Survival in Pulmonary Arterial Hypertension. Journal of Clinical Medicine, 9(5), 1248. https://doi.org/10.3390/jcm9051248