Local and Central Evaluation of HER2 Positivity and Clinical Outcome in Advanced Gastric and Gastroesophageal Cancer—Results from the AGMT GASTRIC-5 Registry
Abstract
1. Introduction
2. Experimental Section
Statistical Analysis
3. Results
3.1. Baseline Characteristics
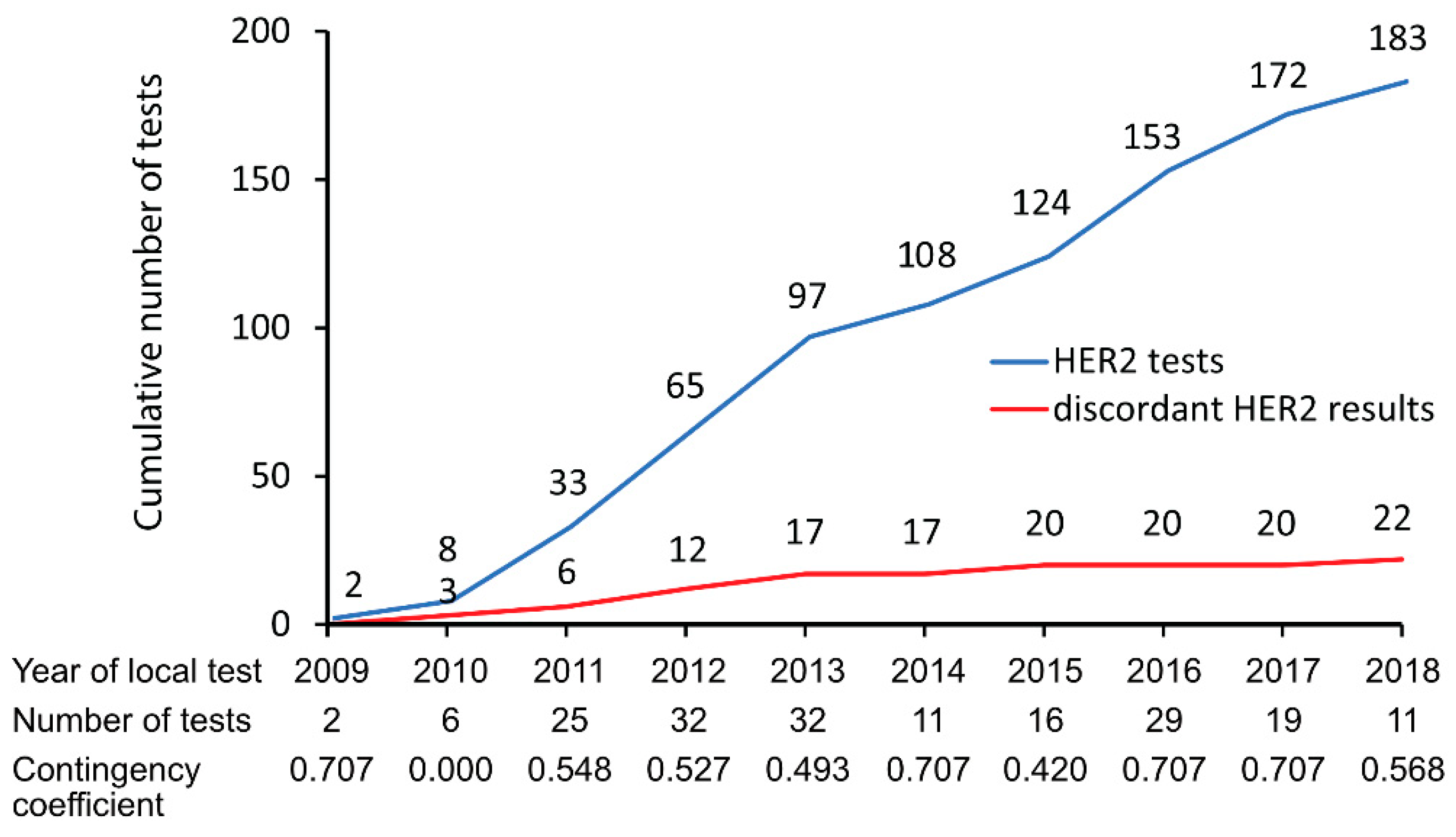
3.2. HER2 Concordance and Discordance Rate
3.3. HER2 Status Assessment by Immunohistochemistry (IHC)
3.4. HER2 Status Assessment by in Situ Hybridization (ISH)
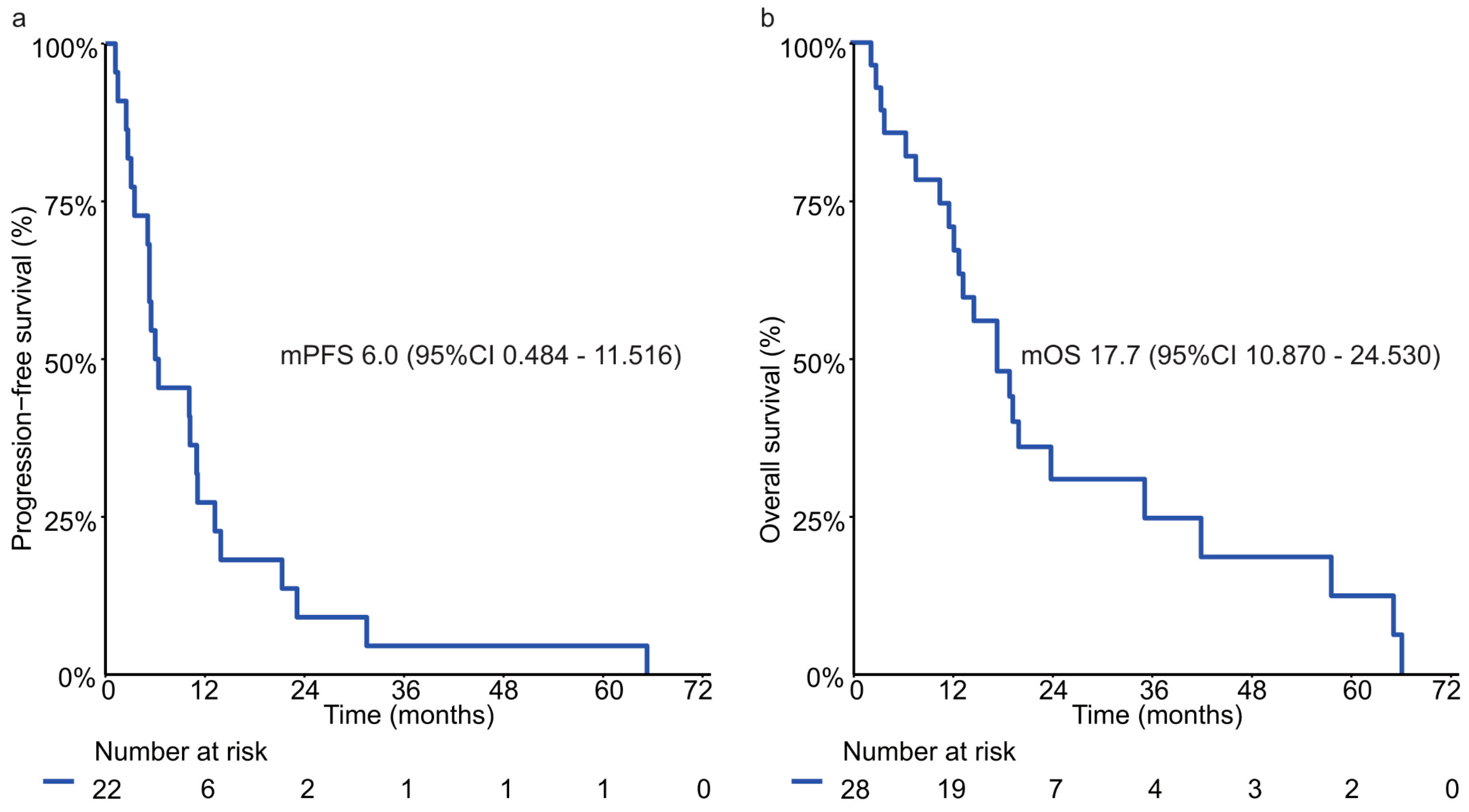
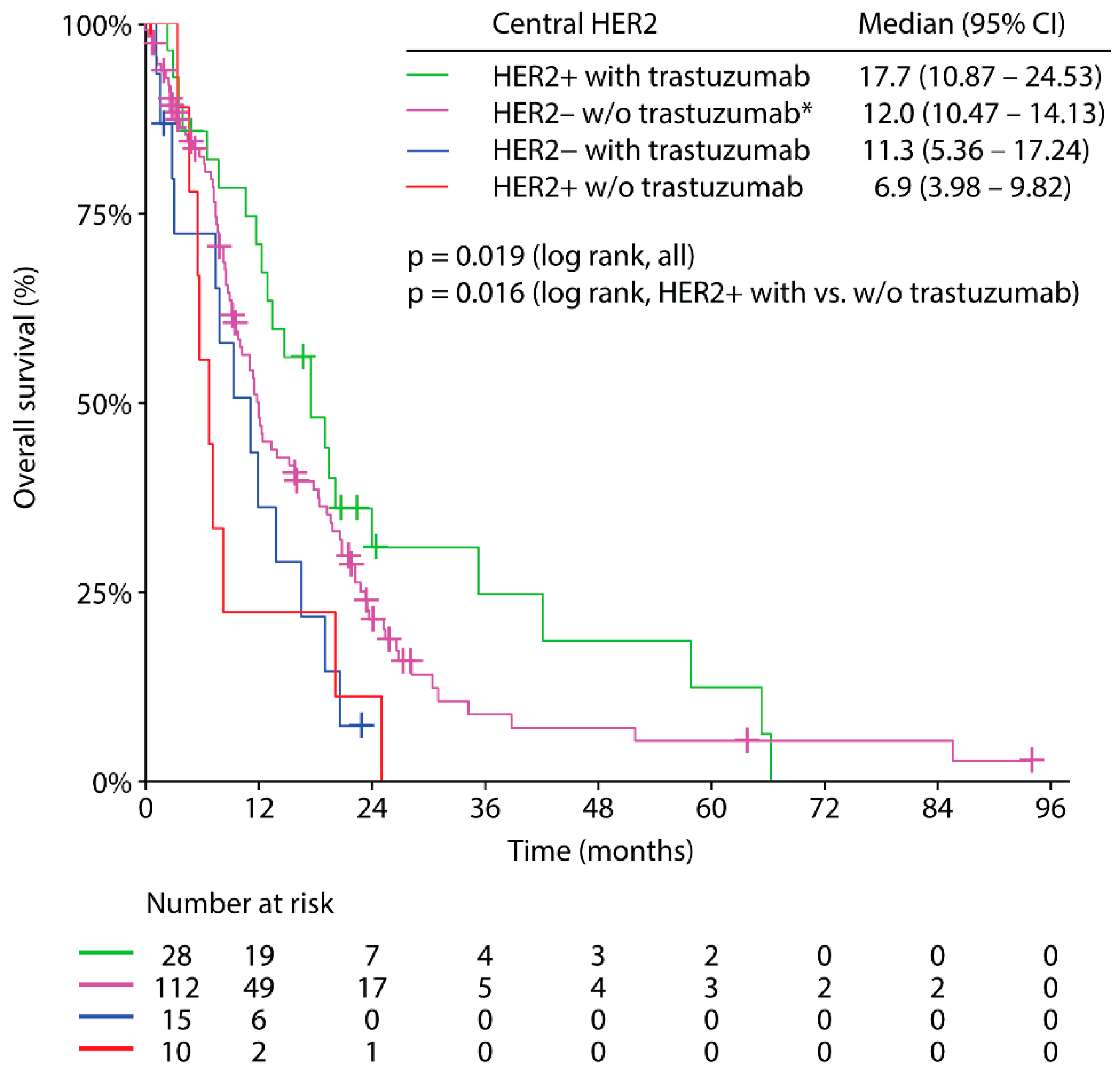
3.5. Trastuzumab Based Palliative First-Line Therapy and Clinical Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, based on November 2017 SEER data submission, posted to SEER web site, April 2018. 1975–2016. Natl. Cancer Inst. 2019. [Google Scholar]
- Jorgensen, J.T.; Hersom, M. HER2 as a Prognostic Marker in Gastric Cancer—A Systematic Analysis of Data from the Literature. J. Cancer 2012, 3, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Allgayer, H.; Babic, R.; Gruetzner, K.U.; Tarabichi, A.; Schildberg, F.W.; Heiss, M.M. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J. Clin. Oncol. 2000, 18, 2201–2209. [Google Scholar] [CrossRef]
- Uchino, S.; Tsuda, H.; Maruyama, K.; Kinoshita, T.; Sasako, M.; Saito, T.; Kobayashi, M.; Hirohashi, S. Overexpression of c-erbB-2 protein in gastric cancer. Its correlation with long-term survival of patients. Cancer 1993, 72, 3179–3184. [Google Scholar] [CrossRef]
- Barros-Silva, J.D.; Leitao, D.; Afonso, L.; Vieira, J.; Dinis-Ribeiro, M.; Fragoso, M.; Bento, M.J.; Santos, L.; Ferreira, P.; Rego, S.; et al. Association of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patients. Br. J. Cancer 2009, 100, 487–493. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Press, M.F.; Ellis, C.E.; Gagnon, R.C.; Grob, T.J.; Buyse, M.; Villalobos, I.; Liang, Z.; Wu, S.; Bang, Y.J.; Qin, S.K.; et al. HER2 Status in Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma for Entry to the TRIO-013/LOGiC Trial of Lapatinib. Mol. Cancer Ther. 2017, 16, 228–238. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Gastric Cancer (Version 2.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 25 August 2019).
- Woll, E.; Eisterer, W.; Gerger, A.; Kuhr, T.; Prager, G.W.; Rumpold, H.; Ulrich-Pur, H.; Vogl, U.; Winder, T.; Weiss, L.; et al. Treatment Algorithm for Patients With Gastric Adenocarcinoma: An Austrian Consensus on Systemic Therapy. Anticancer Res. 2019, 39, 4589–4596. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E.; et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J. Clin. Oncol. 2006, 24, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.S.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Castro Salguero, H.R.; et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. J. Clin. Oncol. 2019, 37, LBA4007. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Hecht, J.R.; Bang, Y.J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Xu, R.H.; Chung, H.C.; Sun, G.P.; Doi, T.; Xu, J.M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.W.; et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—A randomized, phase III study. J. Clin. Oncol. 2014, 32, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Inoue, M.; Sawada, N.; Matsuda, T.; Iwasaki, M.; Sasazuki, S.; Shimazu, T.; Shibuya, K.; Tsugane, S. Attributable causes of cancer in Japan in 2005—Systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann. Oncol. 2012, 23, 1362–1369. [Google Scholar] [CrossRef]
- Huang, D.; Lu, N.; Fan, Q.; Sheng, W.; Bu, H.; Jin, X.; Li, G.; Liu, Y.; Li, X.; Sun, W.; et al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS ONE 2013, 8, e80290. [Google Scholar] [CrossRef]
- Hofmann, M.; Stoss, O.; Shi, D.; Buttner, R.; van de Vijver, M.; Kim, W.; Ochiai, A.; Ruschoff, J.; Henkel, T. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008, 52, 797–805. [Google Scholar] [CrossRef]
- Kim, K.C.; Koh, Y.W.; Chang, H.M.; Kim, T.H.; Yook, J.H.; Kim, B.S.; Jang, S.J.; Park, Y.S. Evaluation of HER2 protein expression in gastric carcinomas: Comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann. Surg. Oncol. 2011, 18, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.W.; Zhang, J.J.; Zhang, T.; Zheng, Z.C. Clinicopathological and prognostic significance of HER2 overexpression in gastric cancer: A meta-analysis of the literature. Tumour Biol. 2014, 35, 4849–4858. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Janjigian, Y.J.; Ilson, D.H. HER2 directed therapy for gastric/esophageal cancers. Curr. Treat. Options Oncol. 2014, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Hollmen, M.; Junttila, T.T.; Kapanen, A.I.; Tommola, S.; Soini, Y.; Helin, H.; Salo, J.; Joensuu, H.; Sihvo, E.; et al. Amplification of HER-2 in gastric carcinoma: Association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 2005, 16, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Riihimaki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 2016, 7, 52307–52316. [Google Scholar] [CrossRef]
- Lordick, F.; Haffner, I.; Luber, B.; Maier, D.; Raimundez, E.; Hasenauer, J.; Kretzschmar, A.; Fischer von Weikersthal, L.; Ahlborn, M.; Knorrenschild, J.R.; et al. Heterogeneity of HER2 expression in gastric cancer (GC) leads to high deviation rates between local and central testing and hampers efficacy of anti-HER2 therapy: Survival results from the VARIANZ study. In Proceedings of the American Association for Cancer Research Annual Meeting 2018, Chicago, IL, USA, 14–18 April 2018. [Google Scholar]
- Monges-Ranchin, G.; Terris, B.; Chenard, M.P.; Bibeau, F.; Penault-Llorca, F.M.; Bougrini, M.; Condé da Silva Fraga, E.; Doucet, L. Concordance of HER2 status between local and central review in gastric and gastroesophageal junction cancers: A French observational study of 394 specimens (HERable study). J. Clin. Oncol. 2016, 34, 30. [Google Scholar] [CrossRef]
- Pfitzner, B.M.; Lederer, B.; Lindner, J.; Solbach, C.; Engels, K.; Rezai, M.; Dohnal, K.; Tesch, H.; Hansmann, M.L.; Salat, C.; et al. Clinical relevance and concordance of HER2 status in local and central testing-an analysis of 1581 HER2-positive breast carcinomas over 12 years. Mod. Pathol. 2018, 31, 607–615. [Google Scholar] [CrossRef]
- Peng, Z.; Zou, J.; Zhang, X.; Yang, Y.; Gao, J.; Li, Y.; Li, Y.; Shen, L. HER2 discordance between paired primary gastric cancer and metastasis: A meta-analysis. Chin. J. Cancer Res. 2015, 27, 163–171. [Google Scholar]
- Ieni, A.; Barresi, V.; Caltabiano, R.; Caleo, A.; Bonetti, L.R.; Lanzafame, S.; Zeppa, P.; Caruso, R.A.; Tuccari, G. Discordance rate of HER2 status in primary gastric carcinomas and synchronous lymph node metastases: A multicenter retrospective analysis. Int. J. Mol. Sci. 2014, 15, 22331–22341. [Google Scholar] [CrossRef]
- Amato, M.; Perrone, G.; Righi, D.; Pellegrini, C.; Rabitti, C.; Di Matteo, F.; Crucitti, P.; Caputo, D.; Coppola, R.; Tonini, G.; et al. HER2 Status in Gastric Cancer: Comparison between Primary and Distant Metastatic Disease. Pathol. Oncol. Res. 2017, 23, 55–61. [Google Scholar] [CrossRef]
- Huang, S.C.; Ng, K.F.; Lee, S.E.; Chen, K.H.; Yeh, T.S.; Chen, T.C. HER2 testing in paired biopsy and excision specimens of gastric cancer: The reliability of the scoring system and the clinicopathological factors relevant to discordance. Gastric Cancer 2016, 19, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, H.; Li, Y.; Li, J.; Cai, Z.; Su, X.; Dai, D.; Du, W.; Chen, T.; Chen, M. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem. Biophys. 2012, 62, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, K.; Imai, H.; Usugi, E.; Shiraishi, T.; Hirokawa, Y.S.; Watanabe, M. Association of HER2 gene amplification and tumor progression in early gastric cancer. Virchows Arch. 2018, 473, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Cardia, R.; Lentini, M.; Tuccari, G. Intratumoral HER2 heterogeneity in early gastric carcinomas: Potential bias in therapeutic management. Virchows Arch. 2019, 474, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Al-Batran, S.E.; Dietel, M.; Gaiser, T.; Hofheinz, R.D.; Kirchner, T.; Kreipe, H.H.; Lorenzen, S.; Mohler, M.; Quaas, A.; et al. HER2 testing in gastric cancer: Results of a German expert meeting. J. Cancer Res. Clin. Oncol. 2017, 143, 835–841. [Google Scholar] [CrossRef]
- Ieni, A.; Angelico, G.; Giuffre, G.; Tuccari, G. Discordance Rate of HER2 Status in Primary Gastric Cancer and Synchronous Lymph Node Metastases: Its Impact on Therapeutic Decision and Clinical Management. Pathol. Oncol. Res. 2018, 24, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Park, Y.S.; Ryu, M.H.; Ryoo, B.Y.; Woo, C.G.; Jung, H.Y.; Lee, J.H.; Lee, G.H.; Kang, Y.K. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur. J. Cancer 2016, 53, 42–50. [Google Scholar] [CrossRef]
- Woo, C.G.; Ho, W.J.; Park, Y.S.; Park, S.R.; Ryu, M.H.; Jung, H.Y.; Kang, Y.K. A potential pitfall in evaluating HER2 immunohistochemistry for gastric signet ring cell carcinomas. Pathology 2017, 49, 38–43. [Google Scholar] [CrossRef]
- Pernot, S.; Voron, T.; Perkins, G.; Lagorce-Pages, C.; Berger, A.; Taieb, J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 2015, 21, 11428–11438. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1437–1448. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Ter Veer, E.; Creemers, A.; de Waal, L.; van Oijen, M.G.H.; van Laarhoven, H.W.M. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: A meta-analysis. Int. J. Cancer 2018, 143, 438–448. [Google Scholar] [CrossRef]

| All Patients n = 183 (100%) | HER2 Negative n = 145 (79%) | HER2 Positive n = 38 (21%) | p-Value | |
|---|---|---|---|---|
| Age (median) | 67 | 67 | 66 | 0.378 * |
| Range | 28–89 | 28–89 | 45–86 | |
| Sex | 0.922 | |||
| Male | 124 (68) | 98 (68) | 26 (68) | |
| Female | 59 (32) | 47 (32) | 12 (32) | |
| Primary Tumor | 0.403 | |||
| Gastric cancer | 93 (55) | 75 (56) | 18 (49) | |
| GEJ cancer | 77 (45) | 58 (44) | 19 (51) | |
| NA | 13 | 12 | 1 | |
| Prior Surgery | 0.211 | |||
| Yes | 74 (40) | 62 (43) | 12 (32) | |
| No | 109 (60) | 83 (57) | 26 (68) | |
| Histology Grading | <0.001 | |||
| 1 | 3 (2) | 2 (2) | 1 (3) | |
| 2 | 48 (33) | 27 (24) | 21 (60) | |
| 3 | 94 (65) | 81 (74) | 13 (37) | |
| NA | 38 | 35 | 3 | |
| Lauren’s Classification | <0.001 | |||
| Intestinal | 86 (55) | 57 (46) | 29 (88) | |
| Diffuse | 60 (39) | 57 (46) | 3 (9) | |
| Mixed | 10 (6) | 9 (8) | 1 (3) | |
| NA | 27 | 22 | 5 | |
| Distribution of Metastases | ||||
| Liver a | 81 (44) | 56 (39) | 25 (66) | 0.003 |
| Peritoneum a | 62 (34) | 57 (39) | 5 (13) | 0.002 |
| Lung a | 26 (14) | 16 (11) | 10 (26) | 0.016 |
| Distant lymph nodes a | 25 (14) | 20 (14) | 5 (13) | 0.919 |
| Other a | 15 (8) | 13 (9) | 2 (5) | 0.459 |
| Local HER2 Test | |||
|---|---|---|---|
| Negative | Positive | ||
| Central HER2 Test | Negative | 128 (70%) | 17 (9%) |
| Positive | 5 (3%) | 33 (18%) | |
| HER2 Result (%) | ||||
|---|---|---|---|---|
| Locally HER2 Negative/ Centrally HER2 Negative | Locally HER2 Positive/ Centrally HER2 Positive | Locally HER2 Negative/ Centrally HER2 Positive | Locally HER2 Positive/ Centrally HER2 Negative | |
| Local IHC < central IHC | 56 (90) | 1 (2) | 5 (8) | 0 (0) |
| Local IHC > central IHC | 17 (50) | 1 (3) | 0 (0) | 16 (47) |
| Local IHC = central IHC | 55 (63) | 31 (36) | 0 (0) | 1 (1) |
| Total | 128 | 33 | 5 | 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huemer, F.; Weiss, L.; Regitnig, P.; Winder, T.; Hartmann, B.; Thaler, J.; Piringer, G.; Schmitt, C.A.; Eisterer, W.; Gänzer, H.; et al. Local and Central Evaluation of HER2 Positivity and Clinical Outcome in Advanced Gastric and Gastroesophageal Cancer—Results from the AGMT GASTRIC-5 Registry. J. Clin. Med. 2020, 9, 935. https://doi.org/10.3390/jcm9040935
Huemer F, Weiss L, Regitnig P, Winder T, Hartmann B, Thaler J, Piringer G, Schmitt CA, Eisterer W, Gänzer H, et al. Local and Central Evaluation of HER2 Positivity and Clinical Outcome in Advanced Gastric and Gastroesophageal Cancer—Results from the AGMT GASTRIC-5 Registry. Journal of Clinical Medicine. 2020; 9(4):935. https://doi.org/10.3390/jcm9040935
Chicago/Turabian StyleHuemer, Florian, Lukas Weiss, Peter Regitnig, Thomas Winder, Bernd Hartmann, Josef Thaler, Gudrun Piringer, Clemens A. Schmitt, Wolfgang Eisterer, Hannes Gänzer, and et al. 2020. "Local and Central Evaluation of HER2 Positivity and Clinical Outcome in Advanced Gastric and Gastroesophageal Cancer—Results from the AGMT GASTRIC-5 Registry" Journal of Clinical Medicine 9, no. 4: 935. https://doi.org/10.3390/jcm9040935
APA StyleHuemer, F., Weiss, L., Regitnig, P., Winder, T., Hartmann, B., Thaler, J., Piringer, G., Schmitt, C. A., Eisterer, W., Gänzer, H., Wüstner, A., Andel, J., Jagdt, B., Ulmer, H., Greil, R., & Wöll, E. (2020). Local and Central Evaluation of HER2 Positivity and Clinical Outcome in Advanced Gastric and Gastroesophageal Cancer—Results from the AGMT GASTRIC-5 Registry. Journal of Clinical Medicine, 9(4), 935. https://doi.org/10.3390/jcm9040935





