Radiofrequency Chondroplasty May Not Have a Long-Lasting Effect in the Treatment of Concomitant Grade II Patellar Cartilage Defects in Humans
Abstract
1. Introduction
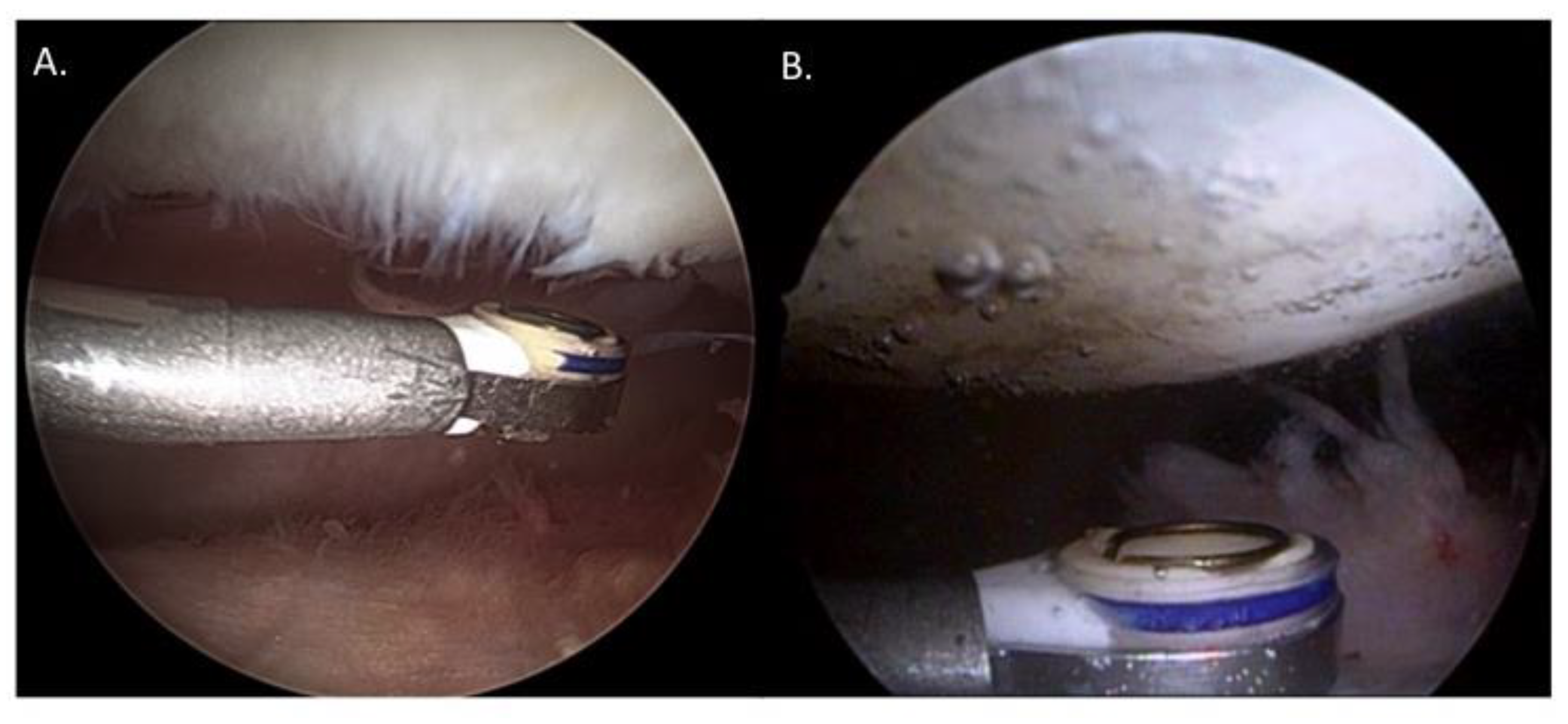
2. Materials and Methods
3. Results
3.1. Clinical Scoring
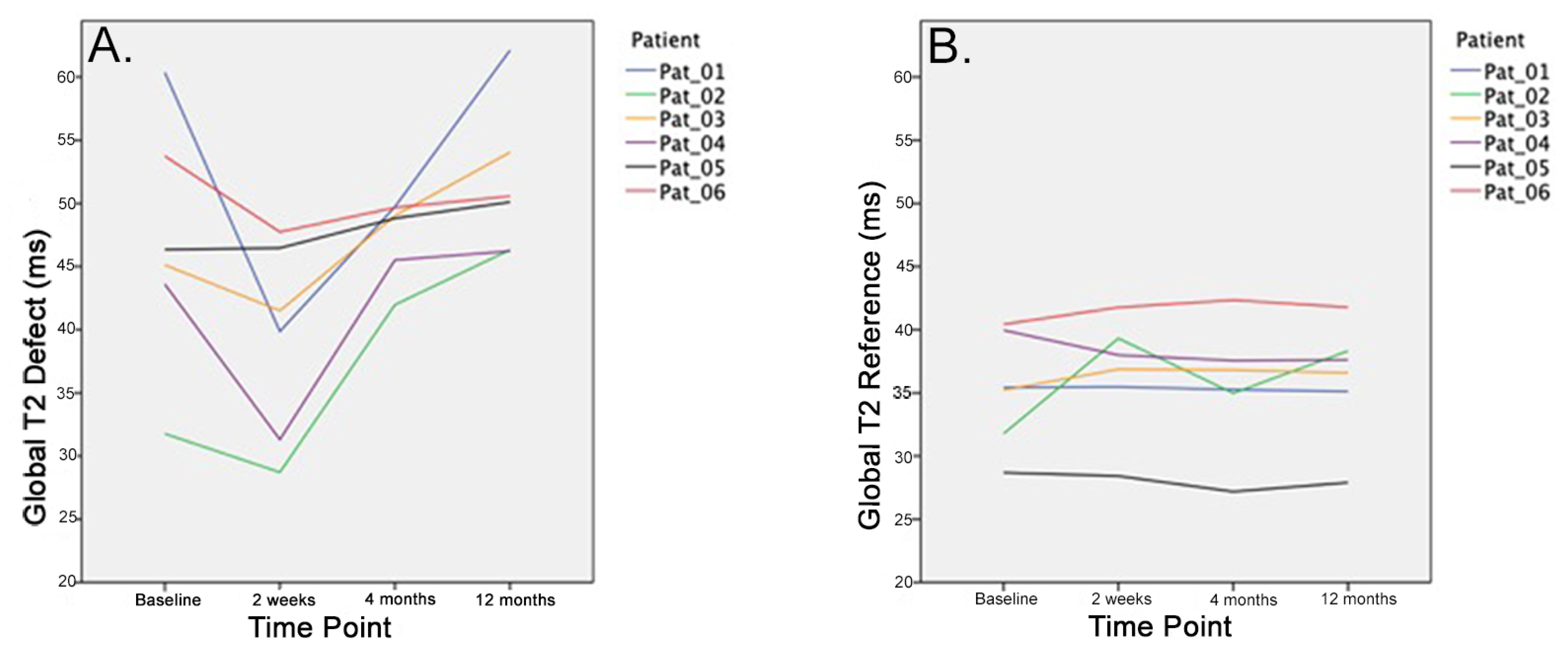
3.2. Global T2 Values
3.3. Zonal T2 Values
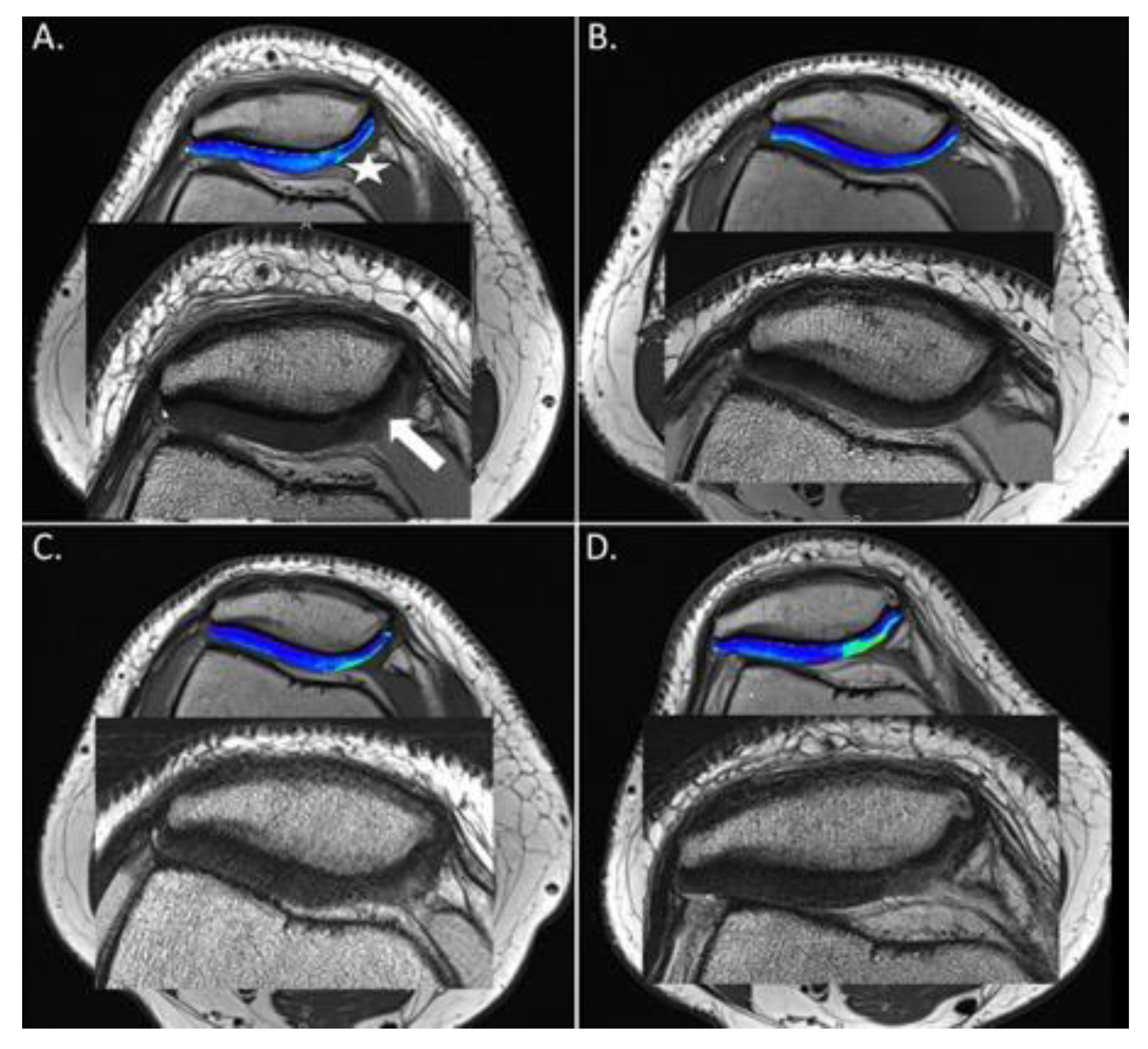
3.4. Morphological MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hjelle, K.; Solheim, E.; Strand, T.; Muri, R.; Brittberg, M. Articular cartilage defects in 1,000 knee arthroscopies. Arthrosc. J. Arthrosc. Relat. Surg. 2002, 18, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Widuchowski, W.; Widuchowski, J.; Trzaska, T. Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee 2007, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.K.; Simpson, A.; Hall, A.C. Iatrogenic articular cartilage injury: The elephant in the operating theatre: The surgeons’ role in chondroprotection. Bone Jt. J. 2017, 99, 1555–1556. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Winalski, C.S. Evaluation of cartilage injuries and repair. J. Bone Jt. Surg. Am. Vol. 2003, 85, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Mundi, R.; Bedi, A.; Chow, L.; Crouch, S.; Simunovic, N.; Sibilsky Enselman, E.; Ayeni, O.R. Cartilage Restoration of the Knee: A Systematic Review and Meta-analysis of Level 1 Studies. Am. J. Sports Med. 2016, 44, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Sgaglione, N.A.; Miniaci, A.; Gillogly, S.D.; Carter, T.R. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthrosc. J. Arthrosc. Relat. Surg. 2002, 18, 9–32. [Google Scholar] [CrossRef]
- Spahn, G.; Hofmann, G.O.; Von Engelhardt, L.V. Mechanical debridement versus radiofrequency in knee chondroplasty with concomitant medial meniscectomy: 10-year results from a randomized controlled study. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1560–1568. [Google Scholar] [CrossRef]
- Liptak, M.G.; Theodoulou, A. Arthroscopic Chondral Debridement Using Radiofrequency Ablation for Patellofemoral Compartment Pathology. Arthrosc. Tech. 2017, 6, e1879–e1883. [Google Scholar] [CrossRef]
- Rocco, P.; Lorenzo, D.B.; Guglielmo, T.; Michele, P.; Nicola, M.; Vincenzo, D. Radiofrequency energy in the arthroscopic treatment of knee chondral lesions: A systematic review. Br. Med. Bull. 2016, 117, 149–156. [Google Scholar] [CrossRef]
- Shellock, F.G.; Shields, C.L., Jr. Radiofrequency energy-induced heating of bovine articular cartilage using a bipolar radiofrequency electrode. Am. J. Sports Med. 2000, 28, 720–724. [Google Scholar] [CrossRef]
- Uthamanthil, R.K.; Edwards, R.B.; Lu, Y.; Manley, P.A.; Athanasiou, K.A.; Markel, M.D. In vivo study on the short-term effect of radiofrequency energy on chondromalacic patellar cartilage and its correlation with calcified cartilage pathology in an equine model. J. Orthop. Res. 2006, 24, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.D.; Stickles, B.J.; Balikian, P.; Busconi, B.D. Prospective analysis of radiofrequency versus mechanical debridement of isolated patellar chondral lesions. Arthrosc. J. Arthrosc. Relat. Surg. 2002, 18, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.D.; Stickles, B.J.; Busconi, B.D. Radiofrequency energy: Applications and basic science. Am. J. Orthop. 2003, 32, 117–120. [Google Scholar] [PubMed]
- Kosy, J.D.; Schranz, P.J.; Toms, A.D.; Eyres, K.S.; Mandalia, V.I. The use of radiofrequency energy for arthroscopic chondroplasty in the knee. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A.; Iwasko, N.G. Treatment of grade III femoral chondral lesions: Mechanical chondroplasty versus monopolar radiofrequency probe. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.R.; Lu, Y.; Edwards, R.B.; Bogdanske, J.J.; Markel, M.D. Effects of thermal energy on chondrocyte viability. Am. J. Vet. Res. 2006, 67, 1708–1712. [Google Scholar] [CrossRef]
- Kaab, M.J.; Bail, H.J.; Rotter, A.; Mainil-Varlet, P.; Apgwynn, I.; Weiler, A. Monopolar radiofrequency treatment of partial-thickness cartilage defects in the sheep knee joint leads to extended cartilage injury. Am. J. Sports Med. 2005, 33, 1472–1478. [Google Scholar] [CrossRef]
- Spahn, G.; Kahl, E.; Muckley, T.; Hofmann, G.O.; Klinger, H.M. Arthroscopic knee chondroplasty using a bipolar radiofrequency-based device compared to mechanical shaver: Results of a prospective, randomized, controlled study. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 565–573. [Google Scholar] [CrossRef]
- Springer, E.; Bohndorf, K.; Juras, V.; Szomolanyi, P.; Zbyn, S.; Schreiner, M.M.; Schmitt, B.; Trattnig, S. Comparison of Routine Knee Magnetic Resonance Imaging at 3 T and 7 T. Investig. Radiol. 2017, 52, 42–54. [Google Scholar] [CrossRef]
- Marlovits, S.; Singer, P.; Zeller, P.; Mandl, I.; Haller, J.; Trattnig, S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur. J. Radiol. 2006, 57, 16–23. [Google Scholar] [CrossRef]
- Mohr, A. The value of water-excitation 3D FLASH and fat-saturated PDw TSE MR imaging for detecting and grading articular cartilage lesions of the knee. Skelet. Radiol. 2003, 32, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Link, T.M.; Neumann, J.; Li, X. Prestructural cartilage assessment using MRI. J. Magn. Reson. Imaging 2017, 45, 949–965. [Google Scholar] [CrossRef]
- Baum, T.; Joseph, G.B.; Karampinos, D.C.; Jungmann, P.M.; Link, T.M.; Bauer, J.S. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthr. Cartil. 2013, 21, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Mosher, T.J.; Dardzinski, B.J.; Collins, B.G.; Collins, C.M.; Yang, Q.X.; Schmithorst, V.J.; Smith, M.B. Spatial variation in cartilage T2 of the knee. J. Magn. Reson. Imaging 2001, 14, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Koller, U.; Apprich, S.; Domayer, S.; Windhager, R.; Trattnig, S. Magnetic resonance mapping of the rim of articular cartilage defects of the patella. Int. Orthop. 2014, 38, 67–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liess, C.; Lusse, S.; Karger, N.; Heller, M.; Gluer, C.C. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthr. Cartil. 2002, 10, 907–913. [Google Scholar] [CrossRef]
- Mosher, T.J.; Dardzinski, B.J.; Smith, M.B. Human articular cartilage: Influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology 2000, 214, 259–266. [Google Scholar] [CrossRef]
- Mosher, T.J.; Liu, Y.; Torok, C.M. Functional cartilage MRI T2 mapping: Evaluating the effect of age and training on knee cartilage response to running. Osteoarthr. Cartil. 2010, 18, 358–364. [Google Scholar] [CrossRef]
- Mosher, T.J.; Liu, Y.; Yang, Q.X.; Yao, J.; Smith, R.; Dardzinski, B.J.; Smith, M.B. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004, 50, 2820–2828. [Google Scholar] [CrossRef]
- Mosher, T.J.; Dardzinski, B.J. Cartilage MRI T2 relaxation time mapping: Overview and applications. Semin. Musculoskelet. Radiol. 2004, 8, 355–368. [Google Scholar] [CrossRef]
- Dardzinski, B.J.; Mosher, T.J.; Li, S.; Van Slyke, M.A.; Smith, M.B. Spatial variation of T2 in human articular cartilage. Radiology 1997, 205, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Gold, G.E.; Burstein, D.; Dardzinski, B.; Lang, P.; Boada, F.; Mosher, T. MRI of articular cartilage in OA: Novel pulse sequences and compositional/functional markers. Osteoarthr. Cartil. 2006, 14, A76–A86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prasad, A.P.; Nardo, L.; Schooler, J.; Joseph, G.B.; Link, T.M. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthr. Cartil. 2013, 21, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Price, A.J.; Haddad, F.S.; Beard, D.J. New guidelines for the use of arthroscopic meniscal knee surgery. Bone Jt. J. 2019, 101-B, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.D.; Li, J.G.; Majumdar, S.; Henkelman, R.M. Image resolution and signal-to-noise ratio requirements for MR imaging of degenerative cartilage. AJR Am. J. Roentgenol. 1997, 169, 1089–1096. [Google Scholar] [CrossRef][Green Version]
- Apprich, S.; Welsch, G.H.; Mamisch, T.C.; Szomolanyi, P.; Mayerhoefer, M.; Pinker, K.; Trattnig, S. Detection of degenerative cartilage disease: Comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthr. Cartil. 2010, 18, 1211–1217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pan, J.; Pialat, J.B.; Joseph, T.; Kuo, D.; Joseph, G.B.; Nevitt, M.C.; Link, T.M. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: A longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology 2011, 261, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Stahl, R.; Blumenkrantz, G.; Carballido-Gamio, J.; Zhao, S.; Muñoz, T.; Le Graverand-Gastineau, M.H.; Li, X.; Majumdar, S.; Link, T.M. MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthr. Cartil. 2007, 15, 1225–1234. [Google Scholar] [CrossRef][Green Version]
- Choi, J.A.; Gold, G.E. MR imaging of articular cartilage physiology. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 249–282. [Google Scholar] [CrossRef]
- Juras, V.; Schreiner, M.; Laurent, D.; Zbyn, S.; Mlynarik, V.; Szomolanyi, P.; Hager, B.; Scotti, C.; Goldhahn, J.; Heule, R.; et al. The comparison of the performance of 3T and 7T T2 mapping for untreated low-grade cartilage lesions. Magn. Reson. Imaging 2019, 55, 86–92. [Google Scholar] [CrossRef]
- Apprich, S.; Mamisch, T.C.; Welsch, G.H.; Stelzeneder, D.; Albers, C.; Totzke, U.; Trattnig, S. Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur. J. Radiol. 2012, 81, e438–e443. [Google Scholar] [CrossRef] [PubMed]
- Soellner, S.T.; Goldmann, A.; Muelheims, D.; Welsch, G.H.; Pachowsky, M.L. Intraoperative validation of quantitative T2 mapping in patients with articular cartilage lesions of the knee. Osteoarthr. Cartil. 2017, 25, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Apprich, S.R.; Schreiner, M.M.; Szomolanyi, P.; Welsch, G.H.; Koller, U.K.; Weber, M.; Windhager, R.; Trattnig, S. Potential predictive value of axial T2 mapping at 3 Tesla MRI in patients with untreated patellar cartilage defects over a mean follow-up of four years. Osteoarthr. Cartil. 2019, 28, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Denti, M.; Filardo, G.; Kon, E.; Engebretsen, L. Definition and classification of early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 401–406. [Google Scholar] [CrossRef]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]

| Mean Values (ms) | Std.-Deviation | 95% Confidence Level | |||
|---|---|---|---|---|---|
| Lower Border | Upper Border | ||||
| Deep T2 Values Defect | Baseline | 38.7 | 7.1 | 31.3 | 46.2 |
| 2 Weeks | 36.6 | 6.5 | 29.7 | 43.4 | |
| 4 Months | 38.4 | 5.4 | 32.7 | 44.1 | |
| 1 Year | 40.5 | 5.9 | 34.3 | 46.8 | |
| P-value | 0.756 | ||||
| Superficial T2 Values Defect | Baseline | 52.6 | 15.3 | 36.5 | 68.8 |
| 2 Weeks | 43.2 | 8.7 | 33.9 | 52.4 | |
| 4 Months | 53.8 | 8.3 | 45.1 | 62.7 | |
| 1 Year | 59.2 | 12.2 | 46.4 | 72.1 | |
| P-value | 0.146 | ||||
| Global T2 Values Defect | Baseline | 46.8 | 9.7 | 36.6 | 57.0 |
| 2 Weeks | 39.2 | 7.7 | 31.0 | 47.4 | |
| 4 Months | 47.4 | 3.1 | 44.2 | 50.7 | |
| 1 Year | 51.5 | 5.9 | 45.3 | 57.8 | |
| P-value | 0.048 | ||||
| Deep T2 Values Reference | Baseline | 33.1 | 4.3 | 28.6 | 37.6 |
| 2 Weeks | 31.8 | 4.2 | 27.4 | 36.3 | |
| 4 Months | 29.6 | 4.8 | 24.5 | 34.7 | |
| 1 Year | 31.1 | 5.1 | 25.8 | 36.4 | |
| P-value | 0.617 | ||||
| Superficial T2 Values Reference | Baseline | 39.3 | 7.1 | 31.8 | 46.8 |
| 2 Weeks | 39.5 | 6.4 | 32.7 | 46.2 | |
| 4 Months | 39.4 | 7.3 | 31.6 | 47.1 | |
| 1 Year | 40.1 | 6.4 | 33.3 | 46.9 | |
| P-value | 0.997 | ||||
| Global T2 Values Reference | Baseline | 35.2 | 4.5 | 30.4 | 40.0 |
| 2 Weeks | 36.6 | 4.5 | 31.8 | 41.4 | |
| 4 Months | 35.6 | 4.9 | 30.5 | 40.8 | |
| 1 Year | 36.2 | 4.6 | 31.3 | 41.0 | |
| P-value | 0.958 | ||||
| Preoperative Values | Postoperative Values | |||||||
|---|---|---|---|---|---|---|---|---|
| ICRS Grade | T2 Deep | T2 sup. | T2 Global | ICRS Grade | T2 Deep | T2 sup. | T2 Global | |
| Patient 1 | 2 | 35.76 ms | 78.17 ms | 60.37 ms | 3 | 41.9 ms | 81.85 ms | 62.13 ms |
| Patient 2 | 2 | 28.12 ms | 31.13 ms | 31.77 ms | 2 | 31.42 ms | 48.71 ms | 46.33 ms |
| Patient 3 | 2 | 39.13 ms | 55.24 ms | 45.13 ms | 2 | 39.12 ms | 63.83 ms | 54.05 ms |
| Patient 4 | 2 | 40.17 ms | 45.21 ms | 43.61 ms | 3 | 40.97 ms | 56.39 ms | 46.23 ms |
| Patient 5 | 2 | 25.87 ms | 31.21 ms | 46.35 ms | 2 | 40.07 ms | 53.66 ms | 50.11 ms |
| Patient 6 | 2 | 50.06 ms | 54.33 ms | 53.75 ms | 2 | 49.95 ms | 50.97 ms | 50.57 ms |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koller, U.; Springer, B.; Rentenberger, C.; Szomolanyi, P.; Waldstein, W.; Windhager, R.; Trattnig, S.; Apprich, S. Radiofrequency Chondroplasty May Not Have a Long-Lasting Effect in the Treatment of Concomitant Grade II Patellar Cartilage Defects in Humans. J. Clin. Med. 2020, 9, 1202. https://doi.org/10.3390/jcm9041202
Koller U, Springer B, Rentenberger C, Szomolanyi P, Waldstein W, Windhager R, Trattnig S, Apprich S. Radiofrequency Chondroplasty May Not Have a Long-Lasting Effect in the Treatment of Concomitant Grade II Patellar Cartilage Defects in Humans. Journal of Clinical Medicine. 2020; 9(4):1202. https://doi.org/10.3390/jcm9041202
Chicago/Turabian StyleKoller, Ulrich, Bernhard Springer, Colleen Rentenberger, Pavol Szomolanyi, Wenzel Waldstein, Reinhard Windhager, Siegfried Trattnig, and Sebastian Apprich. 2020. "Radiofrequency Chondroplasty May Not Have a Long-Lasting Effect in the Treatment of Concomitant Grade II Patellar Cartilage Defects in Humans" Journal of Clinical Medicine 9, no. 4: 1202. https://doi.org/10.3390/jcm9041202
APA StyleKoller, U., Springer, B., Rentenberger, C., Szomolanyi, P., Waldstein, W., Windhager, R., Trattnig, S., & Apprich, S. (2020). Radiofrequency Chondroplasty May Not Have a Long-Lasting Effect in the Treatment of Concomitant Grade II Patellar Cartilage Defects in Humans. Journal of Clinical Medicine, 9(4), 1202. https://doi.org/10.3390/jcm9041202






