Polygenic Score for Body Mass Index Is Associated with Disordered Eating in a General Population Cohort
Abstract
1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Measures
2.2.1. Binary Outcomes
2.2.2. Continuous Outcomes
2.2.3. Body Mass Index.
2.3. Genotyping
2.4. PGS Calculations
2.5. Statistical Analyses
2.5.1. Sensitivity Analyses (Linkage Disequilibrium Score Regression)
2.5.2. Regression Analyses
2.5.3. Generalized Linear Mixed Models (GLMM)
2.5.4. Exploratory Causal Mediation Analysis
2.5.5. Missing Data
3. Results
3.1. Sample Description
3.2. PGS Analyses
3.2.1. BMI
3.2.2. DE Behaviors
3.2.3. DE Cognitions
3.3. Exploratory Causal Mediation Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Micali, N.; De Stavola, B.; Ploubidis, G.; Simonoff, E.; Treasure, J.; Field, A.E. Adolescent eating disorder behaviours and cognitions: Gender-specific effects of child, maternal and family risk factors. Br. J. Psychiatry 2015, 207, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Micali, N.; Horton, N.J.; Crosby, R.D.; Swanson, S.A.; Sonneville, K.R.; Solmi, F.; Calzo, J.P.; Eddy, K.T.; Field, A.E. Eating disorder behaviours amongst adolescents: Investigating classification, persistence and prospective associations with adverse outcomes using latent class models. Eur. Child Adolesc. Psychiatry 2017, 26, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.F.; Fitzsimmons-Craft, E.E.; Karam, A.M.; Jakubiak, J.; Brown, M.L.; Wilfley, D.E. Disordered Eating Attitudes and Behaviors in Youth with Overweight and Obesity: Implications for Treatment. Curr. Obes. Rep. 2018, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.A.; Crow, S.J.; Le Grange, D.; Swendsen, J.; Merikangas, K.R. Prevalence and Correlates of Eating Disorders in Adolescents. Arch. Gen. Psychiatry 2011, 68, 714–723. [Google Scholar] [CrossRef]
- Field, A.E.; Sonneville, K.R.; Micali, N.; Crosby, R.D.; Swanson, S.A.; Laird, N.M.; Treasure, J.; Solmi, F.; Horton, N.J. Prospective Association of Common Eating Disorders and Adverse Outcomes. Pediatrics 2012, 130, e289–e295. [Google Scholar] [CrossRef]
- Solmi, F.; Sonneville, K.R.; Easter, A.; Horton, N.J.; Crosby, R.D.; Treasure, J.; Rodriguez, A.; Jarvelin, M.R.; Field, A.E.; Micali, N. Prevalence of purging at age 16 and associations with negative outcomes among girls in three community-based cohorts. J. Child Psychol. Psychiatry Allied Discip. 2015, 56, 87–96. [Google Scholar] [CrossRef]
- Neumark-Sztainer, D.; Wall, M.; Guo, J.; Story, M.; Haines, J.; Eisenberg, M. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: How do dieters fare 5 years later? J. Am. Diet. Assoc. 2006, 106, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Micali, N.; Solmi, F.; Horton, N.J.; Crosby, R.D.; Eddy, K.T.; Calzo, J.P.; Sonneville, K.R.; Swanson, S.A.; Field, A.E. Adolescent Eating Disorders Predict Psychiatric, High-Risk Behaviors and Weight Outcomes in Young Adulthood. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 652–659.e1. [Google Scholar] [CrossRef]
- Crow, S.; Eisenberg, M.E.; Story, M.; Neumark-Sztainer, D. Are body dissatisfaction, eating disturbance, and body mass index predictors of suicidal behavior in adolescents? A longitudinal study. J. Consult. Clin. Psychol. 2008, 76, 887–892. [Google Scholar] [CrossRef]
- Chamay-Weber, C.; Narring, F.; Michaud, P.-A. Partial eating disorders among adolescents: A review. J. Adolesc. Heal. 2005, 37, 416–426. [Google Scholar] [CrossRef]
- Berkman, N.; Bulik, C.; Brownley, K.; Lohr, K.; Sedway, J.; Rooks, A.; Gartlehner, G. Management of Eating Disorders. Evidence Report/Technology Assessment No. 135; (Prepared by the RTI International-University of North Carolina Evidence-Based Practice Center under Contract No. 290-02-0016.) AHRQ Publication No. 06-E010; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. [Google Scholar]
- Keel, P.K.; Haedt, A. Evidence-Based Psychosocial Treatments for Eating Problems and Eating Disorders. J. Clin. Child Adolesc. Psychol. 2008, 37, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Culbert, K.M.; Racine, S.E.; Klump, K.L. Research Review: What we have learned about the causes of eating disorders—A synthesis of sociocultural, psychological, and biological research. J. Child Psychol. Psychiatry 2015, 56, 1141–1164. [Google Scholar] [CrossRef] [PubMed]
- Culbert, K.M.; Racine, S.E.; Klump, K.L. The influence of gender and puberty on the heritability of disordered eating symptoms. In Behavioral neurobiology of eating disorders; Springer: Berlin/Heidelberg, Germany, 2010; pp. 177–185. [Google Scholar]
- Martin, A.R.; Daly, M.J.; Robinson, E.B.; Hyman, S.E.; Neale, B.M. Predicting Polygenic Risk of Psychiatric Disorders. Biol. Psychiatry 2019, 86, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Shin, T.; Mak, H.; Reilly, P.F.O. A guide to performing Polygenic Risk Score analyses. bioRxiv 2018, 5, 11–13. [Google Scholar]
- Tanofsky-Kraff, M.; Yanovski, S.Z.; Wilfley, D.E.; Marmarosh, C.; Morgan, C.M.; Yanovski, J.A. Eating-Disordered Behaviors, Body Fat, and Psychopathology in Overweight and Normal-Weight Children. J. Consult. Clin. Psychol. 2004, 72, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Torstveit, M.K.; Aagedal-Mortensen, K.; Stea, T.H. More than half of high school students report disordered eating: A cross sectional study among Norwegian boys and girls. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef]
- Flament, M.F.; Henderson, K.; Buchholz, A.; Obeid, N.; Nguyen, H.N.T.; Birmingham, M.; Goldfield, G. Weight Status and DSM-5 Diagnoses of Eating Disorders in Adolescents from the Community. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 403–411.e2. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Gottfredson, N.C.; Zerwas, S.C.; Bulik, C.M.; Micali, N. Developmental Premorbid Body Mass Index Trajectories of Adolescents with Eating Disorders in a Longitudinal Population Cohort. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 191–199. [Google Scholar] [CrossRef]
- Herle, M.; De Stavola, B.; Hübel, C.; Abdulkadir, M.; Ferreira, D.S.; Loos, R.J.F.; Bryant-Waugh, R.; Bulik, C.M.; Micali, N. A longitudinal study of eating behaviours in childhood and later eating disorder behaviours and diagnoses. Br. J. Psychiatry 2019, 1–7. [Google Scholar] [CrossRef]
- Föcker, M.; Bühren, K.; Timmesfeld, N.; Dempfle, A.; Knoll, S.; Schwarte, R.; Egberts, K.M.; Pfeiffer, E.; Fleischhaker, C.; Wewetzer, C.; et al. The relationship between premorbid body weight and weight at referral, at discharge and at 1-year follow-up in anorexia nervosa. Eur. Child Adolesc. Psychiatry 2015, 24, 537–544. [Google Scholar] [CrossRef]
- Albuquerque, D.; Stice, E.; Rodríguez, R.; Licíno, L.-R.; Manco, L.; Nóbrega, C. Current review of genetics of human obesity: From molecular mechanisms to an evolutionary perspective. Mol. Genet. Genomics 2015, 290, 1191–1221. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Chaffin, M.; Wade, K.H.; Zahid, S.; Brancale, J.; Xia, R.; Distefano, M.; Senol-Cosar, O.; Haas, M.E.; Bick, A.; et al. Polygenic Prediction of Weight and Obesity Trajectories from Birth to Adulthood. Cell 2019, 177, 587–596.e9. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.; Yilmaz, Z.; Gaspar, H.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Thornton, L.; Hinney, A.; et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef]
- Nagata, J.M.; Braudt, D.B.; Domingue, B.W.; Bibbins-Domingo, K.; Garber, A.K.; Griffiths, S.; Murray, S.B. Genetic risk, body mass index, and weight control behaviors: Unlocking the triad. Int. J. Eat. Disord. 2019, 52, 825–833. [Google Scholar] [CrossRef]
- Snoek, H.M.; Engels, R.C.M.E.; van Strien, T.; Otten, R. Emotional, external and restrained eating behaviour and BMI trajectories in adolescence. Appetite 2013, 67, 81–87. [Google Scholar] [CrossRef]
- Juarascio, A.S.; Forman, E.M.; Timko, C.A.; Herbert, J.D.; Butryn, M.; Lowe, M. Implicit internalization of the thin ideal as a predictor of increases in weight, body dissatisfaction, and disordered eating. Eat. Behav. 2011, 12, 207–213. [Google Scholar] [CrossRef]
- Fraser, A.; Macdonald-wallis, C.; Tilling, K.; Boyd, A.; Golding, J.; Davey smith, G.; Henderson, J.; Macleod, J.; Molloy, L.; Ness, A.; et al. Cohort profile: The avon longitudinal study of parents and children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013, 42, 97–110. [Google Scholar] [CrossRef]
- Golding; Pembrey; Jones; The Alspac Study Team ALSPAC-The Avon Longitudinal Study of Parents and Children. Paediatr. Perinat. Epidemiol. 2001, 15, 74–87. [CrossRef]
- Golding, J.; Team, S. The Avon Longitudinal Study of Parents and Children (ALSPAC) – Study design and collaborative opportunities. Eur. J. Endocrinol. 2004, 151 (Suppl. 3), U119–U123. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Golding, J.; Macleod, J.; Lawlor, D.A.; Fraser, A.; Henderson, J.; Molloy, L.; Ness, A.; Ring, S.; Smith, G.D. Cohort profile: The ’Children of the 90s’-The index offspring of the avon longitudinal study of parents and children. Int. J. Epidemiol. 2013, 42, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Kann, L.; Warren, C.W.; Harris, W.A.; Collins, J.L.; Williams, B.I.; Ross, J.G.; Kolbe, L.J. Youth Risk Behavior Surveillance-United States, 1995. J. Sch. Health 1996, 66, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Stice, E. Modeling of eating pathology and social reinforcement of the thin-ideal predict onset of bulimic symptoms. Behav. Res. Ther. 1998, 36, 931–944. [Google Scholar] [CrossRef]
- Calzo, J.P.; Austin, S.B.; Micali, N. Sexual orientation disparities in eating disorder symptoms among adolescent boys and girls in the UK. Eur. Child Adolesc. Psychiatry 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Stice, E. A prospective test of the dual-pathway model of bulimic pathology: Mediating effects of dieting and negative affect. J. Abnorm. Psychol. 2001, 110, 124–135. [Google Scholar] [CrossRef]
- van Strien, T.; Frijters, J.E.R.; Bergers, G.P.A.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Schaumberg, K.; Zerwas, S.; Goodman, E.; Yilmaz, Z.; Bulik, C.M.; Micali, N. Anxiety disorder symptoms at age 10 predict eating disorder symptoms and diagnoses in adolescence. J. Child Psychol. Psychiatry Allied Discip. 2018. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.; Flegal, K.; Dietz, W. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef]
- Yengo, L.; Sidorenko, J.; Kemper, K.E.; Zheng, Z.; Wood, A.R.; Weedon, M.N.; Frayling, T.M.; Hirschhorn, J.; Yang, J.; Visscher, P.M. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 2018, 27, 3641–3649. [Google Scholar] [CrossRef]
- Euesden, J.; Lewis, C.M.; O’Reilly, P.F. PRSice: Polygenic Risk Score software. Bioinformatics 2015, 31, 1466–1468. [Google Scholar] [CrossRef]
- Choi, S.W.; O’Reilly, P.F. PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience 2019, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Bulik-Sullivan, B.; Loh, P.R.; Finucane, H.K.; Ripke, S.; Yang, J.; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M.; Corvin, A.; et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Goddard, M.E.; Wray, N.R.; Visscher, P.M. A better coefficient of determination for genetic profile analysis. Genet. Epidemiol. 2012, 36, 214–224. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- VanderWeele, T. Explanation in Causal Inference: Methods for Mediation and Interaction; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- White, I.R.; Carlin, J.B. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat. Med. 2010, 29, 2920–2931. [Google Scholar] [CrossRef]
- Micali, N.; Field, A.E.; Treasure, J.L.; Evans, D.M. Are obesity risk genes associated with binge eating in adolescence? Obesity 2015, 23, 1729–1736. [Google Scholar] [CrossRef]
- Reed, Z.E.; Micali, N.; Bulik, C.M.; Davey Smith, G.; Wade, K.H. Assessing the causal role of adiposity on disordered eating in childhood, adolescence, and adulthood: A Mendelian randomization analysis. Am. J. Clin. Nutr. 2017, ajcn154104. [Google Scholar] [CrossRef]
- Bucchianeri, M.M.; Arikian, A.J.; Hannan, P.J.; Eisenberg, M.E.; Neumark-Sztainer, D. Body dissatisfaction from adolescence to young adulthood: Findings from a 10-year longitudinal study. Body Image 2013, 10, 1–7. [Google Scholar] [CrossRef]
- Keel, P.K.; Baxter, M.G.; Heatherton, T.F.; Joiner, T.E. A 20-year longitudinal study of body weight, dieting, and eating disorder symptoms. J. Abnorm. Psychol. 2007, 116, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Gau, J.M.; Rohde, P.; Shaw, H. Risk factors that predict future onset of each DSM–5 eating disorder: Predictive specificity in high-risk adolescent females. J. Abnorm. Psychol. 2017, 126, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Bentham, J.; Di Cesare, M.; Bilano, V.; Bixby, H.; Zhou, B.; Stevens, G.A.; Riley, L.M.; Taddei, C.; Hajifathalian, K.; Lu, Y.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar]
- Hinney, A.; Kesselmeier, M.; Jall, S.; Volckmar, A.-L.; Föcker, M.; Antel, J.; Heid, I.M.; Winkler, T.W.; Grant, S.F.A.; Guo, Y.; et al. Evidence for three genetic loci involved in both anorexia nervosa risk and variation of body mass index. Mol. Psychiatry 2017, 22, 192–201. [Google Scholar] [CrossRef]
- Field, A.E.; Javaras, K.M.; Aneja, P.; Kitos, N.; Camargo, C.A.; Taylor, C.B.; Laird, N.M. Family, Peer, and Media Predictors of Becoming Eating Disordered. Arch. Pediatr. Adolesc. Med. 2008, 162, 574. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Hellwege, J.N.; Keaton, J.M.; Giri, A.; Gao, X.; Velez Edwards, D.R.; Edwards, T.L. Population Stratification in Genetic Association Studies. Curr. Protoc. Hum. Genet. 2017, 95, 1.22.1–1.22.23. [Google Scholar] [CrossRef]
- Taylor, A.E.; Jones, H.J.; Sallis, H.; Euesden, J.; Stergiakouli, E.; Davies, N.M.; Zammit, S.; Lawlor, D.A.; Munafò, M.R.; Smith, G.D.; et al. Exploring the association of genetic factors with participation in the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2018, 47, 1207–1216. [Google Scholar] [CrossRef]
- Lambert, S.A.; Abraham, G.; Inouye, M. Towards clinical utility of polygenic risk scores. Hum. Mol. Genet. 2019, 00, 1–10. [Google Scholar] [CrossRef]
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| Binary Outcomes | Age Outcome Measured (Years) | Total N | N (%) | % Female | N | % Female |
| Fasting b | 14 | 4584 | 300 (3.4%) | 83.7% | 4284 | 53.3% |
| Fasting b | 16 | 3844 | 516 (5.9%) | 90.7% | 3328 | 53.8% |
| Fasting b | 18 | 2586 | 143 (1.6%) | 93% | 2443 | 62.1% |
| Binge eating b | 14 | 4144 | 257 (2.9%) | 71.6% | 3887 | 53.4% |
| Binge eating b | 16 | 3336 | 434 (5.0%) | 83.2% | 2902 | 55.3% |
| Binge eating b | 18 | 1910 | 365 (4.2%) | 85.2% | 1545 | 62.2% |
| Purging b | 16 | 3871 | 237 (2.7%) | 92.4% | 3634 | 56.7% |
| Purging b | 18 | 2582 | 166 (1.9%) | 90.9% | 2416 | 62.0% |
| Continuous Outcomes | Age Outcome Measured (Years) | Total N | Mean | SD | Observed Range (min) | Observed Range (max) |
| Thin ideal internalization c | 14 | 4496 | 15.33 | 2.69 | 5 | 25 |
| Body dissatisfaction d | 14 | 4625 | 21.85 | 7.75 | 9 | 46.3 |
| Restrained eating e | 14 | 4530 | 0.68 | 1.13 | 0 | 5 |
| Emotional eating e | 14 | 4345 | 5.22 | 5.53 | 0 | 28 |
| External eating e | 14 | 3995 | 8.45 | 3.34 | 0 | 21 |
| Age- and sex-adjusted body mass index (zBMI) f | 11 | 4037 | 0.59 | 1.13 | −3.22 | 3.78 |
| Total sample size g | 8654 | |||||
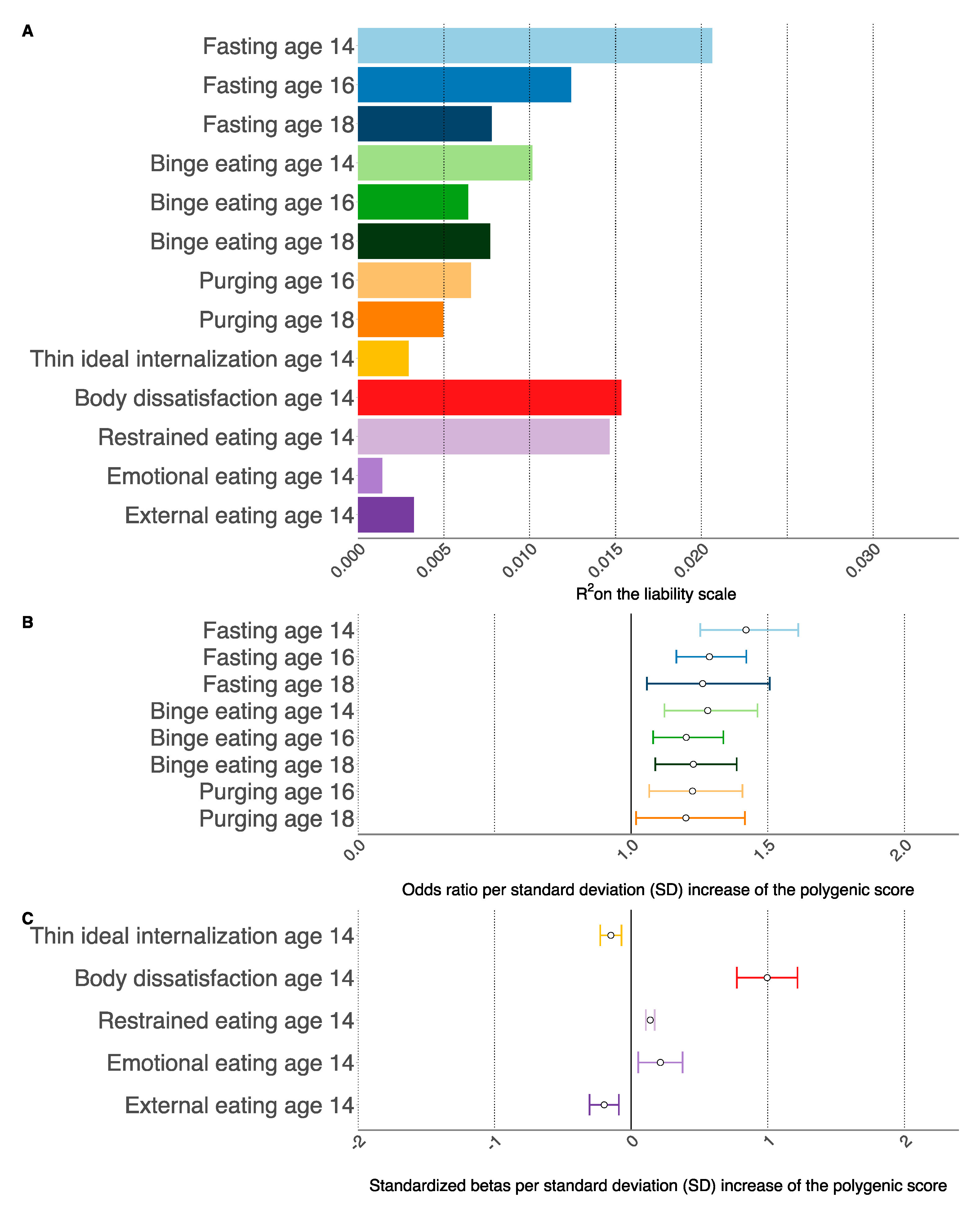
| Binary Outcomes | Age Outcome Measured (Years) | Threshold a | N SNPs | R2 | OR (95% CI) b | Q c |
| Fasting | 14 | 0.085 | 33,379 | 0.021 | 1.42 (1.25, 1.61) | <0.001 |
| Fasting | 16 | 0.17 | 43,956 | 0.012 | 1.29 (1.17, 1.42) | <0.001 |
| Fasting | 18 | 0.1 | 36,088 | 0.008 | 1.26 (1.06, 1.51) | 0.045 |
| Binge eating | 14 | 0.014 | 17,929 | 0.010 | 1.28 (1.12, 1.46) | 0.003 |
| Binge eating | 16 | 0.0016 | 9523 | 0.006 | 1.20 (1.08, 1.34) | 0.006 |
| Binge eating | 18 | 0.0047 | 12,708 | 0.008 | 1.23 (1.09, 1.39) | 0.008 |
| Purging | 16 | 0.00025 | 6115 | 0.007 | 1.22 (1.07, 1.41) | 0.02 |
| Purging | 18 | 0.033 | 23,731 | 0.005 | 1.20 (1.02, 1.42) | 0.10 |
| Continuous Outcomes | Age Outcome Measured (Years) | Threshold a | N SNPs | R2 | β (95% CI) d | Q c |
| Thin ideal internalization | 14 | 0.5 | 66,077 | 0.003 | −0.15 (−0.23, −0.07) | 0.003 |
| Body dissatisfaction | 14 | 0.0013 | 9075 | 0.015 | 0.99 (0.77, 1.22) | <0.001 |
| Restrained eating | 14 | 0.1 | 36,088 | 0.015 | 0.14 (0.10, 0.17) | <0.001 |
| Emotional eating | 14 | 0.0091 | 15,467 | 0.001 | 0.21 (0.052, 0.38) | 0.046 |
| External eating | 14 | 0.0026 | 10,780 | 0.003 | −0.19 (−0.30, −0.09) | 0.003 |
| Phenotype | Age b | Total Effect Estimate βtotal effect (95% CI) | Total Effect p-Value | Average Direct Effect βADE (95% CI) | Average Direct Effect p-Value | Average Causal Mediation Effect βACME (95% CI) | Average Causal Mediation Effect p-Value |
|---|---|---|---|---|---|---|---|
| Fasting | 14 | 0.022 (0.01, 0.032) | <0.0001 | 0.011 (0.001, 0.022) | 0.038 | 0.011 (0.007, 0.014) | <0.0001 |
| Fasting | 16 | 0.035 (0.019, 0.051) | <0.0001 | 0.021 (0.005, 0.037) | 0.004 | 0.014 (0.008, 0.019) | <0.0001 |
| Fasting | 18 | 0.014 (0.002, 0.028) | 0.02 | 0.009 (−0.002, 0.024) | 0.146 | 0.005 (0.001, 0.01) | 0.03 |
| Binge eating | 14 | 0.01 (−0.001, 0.02) | 0.07 | 0.004 (−0.007, 0.014) | 0.464 | 0.006 (0.002, 0.01) | <0.0001 |
| Binge eating | 16 | 0.015 (0.001, 0.031) | 0.04 | −0.001 (−0.015, 0.014) | 0.95 | 0.016 (0.01, 0.022) | <0.0001 |
| Binge eating | 18 | 0.028 (0.006, 0.053) | 0.01 | 0.01 (−0.013, 0.034) | 0.348 | 0.017 (0.009, 0.026) | <0.0001 |
| Purging | 16 | 0.011 (0.002, 0.022) | 0.02 | 0.007 (−0.003, 0.018) | 0.168 | 0.004 (0.001, 0.008) | 0.006 |
| Purging | 18 | 0.014 (0.001, 0.03) | 0.04 | 0.004 (−0.008, 0.019) | 0.494 | 0.009 (0.005, 0.014) | <0.0001 |
| Thin ideal internalization | 14 | −0.133 (−0.245, −0.017) | 0.03 | −0.118 (−0.228, −0.001) | 0.042 | −0.015 (−0.055, 0.027) | 0.47 |
| Body dissatisfaction | 14 | 1.097 (0.819, 1.378) | <0.0001 | 0.171 (−0.097, 0.439) | 0.218 | 0.926 (0.798, 1.061) | <0.0001 |
| Restrained eating | 14 | 0.161 (0.819, 0.208) | <0.0001 | 0.03 (−0.014, 0.072) | 0.162 | 0.13 (0.113, 0.149) | <0.0001 |
| Emotional eating | 14 | 0.129 (−0.071, 0.356) | 0.26 | 0.03 (−0.181, 0.269) | 0.804 | 0.098 (0.023, 0.176) | 0.01 |
| External eating | 14 | −0.195 (−0.335, −0.053) | 0.006 | −0.087 (−0.228, 0.06) | 0.212 | −0.108 (−0.162, −0.057) | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulkadir, M.; Herle, M.; De Stavola, B.L.; Hübel, C.; Santos Ferreira, D.L.; Loos, R.J.F.; Bryant-Waugh, R.; Bulik, C.M.; Micali, N. Polygenic Score for Body Mass Index Is Associated with Disordered Eating in a General Population Cohort. J. Clin. Med. 2020, 9, 1187. https://doi.org/10.3390/jcm9041187
Abdulkadir M, Herle M, De Stavola BL, Hübel C, Santos Ferreira DL, Loos RJF, Bryant-Waugh R, Bulik CM, Micali N. Polygenic Score for Body Mass Index Is Associated with Disordered Eating in a General Population Cohort. Journal of Clinical Medicine. 2020; 9(4):1187. https://doi.org/10.3390/jcm9041187
Chicago/Turabian StyleAbdulkadir, Mohamed, Moritz Herle, Bianca L. De Stavola, Christopher Hübel, Diana L. Santos Ferreira, Ruth J. F. Loos, Rachel Bryant-Waugh, Cynthia M. Bulik, and Nadia Micali. 2020. "Polygenic Score for Body Mass Index Is Associated with Disordered Eating in a General Population Cohort" Journal of Clinical Medicine 9, no. 4: 1187. https://doi.org/10.3390/jcm9041187
APA StyleAbdulkadir, M., Herle, M., De Stavola, B. L., Hübel, C., Santos Ferreira, D. L., Loos, R. J. F., Bryant-Waugh, R., Bulik, C. M., & Micali, N. (2020). Polygenic Score for Body Mass Index Is Associated with Disordered Eating in a General Population Cohort. Journal of Clinical Medicine, 9(4), 1187. https://doi.org/10.3390/jcm9041187





