Serum Omentin Levels in Patients with Prostate Cancer and Associations with Sex Steroids and Metabolic Syndrome
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2012, 63, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Toren, P.; Venkateswaran, V. Periprostatic adipose tissue and prostate cancer progression: New insights into the tumor microenvironment. Clin. Genitourin. Cancer 2014, 12, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Senga, S.; Kobayashi, N.; Kawaguchi, K.; Ando, A.; Fujii, H. Fatty acid-binding protein 5 (FABP5) promotes lipolysis of lipid droplets, de novo fatty acid (FA) synthesis and activation of nuclear factor-kappaB (NF-kB) signaling in cancer cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1057–1067. [Google Scholar] [CrossRef]
- Alshaker, H.; Sacco, K.; Alfraidi, A.; Muhammad, A.; Winkler, M.; Pchejetski, D. Leptin signaling, obesity and prostate cancer: Molecular and clinical perspective on the old dilemma. Oncotarget 2015, 6, 35556–35563. [Google Scholar] [CrossRef]
- Szyszka, M.; Tyczewska, M.; Milecka, P.; Jopek, K.; Celichowski, P.; Malendowicz, L.K.; Rucinski, M. Effects of leptin on leptin receptor isoform expression and proliferative activity in human normal prostate and prostate cancer cell lines. Oncol. Rep. 2018, 39, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Monks, M.; Irakleidis, F.; Tan, P.H. Complex interaction of adiponectin-mediated pathways on cancer treatment: A novel therapeutic target. J. Cancer Metastasis Treat. 2019, 5, 24. [Google Scholar] [CrossRef]
- Alves-Pereira, J.L.; Colli, S.; Marques, D.S.; Sampaio, F.J.; Ramos, C.F. Molecular and morphometric analysis of the rat ventral prostate injected with leptin. Regul. Pept. 2012, 176, 6–12. [Google Scholar] [CrossRef]
- Sieminska, L.; Borowski, A.; Marek, B.; Nowak, M.; Kajdaniuk, D.; Warakomski, J.; Kos-Kudła, B. Serum concentrations of adipokines in men with prostate cancer and benign prostate hyperplasia. Endokrynol. Pol. 2018, 69, 120–127. [Google Scholar] [CrossRef]
- Szydło, B.; Kiczmer, P.; Świętochowska, E.; Ostrowska, Z. Role of omentin and chemerin in metabolic syndrome and tumor diseases. Postepy Hig. Med. Dosw. 2016, 70, 844–849. [Google Scholar] [CrossRef]
- Zheng, L.; Weng, M.; Qi, M.; Qi, T.; Tong, L.; Hou, X.; Tong, Q. Aberrant expression of intelectin-1 in gastric cancer: Its relationship with clinicopathological features and prognosis. J. Cancer Res. Clin. Oncol. 2012, 138, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Deng, L.; Wang, Y.; Li, Y.; Chen, M. The association between Chinese patients’ elevated omentin-1 levels, their clinicopathological features, and the risk of colorectal cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 2264–2274. [Google Scholar] [PubMed]
- Arjmand, M.H.; Moradi, A.; Akbari, A.; Mehrad-Majd, H. Clinical significance of circulating omentin levels in various malignant tumors: Evidence from a systematic review and meta-analysis. Cytokine 2020, 125, 154869. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, W.; Wang, W.; Zhou, D. Altered circulating levels of adipokine omentin-1 in patients with prostate cancer. Onco Targets Ther. 2019, 12, 3313–3319. [Google Scholar] [CrossRef]
- Uyeturk, U.; Sarici, H.; Kin Tekce, B.; Eroglu, M.; Kemahli, E.; Uyeturk, U.; Gucuk, A. Serum omentin level in patients with prostate cancer. Med. Oncol. 2014, 31, 923. [Google Scholar] [CrossRef]
- Fryczkowski, M.; Bułdak, R.J.; Hejmo, T.; Kukla, M.; Żwirska-Korczala, K. Circulating levels of omentin, leptin, VEGF, and HGF and their clinical relevance with PSA marker in prostate cancer. Dis. Markers 2018, 2018, 3852401. [Google Scholar] [CrossRef]
- Heikkila, R.; Aho, K.; Heliovaara, M.; Hakama, M.; Marniemi, J.; Reunanen, A.; Knekt, P. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: A longitudinal study. Cancer 1999, 86, 312–315. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Grossman, A.B. Management of Hypopituitarism. J. Clin. Med. 2019, 8, 2153. [Google Scholar] [CrossRef]
- Tu, H.; Gu, J.; Meng, Q.H.; Kim, J.; Strom, S.; Davis, J.W.; He, Y.; Wagar, E.A.; Thompson, T.C.; Logothetis, C.J.; et al. Low serum testosterone is associated with tumor aggressiveness and poor prognosis in prostate cancer. Oncol. Lett. 2017, 13, 1949–1957. [Google Scholar] [CrossRef]
- Xue, B.; Wu, S.; Sharkey, C.; Tabatabaei, S.; Wu, C.L.; Tao, Z.; Cheng, Z.; Strand, D.; Olumi, A.F.; Wang, Z. Obesity-associated inflammation induces androgenic to estrogenic switch in the prostate gland. Prostate Cancer Prostatic Dis. 2020. [Google Scholar] [CrossRef]
- Washimi, K.; Yokose, T.; Yamashita, M.; Kageyama, T.; Suzuki, K.; Yoshihara, M. Specific expression of human intelectin-1 in malignant pleural mesothelioma and gastrointestinal goblet cells. PLoS ONE 2012, 7, e39889. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, S.; Afsar, C.U.; Karabulut, M.; Alis, H.; Bozkurt, M.A.; Aydogan, F.; Serilmez, M.; Tas, F. Clinical significance of serum omentin-1 levels in patients with pancreatic adenocarcinoma. BBA Clin. 2016, 6, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhou, L.M. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur. J. Pharmacol. 2013, 698, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Mogal, A.P.; van der Meer, R.; Crooke, P.S.; Abdulkadir, S.A. Haploinsufficient prostate tumor suppression by Nkx3.1. A role for chromatina accessibility in dosage-sensitive gene regulation. J. Biol. Chem. 2007, 282, 25790–25800. [Google Scholar] [CrossRef]
- Ji, H.; Wan, L.; Zhang, Q.; Chen, M.; Zhao, X. The effect of omentin-1 on the proliferation and apoptosis of colon cancer stem cells and the potential mechanism. J. BU ON 2019, 24, 91–98. [Google Scholar]
- Yan, C.; Xiaotong, Z.; Mingwei, C. A study on relationship of adipokine omentin-1 involved in colorectal cancer. Acta Univ. Med. Anhui 2015, 1, 75–78. [Google Scholar]
- Zhang, Y.; Zhao, X.; Chen, M. Autocrine action of adipokine omentin-1 in the SW480 colon cancer cell line. Oncol. Lett. 2020, 19, 892–898. [Google Scholar] [CrossRef]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pray, J.; Wu, H.B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Wu, S.S.; Liang, Q.H.; Liu, Y.; Cui, R.R.; Yuan, L.Q.; Liao, E.Y. Omentin-1 stimulates human osteoblast proliferation through PI3K/Akt signal pathway. Int. J. Endocrinol. 2013, 2013, 368970. [Google Scholar] [CrossRef]
- Crumbaker, M.; Khoja, L.; Joshua, A.M. AR Signaling and the PI3K pathway in prostate cancer. Cancers 2017, 9, 34. [Google Scholar] [CrossRef]
- Yin, L.; Huang, D.; Liu, X.; Wang, Y.; Liu, J.; Liu, F.; Yu, B. Omentin-1 effects on mesenchymal stem cells: Proliferation, apoptosis, and angiogenesis in vitro. Stem Cell Res. Ther. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, K.; Zhang, Q.; Yu, Y.; Meng, Q.; Mo, N.; Zhou, Y.; Yi, X.; Ma, C.; Lei, A.; et al. The role of mesenchymal stem cells in promoting the transformation of androgen-dependent human prostate cancer cells into androgen-independent manner. Sci. Rep. 2016, 6, 16993. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Lewandowski, K.C.; O’Hare, P.; Lehnert, H.; Randeva, H.S. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: Ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes 2008, 57, 801. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, N.H.; Sadik, N.A.; Ahmed, N.R.; Fayez, S.E.; Mohammed, N.A.E. Serum omentin-1 levels in type 2 diabetic obese women in relation to glycemic control, insulin resistance and metabolic parameters. J. Clin. Transl. Endocrinol. 2018, 13, 14–19. [Google Scholar] [CrossRef]
- Yan, P.; Liu, D.; Long, M.; Ren, Y.; Pang, J.; Li, R. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2011, 119, 257–263. [Google Scholar] [CrossRef]
- Luque-Ramirez, M.; Martinez-Garcia, M.A.; Montes-Nieto, R.; Fernandez-Duran, E.; Insenser, M.; Alpanes, M.; Escobar-Morreale, H.F. Sexual dimorphism in adipose tissue function as evidenced by circulating adipokine concentrations in the fasting state and after an oral glucose challenge. Hum. Reprod. 2013, 28, 1908–1918. [Google Scholar] [CrossRef]
- Babaei, P.; Damirchi, A.; Pourrahim Ghouroughchi, A. The effect of estrogen on visceral fat, serum omentin-1 and insulin resistance in ovariectomized rats. J. Ardabil Univ. Med. Sci. 2016, 16, 189–199. [Google Scholar]
- Selva, D.M.; Hogeveen, K.N.; Innis, S.M.; Hammond, G.L. Monosaccharid-induced lipogenesis regulates the human hepatic sex hormone-binding globulin gene. J. Clin. Invest. 2007, 117, 3979–3987. [Google Scholar] [CrossRef]
- Miura, A.; Yamagata, K.; Kakei, M.; Hatakeyama, H.; Takahashi, N.; Fukui, K.; Nammo, T.; Yoneda, K.; Inoue, Y.; Sladek, F.M.; et al. Hepatocyte nuclear factor-4α is essential for glucose-stimulated insulin secretion by pancreatic β-cells. J. Biol. Chem. 2006, 281, 5246–5257. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wu, D.; Yu, S.; Wang, Y.; Leung Vhan, F. Nuclear receptor HNF4α performs a tumor suppressor function in prostate cancer via its induction of p21-driven cellular senescence. Oncogene 2020, 39, 1572–1589. [Google Scholar] [CrossRef] [PubMed]
- Selva, D.M.; Hammond, G.L. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4alpha. J. Mol. Endocrinol. 2009, 43, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, X.; Xiao, Y.; Mei, H.; Pu, J.; Xiang, X.; Jiao, W.; Song, H.; Qu, H.; Huang, K.; et al. Intelectin 1 suppresses tumor progression and is associated with improved survival in gastric cancer. Oncotarget 2015, 6, 16168–16182. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, A.; Krzyżanowska-Świniarska, B.; Miazgowski, T.; Jędrzejuk, D.; Arkowska, A.; Mieszczanowicz, U.; Bar-Andziak, E. The reference values of sex hormones and SHBG serum levels in subjects over 65 years old—The PolSenior Study. Endokrynol. Pol. 2013, 64, 82–93. [Google Scholar]
- Gyawali, P.; Martin, S.; Heilbronn, L.K.; Vincent, A.D.; Jenkins, A.J.; Januszewski, A.S.; Adams, R.J.T.; O’Loughlin, P.D.; Wittert, G.A. Higher serum sex hormone-binding globulin levels are associated with incident cardiovascular disease in men. J. Clin. Endocrinol. Metab. 2019, 104, 6301–6305. [Google Scholar] [CrossRef]
- Nishimura, M.; Morioka, T.; Hayashi, M.; Kakutani, Y.; Yamazaki, Y.; Kurajoh, M.; Mori, K.; Fukumoto, S.; Shioi, A.; Shoji, T.; et al. Plasma omentin levels are inversely associated with atherosclerosis in type 2 diabetes patients with increased plasma adiponectin levels: A cross-sectional study. Cardiovasc. Diabetol. 2019, 18, 167. [Google Scholar] [CrossRef]
- Wu, A.; Shi, Z.; Martin, S.; Vincent, A.; Heilbronn, L.; Wittert, G. Age-related changes in estradiol and longitudinal associations with fat mass in men. PLoS ONE 2018, 13, e0201912. [Google Scholar] [CrossRef]
- Onat, A.; Ademoglu, E.; Karadeniz, Y.; Can, G.; Uzun, A.O.; Simsek, B.; Kaya, A. Population-based serum omentin-1 levels: Paradoxical association with cardiometabolic disorders primarily in men. Biomark. Med. 2018, 12, 141–149. [Google Scholar] [CrossRef]
- Niersmann, C.; Carstensen-Kirberg, M.; Maalmi, H.; Holleczek, B.; Roden, M.; Brenner, H.; Herder, C.; Schottker, B. Higher circulating omentin is associated with increased risk of primary cardiovascular events in individuals with diabetes. Diabetologia 2020, 63, 410–418. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Yonal, O.; Kurt, R.; Alahdab, Y.O.; Eren, F.; Ozdogan, O.; Celikel, C.A.; Imeryuz, N.; Kalayci, C.; Avsar, E. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand. J. Gastroenterol. 2011, 46, 91–97. [Google Scholar] [CrossRef]
- Jarecki, P.; Herman, W.A.; Pawliczak, E.; Lacka, K. Can low SHBG serum concentration be a good early marker of male hypogonadism in metabolic syndrome? Diabetes Metab. Syndr. Obes. 2019, 12, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Strange, R.C.; Fryer, A.A.; Saad, F.; Hackett, G.I. The association of sex hormone-binding globulin with mortality is mediated by age and testosterone in men with type 2 diabetes. Andrology 2018, 6, 846–853. [Google Scholar] [CrossRef]
- Grosman, H.; Fabre, B.; Lopez, M.; Scorticati, C.; Lopez Sliva, M.; Mesch, V.; Mazza, O.; Berg, G. Complex relationship between sex hormones, insulin resistance and leptin in men with and without prostatic disease. Aging Male 2016, 19, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.M.; Lee, M.T.; Lam, H.M.; Leung, Y.K. Estrogens and prostate cancer: Etiology, mediators, prevention, and management. Endocrinol. Metab. Clin. N. Am. 2011, 40, 591–614. [Google Scholar] [CrossRef]
- Dobbs, R.W.; Malhotra, N.R.; Greenwald, D.T.; Wang, A.Y.; Prins, G.S.; Abern, M.R. Estrogens and prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 185–194. [Google Scholar] [CrossRef]
| PC (Group I), n = 72 Mean ± SD (Median) | BPH (Group II), n = 65 Mean ± SD (Median) | p | |
|---|---|---|---|
| Age | 67.08 ± 8.36 (67.0) | 61.43 ± 10.17 (60.0) | <0.001 |
| BMI (kg/m2) | 27.90 ± 3.29 (28.0) | 27.23 ± 4.00 (26.57) | Ns |
| Waist circumference (cm) | 102.38 ± 9.93 (102.0) | 101.72 ± 9.33 (101.0) | Ns |
| Fasting glucose (mg/dL) | 104.76 ± 28.20 (103.0) | 108.33 ± 33.57 (98.0) | Ns |
| CHOL (mg/dL) | 185.11 ± 45.99 (182.5) | 187.41 ± 45.41 (187.0) | Ns |
| HDL-C (mg/dL) | 47.26 ± 14.03 (45.0) | 50.75 ± 22.42 (48.0) | Ns |
| TG (mg/dL) | 124.42 ± 49.74 (113.0) | 138.15 ± 92.03 (110.0) | Ns |
| HOMA-I | 3.88 ± 4.27 (3.22) | 5.28 ± 8.20 (2.87) | Ns |
| Omentin (ng/mL) | 594.29 ± 266.85 (565.3) | 379.92 ± 168.05 (360.9) | <0.001 |
| Leptin (ng/mL) | 10.04 ± 8.18 (8.25 | 9.02 ± 7.23 (7.87) | Ns |
| Testosterone (ng/mL) | 3.64 ± 2.79 (3.36) | 3.49 ± 1.37 (3.43) | Ns |
| Estradiol (pg/mL) | 24.85 ± 20.79 (21.1) | 19.27 ± 9.13 (18.4) | <0.01 |
| SHBG (nmol/L) | 30.18 ± 13.09 (27.8) | 26.27 ± 15.14 (24.6) | Ns |
| Testosterone/SHBG ratio | 0.20 ± 0.59 (0.12) | 0.17 ± 0.10 (0.15) | Ns |
| Estradiol/SHBG ratio | 1.37 ± 2.45 (0.72) | 1.06 ± 0.84 (0.88) | Ns |
| Estradiol/Testosterone ratio | 15.63 ± 27.96 (7.87) | 7.36 ± 8.16 (5.56) | <0.01 |
| PSA (ng/mL) | 33.85 ± 91.09 (7.8) | 3.80 ± 5.43 (1.51) | <0.001 |
| PC (Group I), n = 72 | BPH (Group II), n = 65 | Differences between Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Group I MS, n = 47 Mean ± SD (Median) | Group I non-MS, n = 25 Mean ± SD (Median) | Group II MS, n = 30 Mean ± SD (Median) | Group II non-MS, n = 35 Mean ± SD (Median) | Group I MS vs. Group I non-MS (p) | Group II MS vs. Group II non-MS (p) | Group I MS vs. Group II MS (p) | Group I non-MS vs. Group II non-MS (p) | |
| Age | 66.89 ± 8.12 (68.0) | 67.44 ±8.97 (66.0) | 63.90 ± 10.184(62.0) | 59.31 ± 9.82 (57.0) | NS | NS | NS | <0.01 |
| BMI (kg/m2) | 29.11 ± 2.83 (28.40) | 25.61 ± 2.84 (25.84) | 28.79 ± 4.15 (27.93) | 25.95 ± 3.43 (25.66) | <0.001 | <0.01 | NS | NS |
| Waist circumference (cm) | 106.29 ± 8.07 (105.0) | 95.04 ± 8.96 (94.0) | 106.56 ± 7.47 (106.5) | 97.57 ± 8.83 (98.0) | <0.001 | <0.001 | NS | NS |
| Fasting glucose (mg/dL) | 110.65 ± 31.98 (106) | 93.68 ± 13.99 (96.0) | 126.53 ± 41.60 (113.5) | 92.74 ± 10.15 (91.0) | <0.001 | <0.001 | NS | NS |
| CHOL (mg/dL) | 187.40 ± 45.85 (181.0) | 180.80 ± 46.89 (186.0) | 195.06 ± 49.64 (194.5) | 180.85 ± 41.03 (184.0) | NS | NS | NS | NS |
| HDL-C (mg/dL) | 46.40 ± 12.65 (44.0) | 48.88 ± 16.46 (50.0) | 43.16 ± 10.21 (45.5) | 56.43 ± 28.16 (49.0) | NS | <0.05 | NS | NS |
| TG (mg/dL) | 139.59 ± 53.57 (123.0) | 95.88 ± 22.76 (92.0) | 157.06 ± 104.8 (122.0) | 121.94 ± 77.34 (97.0) | <0.001 | NS | NS | NS |
| HOMA-I | 4.76 ± 4.97 (3.56) | 2.22 ± 1.47 (2.01) | 8.25 ± 10.92 (4.80) | 2.41 ± 1.37 (1.88) | <0.05 | <0.01 | NS | NS |
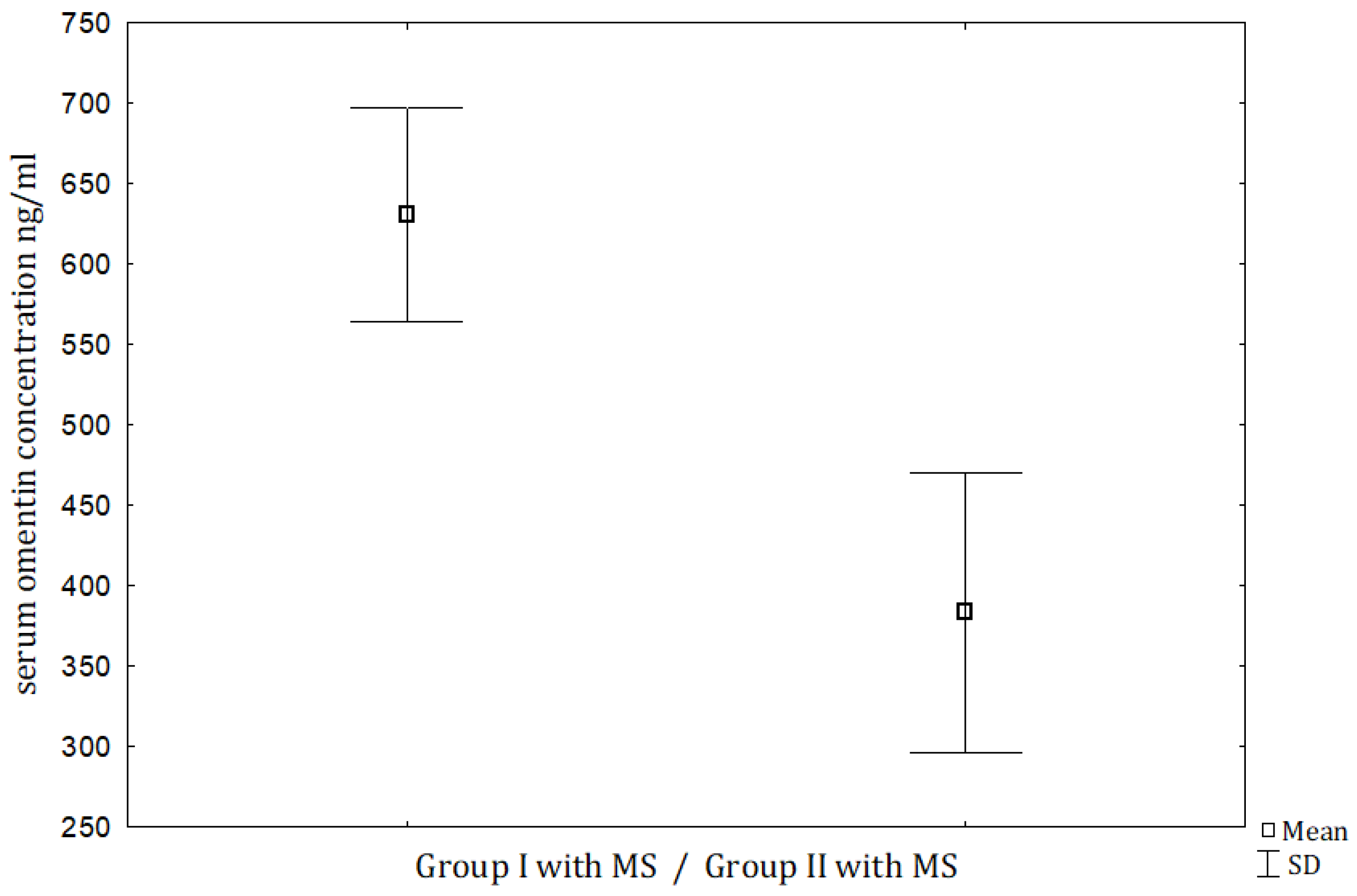
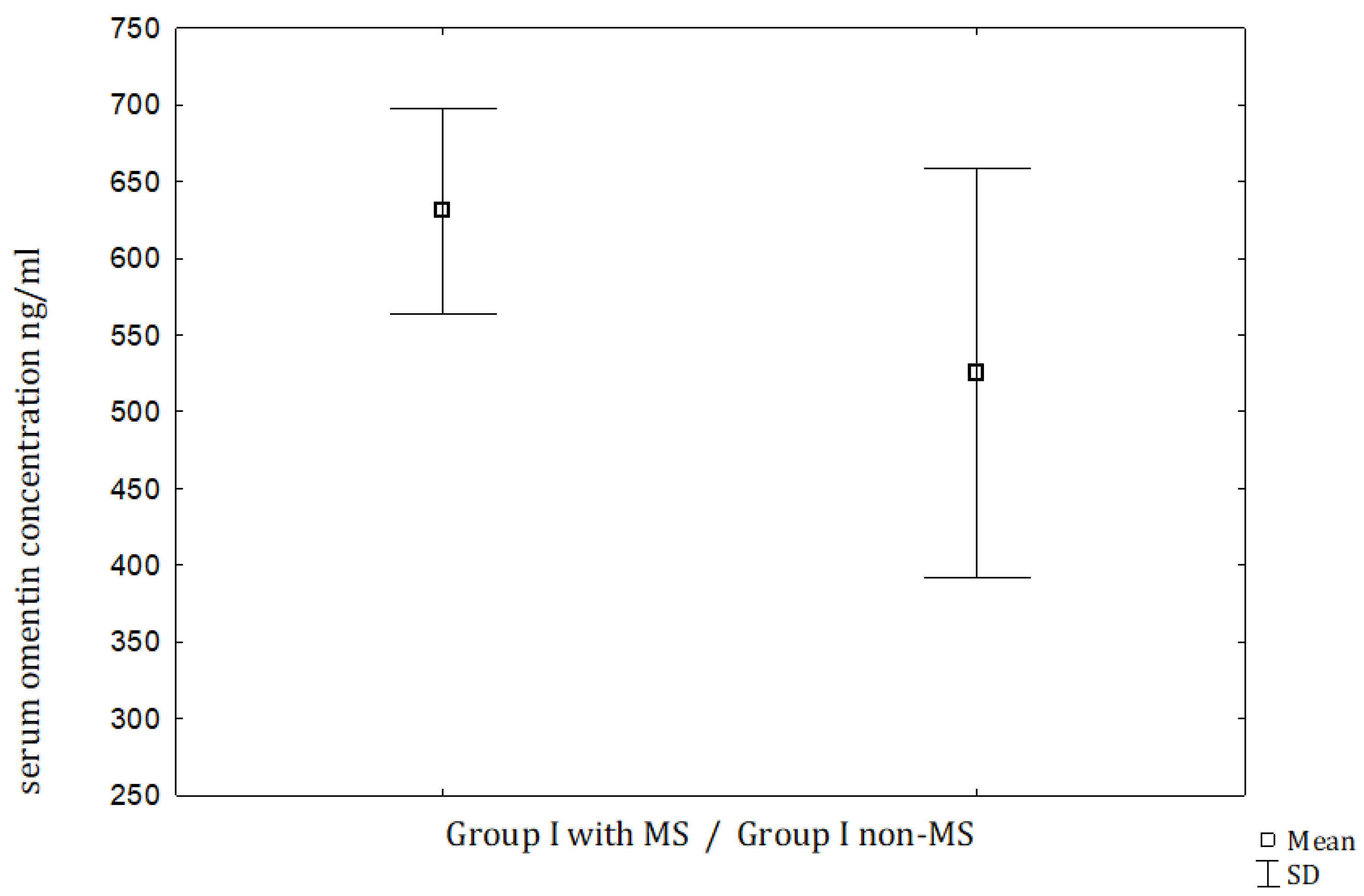
| Omentin (ng/mL) | 630.81 ± 227.06 (576.5) | 525.65 ± 323.0 (453.4) | 383.22 ± 202.1 (383.0) | 377.48 ± 141.11 (341.1) | NS | NS | <0.001 | <0.05 |
| Leptin (ng/mL) | 12.20 ± 8.18 (10.09) | 5.96 ± 6.58 (4.58) | 11.54 ± 8.34 (10.37) | 6.86 ± 5.35 (5.41) | <0.01 | <0.01 | NS | NS |
| Testosterone (ng/mL) | 3.31 ± 2.85 (2.91) | 4.25 ± 2.62 (3.51) | 3.20 ± 1.33 (2.97) | 3.74 ± 1.37 (3.58) | NS | NS | NS | NS |
| Estradiol (pg/mL) | 23.59 ± 20.43 (18.2) | 27.22 ± 21.68 (24.6) | 21.30 ± 9.34 (18.85) | 17.53 ± 8.71 (17.1) | NS | NS | NS | <0.05 |
| SHBG (nmol/L) | 30.08 ± 11.96 (27.0) | 30.38 ± 15.26 (30.6) | 25.37 ± 13.86 (23.35) | 27.04 ± 16.33 (24.6) | NS | NS | NS | NS |
| Testosterone/SHBG ratio | 0.12 ± 0.12 (0.10) | 0.35 ± 0.98 (0.14) | 0.16 ± 0.09 (0.15) | 0.18 ± 0.11 (0.16) | NS | NS | NS | NS |
| Estradiol/SHBG ratio | 1.47 ± 2.87 (0.68) | 1.19 ± 1.43 (0.81) | 1.13 ± 0.92 (0.97) | 0.96 ± 0.77 (0.73) | NS | NS | NS | NS |
| Estradiol/Testosterone ratio | 15.98 ± 18.97 (8.37) | 14.99 ± 40.27 (6.76) | 8.25 ± 7.39 (6.67) | 6.58 ± 8.82 (5.12) | NS | NS | <0.01 | NS |
| PSA (ng/mL) | 39.66 ± 107.03 (7.8) | 22.48 ± 46.24 (7.41) | 3.34 ± 3.38 (1.55) | 4.16 ± 6.63 (1.27) | NS | NS | <0.01 | <0.01 |
| Omentin | Leptin | Testosterone | Age | |||||
|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | |
| Age | 0.38 | <0.001 | −0.17 | <0.05 | ||||
| BMI | 0.56 | <0.001 | ||||||
| HOMA−I | 0.44 | <0.001 | −0.22 | <0.01 | ||||
| Omentin | 0.34 | <0.001 | 0.38 | <0.001 | ||||
| Leptin | 0.34 | <0.001 | −0.20 | <0.01 | ||||
| Testosterone | −0.20 | <0.01 | −0.17 | <0.05 | ||||
| Estradiol | 0.28 | <0.001 | ||||||
| SHBG | 0.34 | <0.001 | 0.21 | <0.01 | 0.29 | <0.01 | ||
| Testosterone/SHBG | −0.41 | <0.001 | −0.41 | <0.001 | ||||
| Estradiol/SHBG | 0.17 | <0.05 | ||||||
| Estradiol/testosterone | 0.21 | <0.01 | 0.28 | <0.001 | −0.62 | <0.001 | 0.27 | <0.001 |
| PSA | 0.21 | <0.01 | ||||||
| Independent Variable | Regression Coefficient | p |
|---|---|---|
| Leptin | 0.38 | <0.001 |
| SHBG | 0.16 | <0.05 |
| Age | 0.11 | Ns |
| Estradiol/testosterone ratio | −0.05 | Ns |
| Testosterone/SHBG ratio | 0.05 | Ns |
| PC, yes/no | 0.34 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowski, A.; Siemińska, L. Serum Omentin Levels in Patients with Prostate Cancer and Associations with Sex Steroids and Metabolic Syndrome. J. Clin. Med. 2020, 9, 1179. https://doi.org/10.3390/jcm9041179
Borowski A, Siemińska L. Serum Omentin Levels in Patients with Prostate Cancer and Associations with Sex Steroids and Metabolic Syndrome. Journal of Clinical Medicine. 2020; 9(4):1179. https://doi.org/10.3390/jcm9041179
Chicago/Turabian StyleBorowski, Artur, and Lucyna Siemińska. 2020. "Serum Omentin Levels in Patients with Prostate Cancer and Associations with Sex Steroids and Metabolic Syndrome" Journal of Clinical Medicine 9, no. 4: 1179. https://doi.org/10.3390/jcm9041179
APA StyleBorowski, A., & Siemińska, L. (2020). Serum Omentin Levels in Patients with Prostate Cancer and Associations with Sex Steroids and Metabolic Syndrome. Journal of Clinical Medicine, 9(4), 1179. https://doi.org/10.3390/jcm9041179






