Prognostic Value of Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio for Renal Outcomes in Patients with Rapidly Progressive Glomerulonephritis
Abstract
1. Introduction
2. Materials and Methods
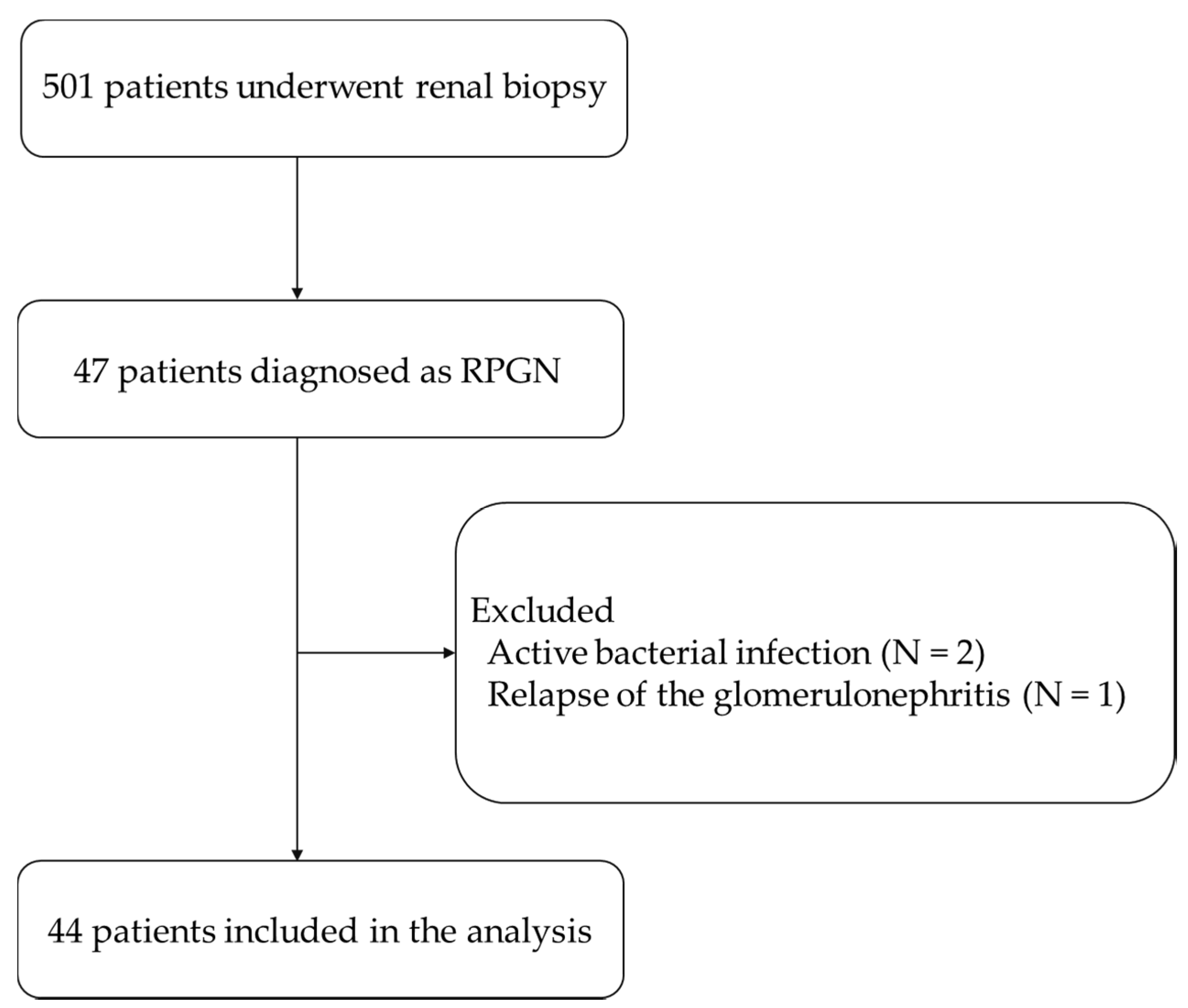
2.1. Study Population
2.2. Clinical and Laboratory Findings
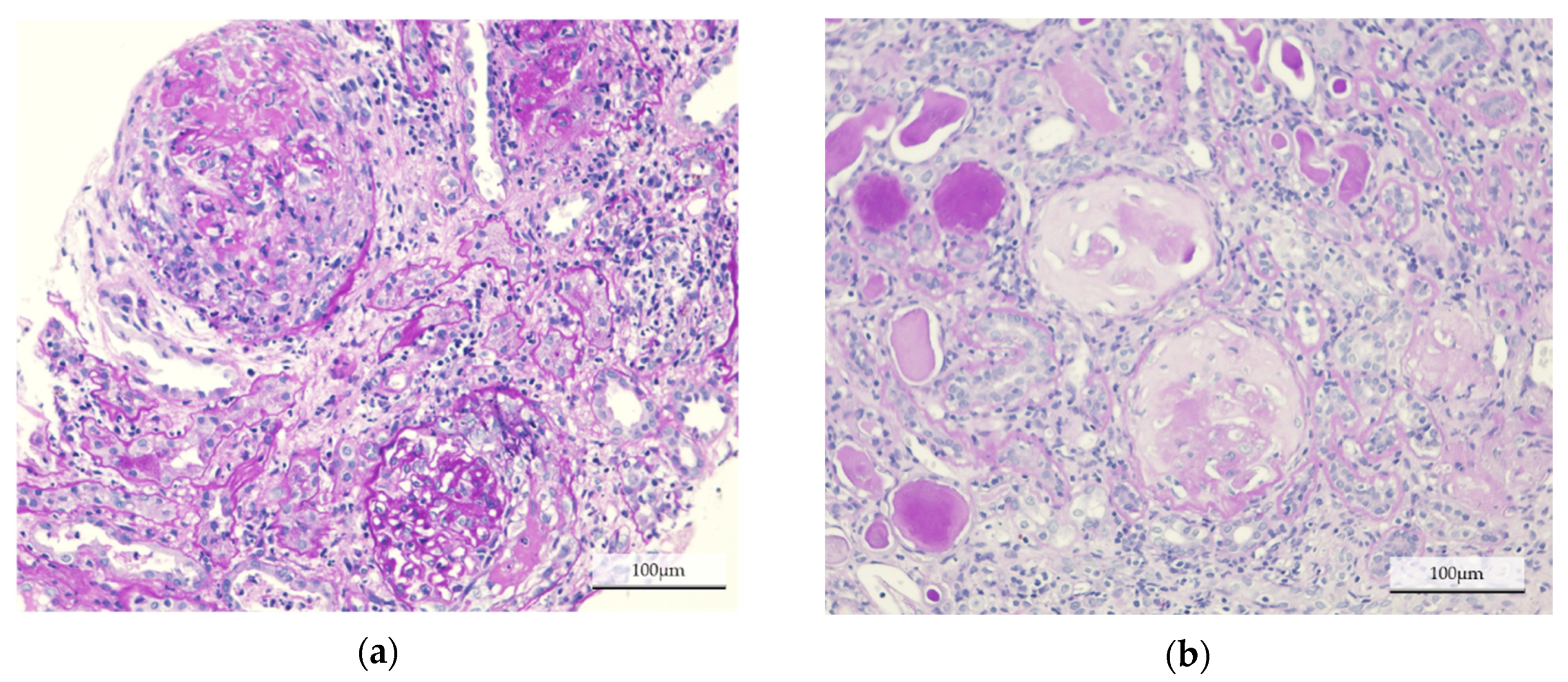
2.3. Histological Findings
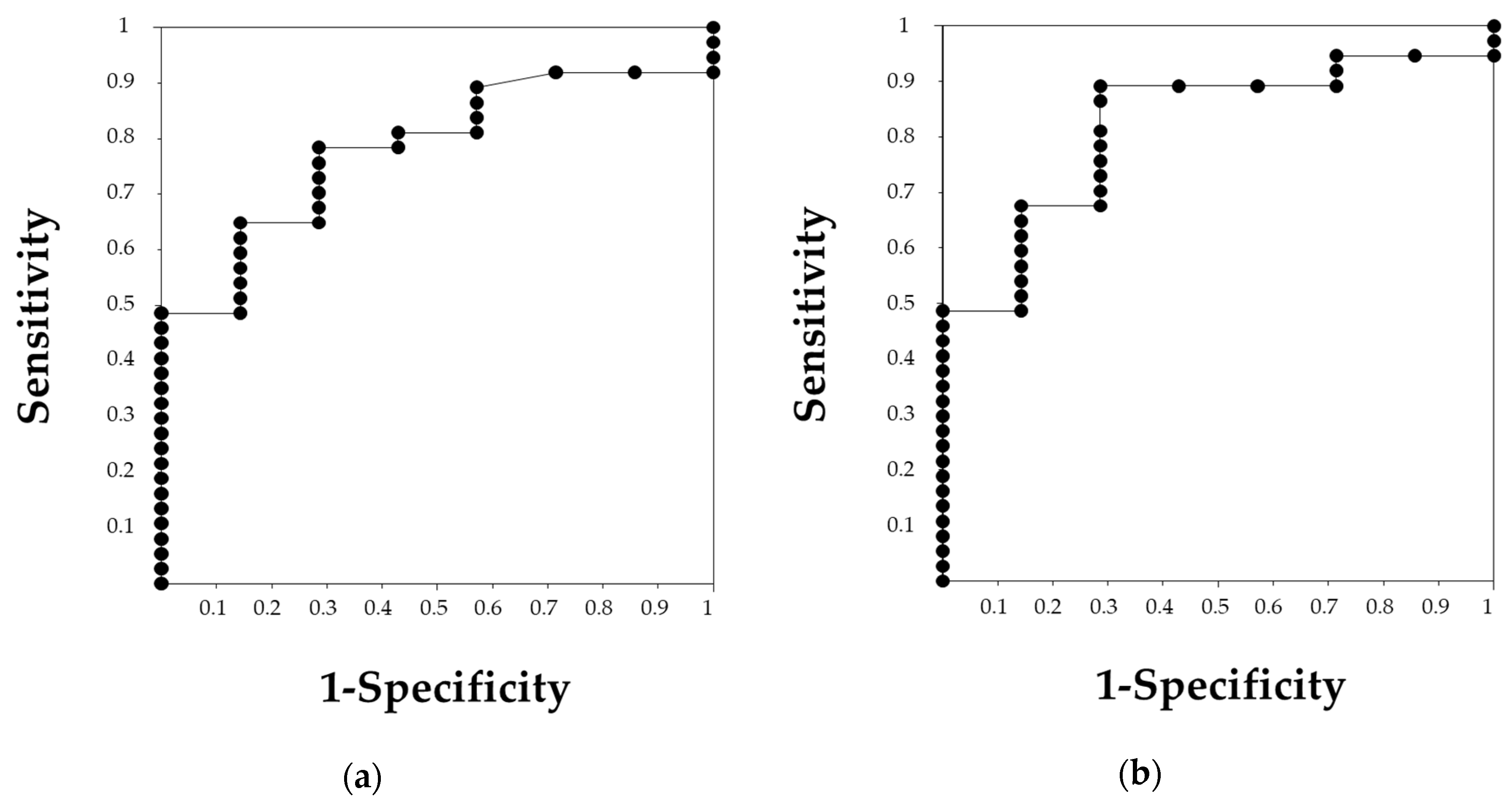
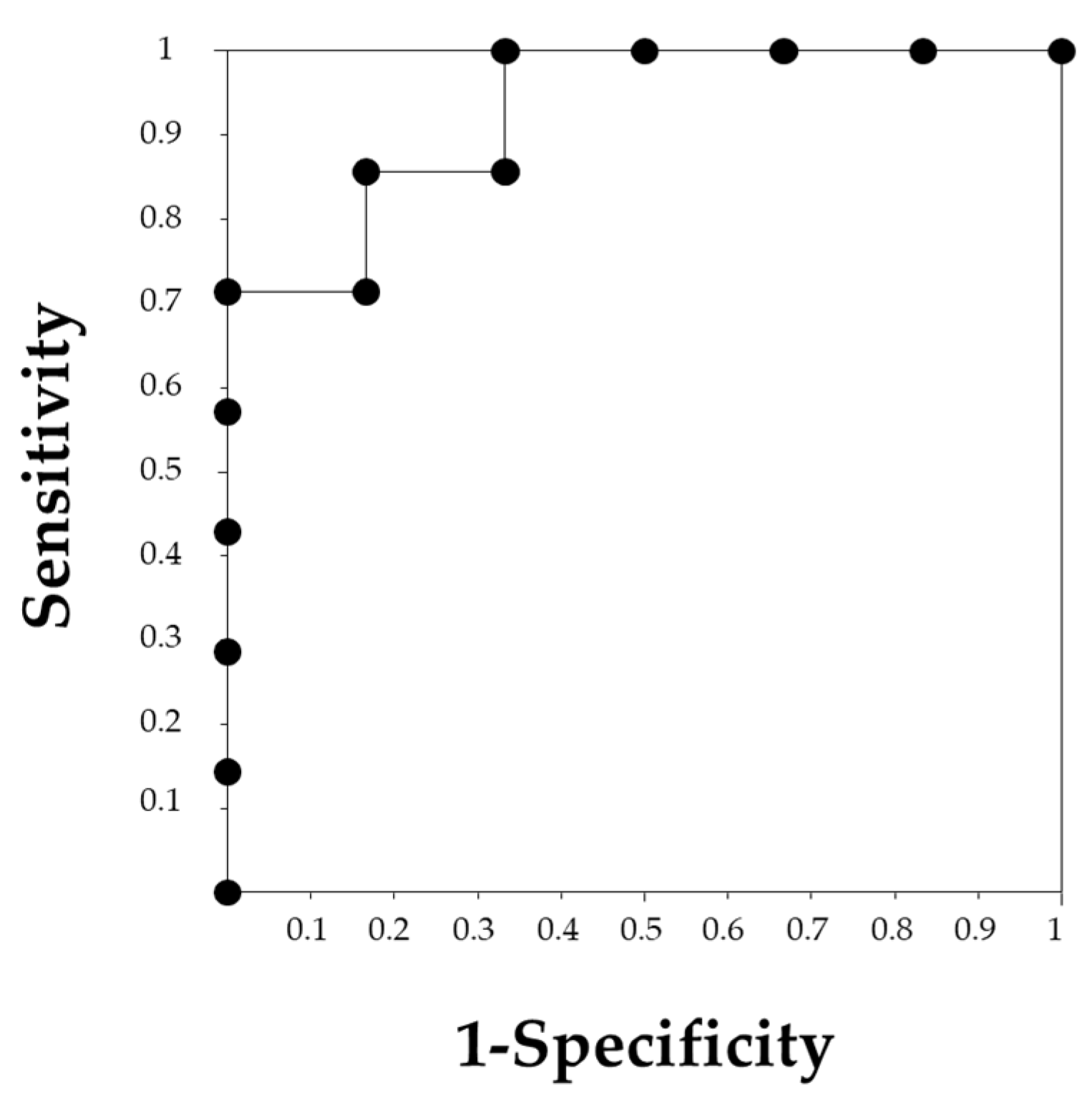
2.4. Statistical Analyses
3. Results
3.1. Differences between Patients with Preserved Renal Function and Renal Failure
3.2. Differences between Patients with Temporary Hemodialysis and Maintenance Hemodialysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nachman, P.H.; Jennette, J.C.; Falk, R.J. Primary glomerular disease. In Brenner and Rector’s The Kidney, 9th ed.; Taal, M.W., Chertow, G.M., Marsden, P.A., Skorecki, K., Yu, A.S.L., Brenner, B.M., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2012; Volume 1, pp. 1153–1154. [Google Scholar]
- Usui, J.; Yamagata, K. Progressive renal diseases: Recent advances in diagnosis and treatments. Topics: II. Pathophysiology and treatments; 4. Crescentic glomerulonephritis. J. Jpn. Soc. Int. Med. 2013, 102, 1128–1135. (In Japanese) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nitta, K.; Masakane, I.; Hanafusa, N.; Goto, S.; Abe, M.; Nakai, S.; Taniguchi, M.; Hasegawa, T.; Wada, A.; Hamano, T.; et al. 2018 annual dialysis data report, JSDT renal data registry. J. Jpn. Soc. Dial. Ther. 2018, 52, 679–754. (In Japanese) [Google Scholar] [CrossRef]
- Toraman, A.; Neşe, N.; Özyurt, B.C.; Kürşat, S. Association between neutrophil-lymphocyte & platelet lymphocyte ratios with prognosis & mortality in rapidly progressive glomerulonephritis. Indian J. Med. Res. 2019, 150, 399–406. [Google Scholar] [PubMed]
- Xu, P.C.; Chen, T.; Wu, S.J.; Yang, X.; Gao, S.; Hu, S.Y.; Wei, L.; Yan, T.K. Pathological severity determines the renal recovery for anti-myeloperoxidase antibody-associated vasculitis requiring dialysis at disease onset: A retrospective study. BMC Nephrol. 2019, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Du, J.; Li, T.; Liao, H. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio associated with disease activity in patients with Takayasu’s arteritis: A case-control study. BMJ Open 2017, 7, e014451. [Google Scholar] [CrossRef]
- Yuksel, M.; Yildiz, A.; Oylumlu, M.; Turkcu, F.M.; Bilik, M.Z.; Ekinci, A.; Elbey, B.; Tekbas, E.; Alan, S. Novel markers of endothelial dysfunction and inflammation in Behçet’s disease patients with ocular involvement: Epicardial fat thickness, carotid intima media thickness, serum ADMA level, and neutrophil-to-lymphocyte ratio. Clin. Rheumatol. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Chantasiriwan, N.; Silvilairat, S.; Makonkawkeyoon, K.; Pongprot, Y.; Sittiwangkul, R. Predictors of intravenous immunoglobulin resistance and coronary artery aneurysm in patients with Kawasaki Disease. Paediatr. Int. Child. Health 2018, 38, 209–212. [Google Scholar] [CrossRef]
- Park, C.H.; Han, D.S.; Jeong, J.Y.; Eun, C.S.; Yoo, K.S.; Jeon, Y.C.; Sohn, J.H. The optimal cut-off value of neutrophil-to-lymphocyte ratio for predicting prognosis in adult patients with Henoch-Schönlein purpura. PLoS ONE 2016, 11, e0153238. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Yang, X.; Chen, L.; Yang, Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int. Immunopharmacol. 2016, 36, 94–99. [Google Scholar] [CrossRef]
- Küçük, H.; Göker, B.; Varan, Ö.; Dumludag, B.; Haznedaroğlu, Ş.; Öztürk, M.A.; Tufan, A.; Emiroglu, T.; Erten, Y. Predictive value of neutrophil/lymphocyte ratio in renal prognosis of patients with granulomatosis with polyangiitis. Ren. Fail. 2017, 39, 273–276. [Google Scholar] [CrossRef]
- Abaza, N.M.; El-Latif, E.M.; Gheita, T.A. Clinical significance of neutrophil/lymphocyte ratio in patients with granulomatosis with polyangiitis. Reumatol. Clin. 2019, 15, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Guney, I.; Yerlikaya, F.H.; Tonbul, H.Z. The relationship between neutrophil-to- lymphocyte ratio and inflammation in end-stage renal disease patients. Ren. Fail. 2012, 34, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Erdur, F.M.; Ozcicek, F.; Ozcicek, A.; Akbas, E.M.; Ozbicer, A.; Demirtas, L.; Turk, S.; Tonbul, H.Z. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial. Int. 2013, 17, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Kurokawa, K.; Koyama, A.; Arimura, Y.; Kida, H.; Shigematsu, H.; Suzuki, S.; Nihei, H.; Makino, H.; Ueda, N.; et al. Guidelines for the management of rapidly progressive glomerulonephritis. Nihon Jinzo Gakkai Shi 2002, 44, 55–82. (In Japanese) [Google Scholar] [PubMed]
- Japanese Society of Nephrology. Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi 2012, 54, 1034–1191. [Google Scholar]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. kidney. Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Iyama, T.; Takata, T.; Koda, M.; Fukuda, S.; Hoi, S.; Mae, Y.; Fukui, T.; Munemura, C.; Isomoto, H. Renal shear wave elastography for the assessment of nephron hypertrophy: A cross-sectional study in chronic kidney disease. J. Med. Ultrason. 2018, 45, 571–576. [Google Scholar] [CrossRef]
- Jennette, J.C. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003, 63, 1164–1177. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. Platelet to lymphocyte ratio is associated with the current activity of ANCA-associated vasculitis at diagnosis: A retrospective monocentric study. Rheumatol. Int. 2018, 38, 1865–1871. [Google Scholar] [CrossRef]
- Schulze-Koops, H. Lymphopenia and autoimmune diseases. Arthritis Res. Ther. 2004, 6, 178–180. [Google Scholar] [CrossRef]
- Ahn, S.S.; Jung, S.M.; Song, J.J.; Park, Y.B.; Lee, S.W. Neutrophil to lymphocyte ratio at diagnosis can estimate vasculitis activity and poor prognosis in patients with ANCA-associated vasculitis: A retrospective study. BMC Nephrol. 2018, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Allen, L.A. Regulation of human neutrophil apoptosis and lifespan in health and disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lebois, M.; Josefsson, E.C. Regulation of platelet lifespan by apoptosis. Platelets 2016, 27, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Kimura, K.; Muso, E.; Fujimoto, S.; Hasegawa, M.; Kaname, S.; Usui, J.; Inohara, T.; Kobayashi, M.; Itabashi, M.; et al. Clinical guideline for rapidly progressive glomerulonephritis in Japan 2014. Jpn. J. Nephrol. 2015, 57, 139–232. (In Japanese) [Google Scholar]

| Pre-dialysis (n = 37) | Maintenance HD (n = 7) | p Value | |
|---|---|---|---|
| Sex (Male/Female) | 22/15 | 5/2 | 0.132 |
| Age (years) | 71.4 ± 11.6 | 65.7 ± 7.8 | 0.222 |
| Classifications of RPGN | |||
| Immune complex CGN | 33 (89.2%) | 1 (14.3%) | |
| Pauci-immune CGN | 4 (10.8%) | 2 (28.6%) | |
| Anti-GBM CGN | 0 (0%) | 4 (57.1%) | |
| Immunosuppressive therapy | |||
| Pulse corticosteroids | 29/8 | 6/1 | 0.557 |
| Cyclophosphamide | 13/24 | 0/7 | 0.069 |
| Plasma exchange | 0/37 | 3/4 | 0.003 |
| White blood cell count (103/μL) | 10.2 (4.9–23.5) | 6.2 (4.7–12.4) | 0.037 |
| Neutrophil count (103/μL) | 8.5 (3.6–22.1) | 4.6 (3.3–8.4) | 0.012 |
| Lymphocyte count (103/μL) | 1.17 ± 0.59 | 1.31 ± 0.43 | 0.550 |
| Platelet count (103/μL) | 327 (98–808) | 189 (117–269) | 0.015 |
| Creatinine (mg/dL) | 2.80 ± 2.01 | 8.74± 1.80 | <0.001 |
| eGFR (mL/min/1.73 m2) | 27.3 ± 21.2 | 5.4 ± 2.0 | <0.001 |
| CRP (mg/dL) | 5.0 (0–24.8) | 4.0 (0.4–26.9) | 0.987 |
| ESR (mm/h) | 99 (10–140) | 111 (62–134) | 0.771 |
| MPO-ANCA (U/mL) | 166 (0–860) | 0 (0–2440) | 0.109 |
| PR3-ANCA (U/mL) | 0 (0–35.8) | 0 (0–0) | 0.308 |
| anti-GBM antibody (U/mL) | 0 (0–0) | 42.3 (0–858.0) | <0.001 |
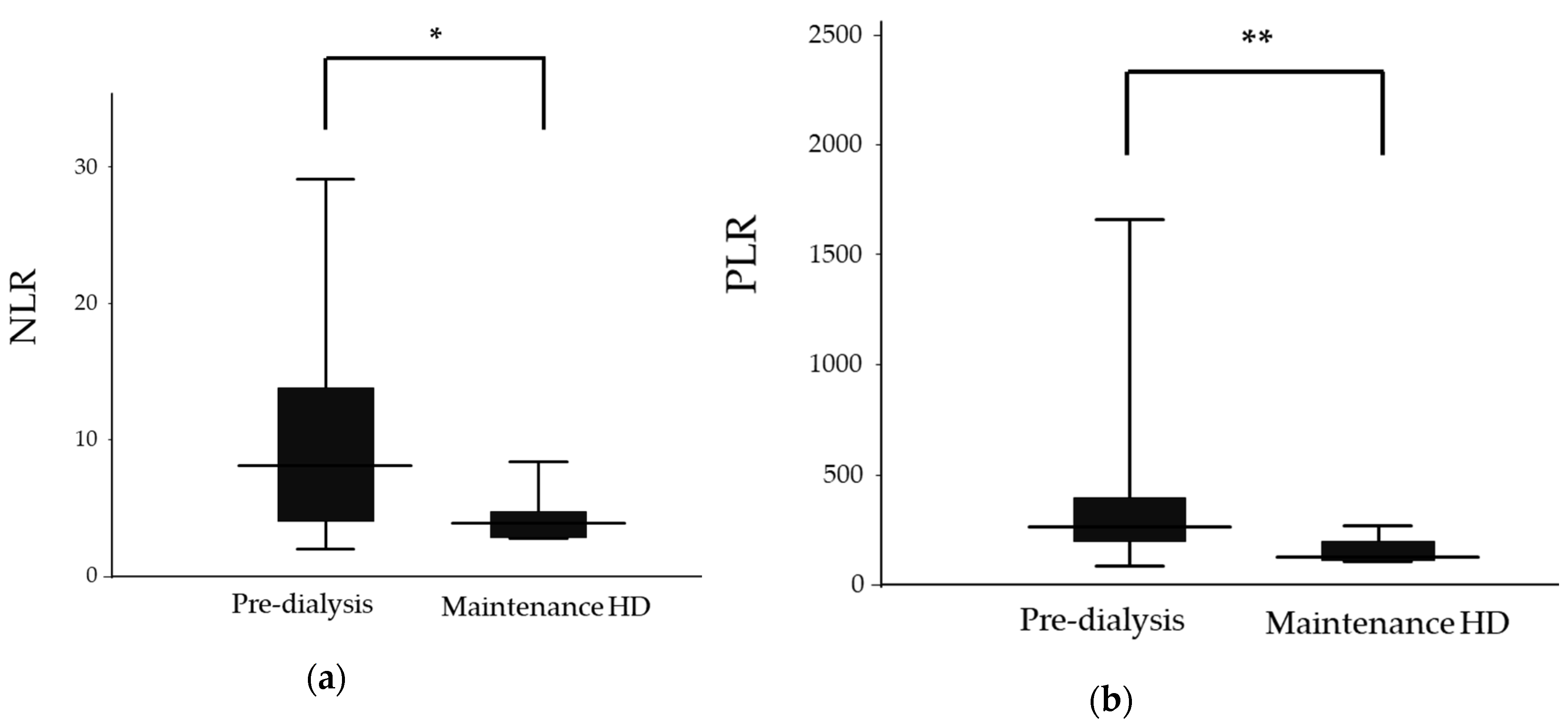
| NLR | 8.2 (2.0–32.0) | 3.9 (2.8–8.4) | 0.019 |
| PLR | 265.7 (82.9–2255.0) | 126.0 (107.1–269.0) | 0.008 |
| Temporary HD (n = 6) | Maintenance HD (n = 7) | p Value | |
|---|---|---|---|
| Sex (Male/Female) | 1/5 | 5/2 | 0.048 |
| Age (years) | 72.7 ± 18.4 | 65.7 ± 7.8 | 0.381 |
| Classifications of RPGN | |||
| Immune complex CGN | 0 (0%) | 1 (14.3%) | |
| Pauci-immune CGN | 6 (100%) | 2 (28.6%) | |
| Anti-GBM CGN | 0 (0%) | 4 (57.1%) | |
| Immunosuppressive therapy | |||
| Pulse corticosteroids | 6/0 | 6/1 | 0.538 |
| Cyclophosphamide | 1/5 | 0/7 | 0.462 |
| Plasma exchange | 6/0 | 3/4 | 0.049 |
| White blood cell count (103/μL) | 12.6 ± 5.1 | 7.6 ± 2.9 | 0.048 |
| Neutrophil count (103/μL) | 11.0 ± 5.0 | 5.3 ± 2.1 | 0.018 |
| Lymphocyte count (103/μL) | 1.10 ± 0.58 | 1.31 ± 0.43 | 0.470 |
| Platelet count (103/μL) | 289.7 ± 112.7 | 191.4 ± 55.6 | 0.066 |
| CRP (mg/dL) | 11.2 ± 7.0 | 8.1 ± 10.3 | 0.554 |
| ESR (mm/h) | 104 ± 32 | 103 ± 27 | 0.985 |
| MPO-ANCA (U/mL) | 160 (17.0–469.0) | 0 (0–2440) | 0.138 |
| anti-GBM antibody (U/mL) | 0 (0–0) | 42.3 (0–858.0) | 0.065 |
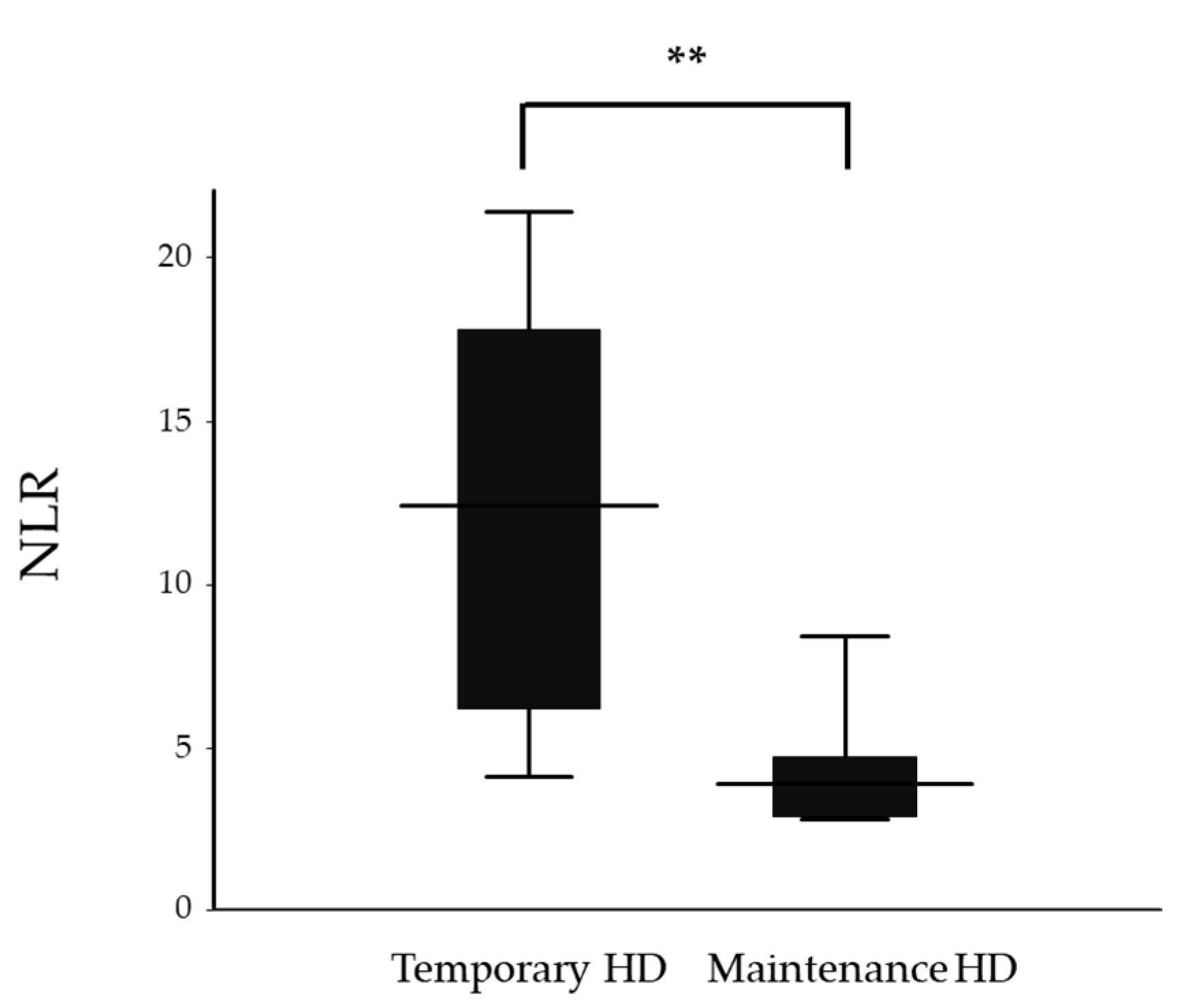
| NLR | 12.4 (4.1–21.4) | 3.9 (2.8–8.4) | 0.008 |
| PLR | 341.7 ± 217.7 | 156.1 ± 62.6 | 0.053 |
| Temporary HD (n = 6) | Maintenance HD (n = 7) | p Value | |
|---|---|---|---|
| Cellular crescent (%) | 30.4 ± 24.1 | 4.4 ± 5.7 | 0.022 |
| Fibrocellular crescent (%) | 11.9 ± 14.1 | 10.2 ± 9.4 | 0.945 |
| Fibrous crescent (%) | 19.0 ± 22.9 | 20.1 ± 16.3 | 0.921 |
| Global sclerosis (%) | 9.0 ± 10.1 | 53.0 ± 9.7 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mae, Y.; Takata, T.; Ida, A.; Ogawa, M.; Taniguchi, S.; Yamamoto, M.; Iyama, T.; Fukuda, S.; Isomoto, H. Prognostic Value of Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio for Renal Outcomes in Patients with Rapidly Progressive Glomerulonephritis. J. Clin. Med. 2020, 9, 1128. https://doi.org/10.3390/jcm9041128
Mae Y, Takata T, Ida A, Ogawa M, Taniguchi S, Yamamoto M, Iyama T, Fukuda S, Isomoto H. Prognostic Value of Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio for Renal Outcomes in Patients with Rapidly Progressive Glomerulonephritis. Journal of Clinical Medicine. 2020; 9(4):1128. https://doi.org/10.3390/jcm9041128
Chicago/Turabian StyleMae, Yukari, Tomoaki Takata, Ayami Ida, Masaya Ogawa, Sosuke Taniguchi, Marie Yamamoto, Takuji Iyama, Satoko Fukuda, and Hajime Isomoto. 2020. "Prognostic Value of Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio for Renal Outcomes in Patients with Rapidly Progressive Glomerulonephritis" Journal of Clinical Medicine 9, no. 4: 1128. https://doi.org/10.3390/jcm9041128
APA StyleMae, Y., Takata, T., Ida, A., Ogawa, M., Taniguchi, S., Yamamoto, M., Iyama, T., Fukuda, S., & Isomoto, H. (2020). Prognostic Value of Neutrophil-To-Lymphocyte Ratio and Platelet-To-Lymphocyte Ratio for Renal Outcomes in Patients with Rapidly Progressive Glomerulonephritis. Journal of Clinical Medicine, 9(4), 1128. https://doi.org/10.3390/jcm9041128






