The Clinical Role of Insulin Degludec/Insulin Aspart in Type 2 Diabetes: An Empirical Perspective from Experience in Australia
Abstract
1. Introduction
1.1. Glycaemic Control in T2D
1.2. Pharmacological Management of T2D
1.3. The IDegAsp Co-Formulation
1.4. Current Guidance on the Practical Use of IDegAsp
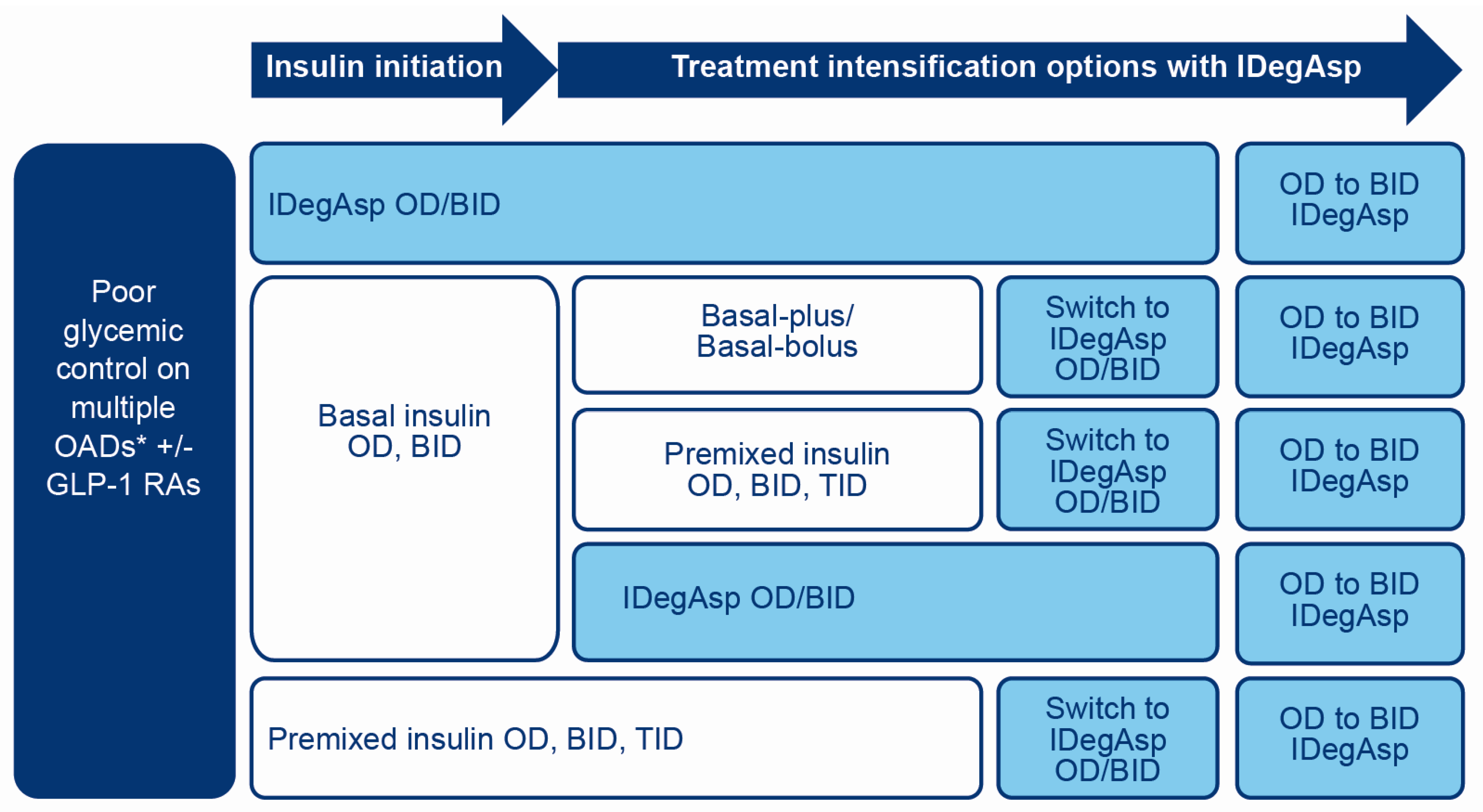
2. Clinical Guidance for the Use of IDegAsp in People with T2D
2.1. Initiation of IDegAsp in Insulin-Naïve People with T2D
2.1.1. Clinical Evidence of Efficacy
2.1.2. Practical Recommendations
2.2. Insulin Intensification from Basal Insulin to IDegAsp
2.2.1. Clinical Evidence of Efficacy
2.2.2. Practical Recommendations
2.3. Intensification from IDegAsp OD to IDegAsp BID
2.3.1. Clinical Evidence of Efficacy
2.3.2. Practical Recommendations
2.4. Switching from Premixed Insulin to IDegAsp
2.4.1. Clinical Evidence of Efficacy
2.4.2. Practical Recommendations
2.5. Switching from Basal-Plus or Basal-Bolus Insulin to IDegAsp
2.5.1. Clinical Evidence of Efficacy
2.5.2. Practical Recommendations
3. Use of IDegAsp in Special Populations or Situations
3.1. Elderly Patients
3.2. Patients on a Very-Low-Calorie, Reduced-Carbohydrate or Erratic Diet
3.3. Patients with Hepatic or Renal Impairment
3.4. Hospitalised Patients
3.5. Use in Pregnancy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 2 December 2019).
- Skyler, J.S.; Ricordi, C. Stopping type 1 diabetes: Attempts to prevent or cure type 1 diabetes in man. Diabetes 2011, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ryzodeg, P.I. Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203313s002lbl.pdf (accessed on 2 December 2019).
- Nathan, D.M. Long-term complications of diabetes mellitus. New Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Seshasai, S.R.; Wijesuriya, S.; Sivakumaran, R.; Nethercott, S.; Preiss, D.; Erqou, S.; Sattar, N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009, 373, 1765–1772. [Google Scholar] [CrossRef]
- IDF. IDF Diabetes Atlas 9th Edition. Available online: https://www.diabetesatlas.org/en/resources/ (accessed on 2 December 2019).
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes. Diabetes Care. 2019, 42, S90–S102. [Google Scholar] [CrossRef]
- IDF. IDF Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care. Available online: File:///C:/Users/Jane.Blackburn/Downloads/IDF-T2D-CPR-2017-print%20(1).pdf (accessed on 2 December 2019).
- ADS Treatment Algorithm. A New Blood Glucose Management Algorithm for Type 2 Diabetes: A Position Statement of the Australian Diabetes Society (Version 2.4). Available online: https://diabetessociety.com.au/position-statements.asp (accessed on 2 December 2019).
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm--2016 Executive Summary. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22, 84–113. [Google Scholar] [CrossRef]
- Royal Australian College of General Practitioners (RACGP). General Practice Management of Type 2 Diabetes 2016–2018. Available online: https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Guidelines/Diabetes/General-practice-management-of-type-2-diabetes_1.pdf (accessed on 2 December 2019).
- Woerle, H.J.; Neumann, C.; Zschau, S.; Tenner, S.; Irsigler, A.; Schirra, J.; Gerich, J.E.; Goke, B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res. Clin. Pract. 2007, 77, 280–285. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef]
- Garber, A.J.; Ligthelm, R.; Christiansen, J.S.; Liebl, A. Premixed insulin treatment for type 2 diabetes: Analogue or human? Diabetes Obes. Metab. 2007, 9, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Nosek, L.; Klein, O.; Coester, H.; Svendsen, A.L.; Haahr, H. Insulin degludec/insulin aspart produces a dose-proportional glucose-lowering effect in subjects with type 1 diabetes mellitus. Diabetes Obes. Metab. 2015, 17, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Awata, T.; Bain, S.C.; Ceriello, A.; Fulcher, G.R.; Unnikrishnan, A.G.; Arechavaleta, R.; Gonzalez-Galvez, G.; Hirose, T.; Home, P.D.; et al. Clinical use of the co-formulation of insulin degludec and insulin aspart. Int. J. Clin. Pract. 2016, 70, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Millar-Jones, D. Clinical inertia to insulin initiation and intensification in the UK: A focused literature review. Prim. Care Diabetes 2017, 11, 3–12. [Google Scholar] [CrossRef]
- Khunti, K.; Wolden, M.L.; Thorsted, B.L.; Andersen, M.; Davies, M.J. Clinical inertia in people with type 2 diabetes: A retrospective cohort study of more than 80,000 people. Diabetes Care 2013, 36, 3411–3417. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Pouwer, F.; Khunti, K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes. Metab. 2018, 20, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ryzodeg SmPC. Available online: https://www.ema.europa.eu/en/documents/product-information/ryzodeg-epar-product-information_en.pdf (accessed on 2 December 2019).
- Ryzodeg® 70/30 FlexTouch®/Penfill® PI. Available online: http://www.novonordisk.com.au/content/dam/australia/affiliate/www-novonordisk-au/Health%20Care%20Professionals/Documents/Ryzodeg%2070-30_pi1.pdf (accessed on 2 December 2019).
- Haahr, H.; Heise, T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin. Pharm. 2014, 53, 787–800. [Google Scholar] [CrossRef]
- Heise, T.; Nosek, L.; Bottcher, S.G.; Hastrup, H.; Haahr, H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes. Metab. 2012, 14, 944–950. [Google Scholar] [CrossRef]
- Heise, T.; Nosek, L.; Roepstorff, C.; Chenji, S.; Klein, O.; Haahr, H. Distinct Prandial and Basal Glucose-Lowering Effects of Insulin Degludec/Insulin Aspart (IDegAsp) at Steady State in Subjects with Type 1 Diabetes Mellitus. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2014, 5, 255–265. [Google Scholar] [CrossRef]
- Kurtzhals, P.; Heise, T.; Strauss, H.M. Multi-hexamer formation is the underlying basis for the ultra-long glucose-lowering effect of insulin degludec. Diabetes 2011, 60. Available online: https://diabetes.diabetesjournals.org/content/60/Supplement_1 (accessed on 20 March 2020).
- Thuillier, P.; Alavi, Z.; Kerlan, V. Long-term safety and efficacy of insulin degludec in the management of type 2 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 483–493. [Google Scholar] [CrossRef]
- Heise, T.; Meneghini, L.F. Insulin stacking versus therapeutic accumulation: Understanding the differences. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2014, 20, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Korsatko, S.; Nosek, L.; Coester, H.V.; Deller, S.; Roepstorff, C.; Segel, S.; Kapur, R.; Haahr, H.; Hompesch, M.; et al. Steady state is reached within 2–3 days of once-daily administration of degludec, a basal insulin with an ultralong duration of action. J. Diabetes 2016, 8, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Haahr, H.; Fita, E.G.; Heise, T. A Review of Insulin Degludec/Insulin Aspart: Pharmacokinetic and Pharmacodynamic Properties and Their Implications in Clinical Use. Clin. Pharmacokinet. 2017, 56, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Hermanski, L.; Nosek, L.; Feldman, A.; Rasmussen, S.; Haahr, H. Insulin degludec: Four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes. Metab. 2012, 14, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; McGuire, D.K.; Zinman, B.; Poulter, N.R.; Emerson, S.S.; Pieber, T.R.; Pratley, R.E.; Haahr, P.M.; Lange, M.; Brown-Frandsen, K.; et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. New Engl. J. Med. 2017, 377, 723–732. [Google Scholar] [CrossRef]
- Ratner, R.E.; Gough, S.C.; Mathieu, C.; Del Prato, S.; Bode, B.; Mersebach, H.; Endahl, L.; Zinman, B. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: A pre-planned meta-analysis of phase 3 trials. Diabetes Obes. Metab. 2013, 15, 175–184. [Google Scholar] [CrossRef]
- Wysham, C.; Bhargava, A.; Chaykin, L.; de la Rosa, R.; Handelsman, Y.; Troelsen, L.N.; Kvist, K.; Norwood, P. Effect of insulin degludec vs insulin gargine U100 on hypoglycemia in patients with type 2 diabetes: The SWITCH 2 randomized clinical trial. JAMA 2017, 318, 45–56. [Google Scholar] [CrossRef]
- Mathieu, C.; Hollander, P.; Miranda-Palma, B.; Cooper, J.; Franek, E.; Russell-Jones, D.; Larsen, J.; Tamer, S.C.; Bain, S.C. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): A 26-week randomized, treat-to-target trial with a 26-week extension. J. Clin. Endocrinol. Metab. 2013, 98, 1154–1162. [Google Scholar] [CrossRef]
- Heinemann, L.; Kapitza, C.; Starke, A.A.; Heise, T. Time-action profile of the insulin analogue B28Asp. Diabet. Med. J. Br. Diabet. Assoc. 1996, 13, 683–684. [Google Scholar] [CrossRef]
- Mudaliar, S.R.; Lindberg, F.A.; Joyce, M.; Beerdsen, P.; Strange, P.; Lin, A.; Henry, R.R. Insulin aspart (B28 asp-insulin): A fast-acting analog of human insulin: Absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care 1999, 22, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Nosek, L.; Klein, O.; Coester, H.V.; Roepstorff, C.; Svendsen, A.L. IDegAsp Produces Dose-proportional Glucose-lowering Effect in Subjects with Type 1 Diabetes. Diabetes 2013, 62. Available online: https://diabetes.diabetesjournals.org/content/62/Supplement_1 (accessed on 20 March 2020).
- Fulcher, G.R.; Christiansen, J.S.; Bantwal, G.; Polaszewska-Muszynska, M.; Mersebach, H.; Andersen, T.H.; Niskanen, L.K. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin-treated type 2 diabetes: A phase 3a, randomized, treat-to-target trial. Diabetes Care 2014, 37, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Atkin, S.; Cervera, A.; Das, A.K.; Demir, O.; Demir, T.; Fariduddin, M.; Vo, K.T.; Ku, B.J.; Kumar, A.; et al. Multinational Consensus: Insulin Initiation with Insulin Degludec/Aspart (IDegAsp). Adv. Ther. 2018, 35, 928–936. [Google Scholar] [CrossRef]
- Philis-Tsimikas, A.; Astamirova, K.; Gupta, Y.; Haggag, A.; Roula, D.; Bak, B.A.; Fita, E.G.; Nielsen, A.M.; Demir, T. Similar glycaemic control with less nocturnal hypoglycaemia in a 38-week trial comparing the IDegAsp co-formulation with insulin glargine U100 and insulin aspart in basal insulin-treated subjects with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2019, 147, 157–165. [Google Scholar] [CrossRef]
- Kalra, S.; Latif, Z.A.; Comlekci, A.; Galvez, G.G.; Malik, R.; Pathan, M.F.; Kumar, A. Pragmatic use of insulin degludec/insulin aspart co-formulation: A multinational consensus statement. Indian J. Endocrinol. Metab. 2016, 20, 542–545. [Google Scholar] [CrossRef]
- Onishi, Y.; Ono, Y.; Rabol, R.; Endahl, L.; Nakamura, S. Superior glycaemic control with once-daily insulin degludec/insulin aspart versus insulin glargine in Japanese adults with type 2 diabetes inadequately controlled with oral drugs: A randomized, controlled phase 3 trial. Diabetes Obes. Metab. 2013, 15, 826–832. [Google Scholar] [CrossRef]
- Franek, E.; Haluzik, M.; Canecki Varzic, S.; Sargin, M.; Macura, S.; Zacho, J.; Christiansen, J.S. Twice-daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin-naive adults with Type 2 diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2016, 33, 497–505. [Google Scholar] [CrossRef]
- Kumar, A.; Franek, E.; Wise, J.; Niemeyer, M.; Mersebach, H.; Simo, R. Efficacy and Safety of Once-Daily Insulin Degludec/Insulin Aspart versus Insulin Glargine (U100) for 52 Weeks in Insulin-Naive Patients with Type 2 Diabetes: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0163350. [Google Scholar] [CrossRef]
- Park, S.W.; Bebakar, W.M.; Hernandez, P.G.; Macura, S.; Herslov, M.L.; de la Rosa, R. Insulin degludec/insulin aspart once daily in Type 2 diabetes: A comparison of simple or stepwise titration algorithms (BOOST((R)) : SIMPLE USE). Diabet. Med. J. Br. Diabet. Assoc. 2017, 34, 174–179. [Google Scholar] [CrossRef]
- Gupta, Y.; Astamirova, K.; Fita, E.G.; Haggag, A.; Roula, D.; Bak, B.A.; Nielsen, A.M.; Tsimikas, A. Similar glycaemic control and less nocturnal hypoglycaemia with intensification of IDegAsp QD or BD vs glargine U100 QD + IAsp 1-3 in adults with type 2 diabetes. In Proceedings of the EASD 2018, Berlin, Germany, 1–5 October 2018. [Google Scholar]
- Kumar, S.; Jang, H.C.; Demirag, N.G.; Skjoth, T.V.; Endahl, L.; Bode, B. Efficacy and safety of once-daily insulin degludec/insulin aspart compared with once-daily insulin glargine in participants with Type 2 diabetes: A randomized, treat-to-target study. Diabet. Med. J. Br. Diabet. Assoc. 2017, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Chow, F.; Choi, D.S.; Taneda, S.; Hirao, K.; Park, Y.; Andersen, T.H.; Gall, M.A.; Christiansen, J.S. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre-/self-mixed insulin: A 26-week, randomised, treat-to-target trial. Diabetes Res. Clin. Pract. 2015, 107, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.S.; Niskanen, L.; Rasmussen, S.; Johansen, T.; Fulcher, G. Lower rates of hypoglycemia during maintenance treatment with insulin degludec/insulin aspart versus biphasic insulin aspart 30: A combined analysis of two Phase 3a studies in type 2 diabetes. J. Diabetes 2016, 8, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, J.; Hong, T.; Liu, M.; Miao, H.; Peng, Y.; Wang, C.; Xu, X.; Yang, T.; Nielsen, A.M.; et al. Efficacy and safety of insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Chinese adults with type 2 diabetes: A phase III, open-label, 2:1 randomized, treat-to-target trial. Diabetes Obes. Metab. 2019, 21, 1652–1660. [Google Scholar] [CrossRef]
- Gerety, G.; Bebakar, W.M.; Chaykin, L.; Ozkaya, M.; Macura, S.; Herslov, M.L.; Behnke, T. Treatment intensification with insulin degludec/insulin aspart twice daily: Randomized study to compare simple and step-wise titration algorithms. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22, 546–554. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Cariou, B.; Pieber, T.R.; Endahl, L.A.; Zacho, J.; Cooper, J.G. Treatment intensification with an insulin degludec (IDeg)/insulin aspart (IAsp) co-formulation twice daily compared with basal IDeg and prandial IAsp in type 2 diabetes: A randomized, controlled phase III trial. Diabetes Obes. Metab. 2016, 18, 274–280. [Google Scholar] [CrossRef]
- Ryzodeg® 70/30 FlexTouch®. Consumer Medicine Information. Available online: http://www.novonordisk.com.au/content/dam/australia/affiliate/www-novonordisk-au/Health%20Care%20Professionals/Documents/Ryzodeg%2070-30%20FlexTouch_cmi1.pdf (accessed on 2 December 2019).
- Fulcher, G.; Mehta, R.; Fita, E.G.; Ekelund, M.; Bain, S.C. Efficacy and Safety of IDegAsp Versus BIAsp 30, Both Twice Daily, in Elderly Patients with Type 2 Diabetes: Post Hoc Analysis of Two Phase 3 Randomized Controlled BOOST Trials. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2019, 10, 107–118. [Google Scholar] [CrossRef]
- Korsatko, S.; Deller, S.; Mader, J.K.; Glettler, K.; Koehler, G.; Treiber, G.; Urschitz, M.; Wolf, M.; Hastrup, H.; Sondergaard, F.; et al. Ultra-long pharmacokinetic properties of insulin degludec are comparable in elderly subjects and younger adults with type 1 diabetes mellitus. Drugs Aging 2014, 31, 47–53. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Rodriguez-Manas, L.; Morley, J.E.; Sinclair, A.J. Hypoglycemia in older people - a less well recognized risk factor for frailty. Aging Dis. 2015, 6, 156–167. [Google Scholar] [CrossRef]
- Wallace, J.I. Management of diabetes in the elderly. Clin. Diabetes 1999, 17, 1–12. [Google Scholar]
- Mohan, V.; Kalra, S.; Kesavadev, J.; Singh, A.K.; Kumar, A.; Unnikrishnan, A.G.; Chawla, R.; Mukherjee, J.J.; Sahay, R.K.; Kumar, J.S.; et al. Consensus on Initiation and Intensification of Premix Insulin in Type 2 Diabetes Management. J. Assoc. Physicians India 2017, 65, 59–73. [Google Scholar]
- Hassanein, M.; Echtay, A.S.; Malek, R.; Omar, M.; Shaikh, S.S.; Ekelund, M.; Kaplan, K.; Kamaruddin, N.A. Original paper: Efficacy and safety analysis of insulin degludec/insulin aspart compared with biphasic insulin aspart 30: A phase 3, multicentre, international, open-label, randomised, treat-to-target trial in patients with type 2 diabetes fasting during Ramadan. Diabetes Res. Clin. Pract. 2018, 135, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kiss, I.; Arold, G.; Roepstorff, C.; Bottcher, S.G.; Klim, S.; Haahr, H. Insulin degludec: Pharmacokinetics in patients with renal impairment. Clin. Pharmacokinet. 2014, 53, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kupcova, V.; Arold, G.; Roepstorff, C.; Hojbjerre, M.; Klim, S.; Haahr, H. Insulin degludec: Pharmacokinetic properties in subjects with hepatic impairment. Clin. Drug Investig. 2014, 34, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.; Galitz, L.; Hu, P.; Lyness, W. Pharmacokinetics of insulin aspart in obesity, renal impairment, or hepatic impairment. Br. J. Clin. Pharmacol. 2005, 60, 469–476. [Google Scholar] [CrossRef]
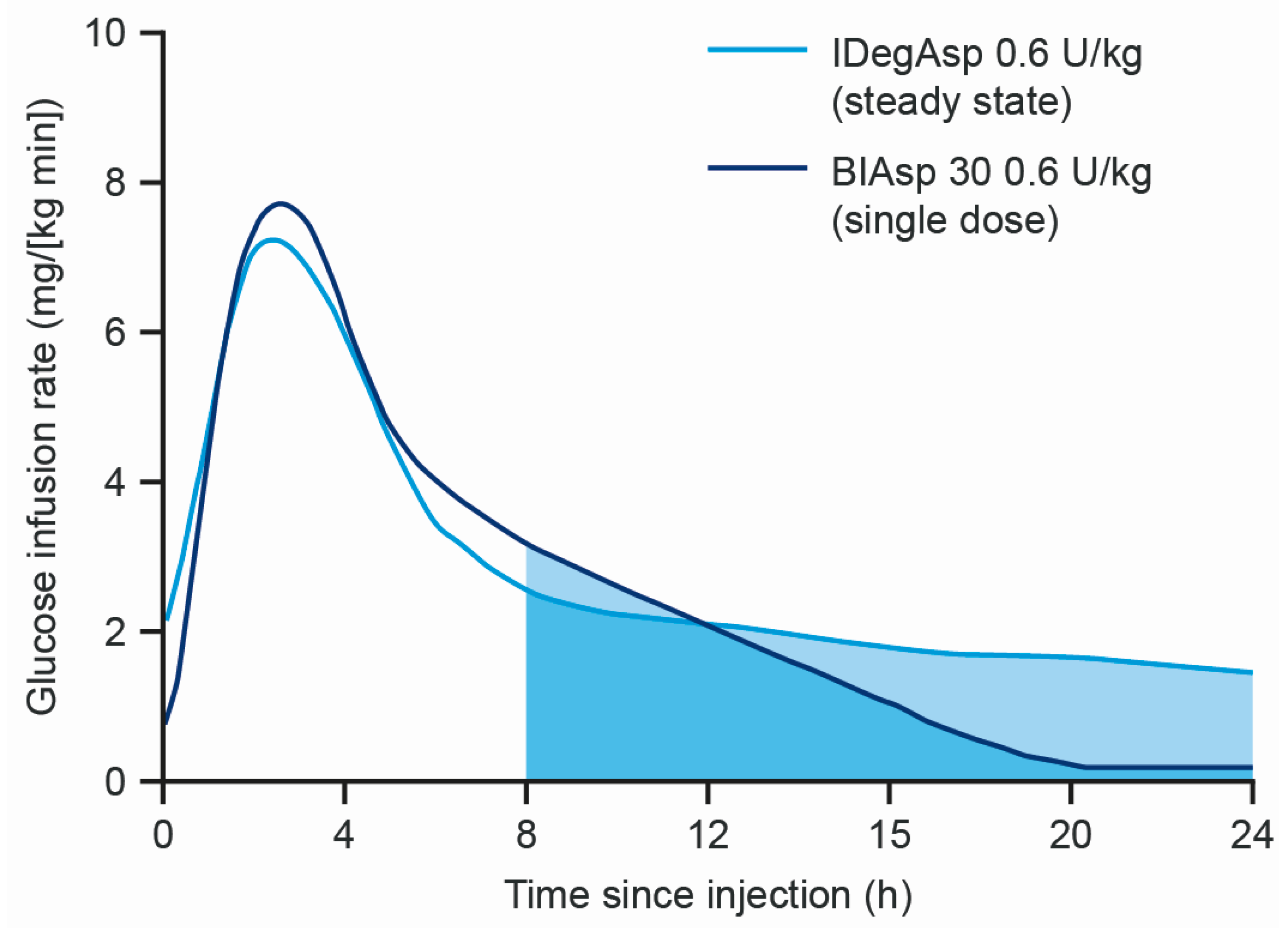

| Study | Study Design | Mean HbA1c | Mean FPG (mmol/L) | Hypoglycaemia (Overall Confirmed or Nocturnal Confirmed) |
|---|---|---|---|---|
| Initiation of IDegAsp (Insulin-Naïve Patients) | ||||
| BOOST JAPAN (Onishi et al.) [45] NCT01272193 | Phase 3a 26-week, open-label, treat-to-target n = 296 (Japanese) IDegAsp OD vs. glargine U100 OD | ETD IDegAsp/glargine: −0.28% [95% CI −0.46, −0.10]; p < 0.01 | ETD IDegAsp/glargine: 0.15 [95% CI −0.29, 0.60]; p = ns | Overall: ERR IDegAsp/glargine 0.73 [95% CI 0.50, 1.08]; p = ns Nocturnal: ERR IDegAsp/glargine 0.75 [95% CI 0.34, 1.64]; p = ns |
| START TWICE DAILY (Franek et al.) [46] NCT01513590 | Phase 3b 26-week, open-label, parallel-group, treat-to-target n = 394 IDegAsp BID vs. BIAsp 30 BID | ETD IDegAsp/BIAsp 30: 0.02% [95% CI −0.12, 0.17] | ETD IDegAsp/BIAsp 30 BID: −1.00 [95% CI −1.4, −0.6]; p < 0.001 | Overall: ERR IDegAsp/BIAsp 30: 0.46 [95% CI 0.35, 0.61]; p < 0.001 Nocturnal: ERR IDegAsp/BIAsp 30: 0.25 [95% CI 0.16, 0.38]; p < 0.001 |
| Kumar et al. PLoS One 2016 [47] NCT01045707 [core] NCT01169766 [ext] | Phase 3 26-week core trial; 26-week extension; open-label, parallel-group, treat-to-target n = 530 IDegAsp OD vs. glargine OD | ETD IDegAsp/glargine: −0.08% [95% CI −0.26, 0.09] at week 52 | ETD IDegAsp/glargine: 0.28 [95% CI −0.14, 0.69] at week 52 | Overall: ERR IDegAsp/glargine: 1.86 [95% CI 1.42, 2.44]; p < 0.0001 Nocturnal: ERR IDegAsp/glargine: 0.25 [95% CI 0.14, 0.47]; p < 0.0001 |
| SIMPLE USE (Park et al.) [48] NCT01365507 | Phase 3b 26-week, open-label, parallel-group, treat-to-target n = 276 IDegAsp OD titrated Q2W using simple algorithm vs. IDegAsp OD titrated OW using stepwise algorithm | ETD IDegAspSimple/Stepwise: −0.2% [95% CI −0.4, 0.02] | ETD IDegAspSimple/ Stepwise: −0.4 [95% CI −0.9, 0.09] | Overall: ERR IDegAspSimple/Stepwise: 1.8 [95% CI 1.1, 2.9] Nocturnal: ERR IDegAspSimple/Stepwise: 1.1 [95% CI 0.5, 2.4] |
| Intensification from Basal Insulin to IDegAsp | ||||
| Step-by-Step trial (Gupta et al.) [49] NCT02906917 | Phase 3 38-week, open-label, treat-to-target n = 532 Inadequately controlled on basal insulin ± OADs IDegAsp OD vs. glargine U100 OD + IAsp OD for 26 weeks then IDegAsp OD/BID vs. glargine U100 OD + IAsp OD/BID/TID, for 12 weeks | Weeks 0–26: ETD 0.07% [95% CI −0.06, 0.21] Week 38: ETD 0.09% [95% CI −0.04, 0.22] | Week 26: IDegAsp: −2.3 (SD 2.9) glargine U100 + IAsp: −2.3 (SD 3.3) Week 38: IDegAsp: −2.7 (SD 3.0) glargine U100 + IAsp: −2.3 (SD 3.1) | Overall: IDegAsp/glargine: Weeks 0–26: ERR 0.90 [95% CI 0.67, 1.02] Weeks 0–38: ERR 0.86 [95% CI 0.65, 1.14] Nocturnal: Weeks 0–26: ERR 0.55 [95% CI 0.34, 0.90] Weeks 0–38: ERR 0.61 [95% CI 0.40, 0.93] |
| Kumar et al. Diabet Med 2017 [50] NCT01045447 | Phase 3 26-week, open-label, treat-to-target n = 465 IDegAsp OD vs. glargine OD | ETD IDegAsp/glargine: −0.03% [95% CI −0.20, 0.14]; p = ns | ETD IDegAsp/glargine: 0.33 [95% CI −0.11, 0.77]; p = ns | Overall: ERR IDegAsp/glargine: 1.43 [95% CI 1.07, 1.92]; p < 0.05 Nocturnal: ERR IDegAsp/glargine: 0.80 [95% CI 0.49, 1.30]; p = ns |
| Intensify All (Kaneko et al.) [51] NCT01059812 | Phase 3 26-week, open-label, treat-to-target n = 424 (Asian) Inadequately controlled on basal, premixed or self-mixed (20%–40% rapid-acting) insulin ± metformin IDegAsp BID vs. BIAsp 30 BID | ETD IDegAsp/BIAsp 30: 0.05% [95% CI −0.10, 0.20] | ETD IDegAsp/BIAsp 30: –1.06 [95% CI −1.43, −0.70]; p < 0.001 | Overall: ERR IDegAsp/BIAsp 30: 1.00 [95% CI 0.76, 1.32]; p = ns Nocturnal: ERR IDegAsp/BIAsp 30: 0.67 [95% CI 0.43, 1.06]; p = ns |
| Intensification from IDegAsp OD to IDegAsp BID | ||||
| Step-by-Step trial (Philis-Tsimikas et al.) [43] NCT02906917 | As above | As above | As above | As above |
| Switching from Premixed or Basal-bolus Insulin to IDegAsp | ||||
| Intensify Premix I (Fulcher et al.) [41] NCT01009580 | Phase 3a 26-week, open-label, treat-to-target n = 447 Inadequately controlled with premixed or self-mixed insulin ± OADs IDegAsp BID vs. BIAsp 30 BID | IDegAsp/BIAsp30 ETD: −0.03% [95% CI −0.18, 0.13] | IDegAsp/BIAsp30: ETD: −1.14 [95% CI −1.53, −0.76]; p < 0.001 | Overall: ERR IDegAsp/BIAsp30: 0.68 [95% CI 0.52, 0.89]; p = 0.0049 Nocturnal: ERR IDegAsp/BIAsp30: 0.27 [95% CI 0.18, 0.41]; p < 0.0001 |
| Intensify All (Kaneko et al.) [51] NCT01059812 | As above | As above | As above | As above |
| Intensify Premix I/Intensify All pooled analysis (Christiansen et al.) [52] NCT01513590 | Pooled analysis of Intensify Premix I and Intensify All Inadequately controlled with premixed insulin ± OADs OR basal or premixed or self-mixed insulin ± metformin, respectively IDegAsp BID vs. BIAsp 30 BID | IDegAsp vs. BIAsp 30: ETD 0.00% [95% CI −0.11, 0.10]; p = ns | IDegAsp vs. BIAsp 30: ETD −1.12 [95% CI −1.38, −0.85]; p < 0.0001 | Overall: ERR IDegAsp vs. BIAsp 30: 0.81 [95% CI 0.67, 0.98]; p = 0.03 Nocturnal: ERR IDegAsp vs. BIAsp 30: 0.43 [95% CI 0.31, 0.59]; p < 0.0001 |
| Step-by-Step trial (Philis-Tsimikas et al.) [43] NCT02906917 | As above | As above | As above | As above |
| Yang et al. Diab Obesity Metab 2019 [53] NCT02762578 | Phase 3a 26-week, open-label, treat-to-target n = 543 IDegAsp BID vs. BIAsp 30 BID | IDegAsp vs. BIAsp 30: ETD 0.08% [95% CI −0.20, 0.05]; p < 0.0001 | IDegAsp vs. BIAsp 30: ETD −1.42 [95% CI −1.74, −1.10]; p < 0.0001 | Overall: ERR IDegAsp vs. BIAsp30: 0.57 [95% CI 0.42, 0.77]; p = 0.0002 Nocturnal: ERR IDegAsp vs. BIAsp30: 0.53 [95% CI 0.33, 0.87]; p = 0.0112 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glastras, S.J.; Cohen, N.; Dover, T.; Kilov, G.; MacIsaac, R.J.; McGill, M.; Fulcher, G.R. The Clinical Role of Insulin Degludec/Insulin Aspart in Type 2 Diabetes: An Empirical Perspective from Experience in Australia. J. Clin. Med. 2020, 9, 1091. https://doi.org/10.3390/jcm9041091
Glastras SJ, Cohen N, Dover T, Kilov G, MacIsaac RJ, McGill M, Fulcher GR. The Clinical Role of Insulin Degludec/Insulin Aspart in Type 2 Diabetes: An Empirical Perspective from Experience in Australia. Journal of Clinical Medicine. 2020; 9(4):1091. https://doi.org/10.3390/jcm9041091
Chicago/Turabian StyleGlastras, Sarah J., Neale Cohen, Thomas Dover, Gary Kilov, Richard J. MacIsaac, Margaret McGill, and Greg R. Fulcher. 2020. "The Clinical Role of Insulin Degludec/Insulin Aspart in Type 2 Diabetes: An Empirical Perspective from Experience in Australia" Journal of Clinical Medicine 9, no. 4: 1091. https://doi.org/10.3390/jcm9041091
APA StyleGlastras, S. J., Cohen, N., Dover, T., Kilov, G., MacIsaac, R. J., McGill, M., & Fulcher, G. R. (2020). The Clinical Role of Insulin Degludec/Insulin Aspart in Type 2 Diabetes: An Empirical Perspective from Experience in Australia. Journal of Clinical Medicine, 9(4), 1091. https://doi.org/10.3390/jcm9041091






