Associations of Objectively-Assessed Physical Activity and Sedentary Time with Hippocampal Gray Matter Volume in Children with Overweight/Obesity
Abstract
1. Introduction
2. Material and Methods
2.1. Participants and Study Design
2.2. Accelerometer Data Collection and Processing
2.3. Magnetic Resonance Imaging (MRI) Data Acquisition and Processing
2.4. Confounders
2.5. Statistics
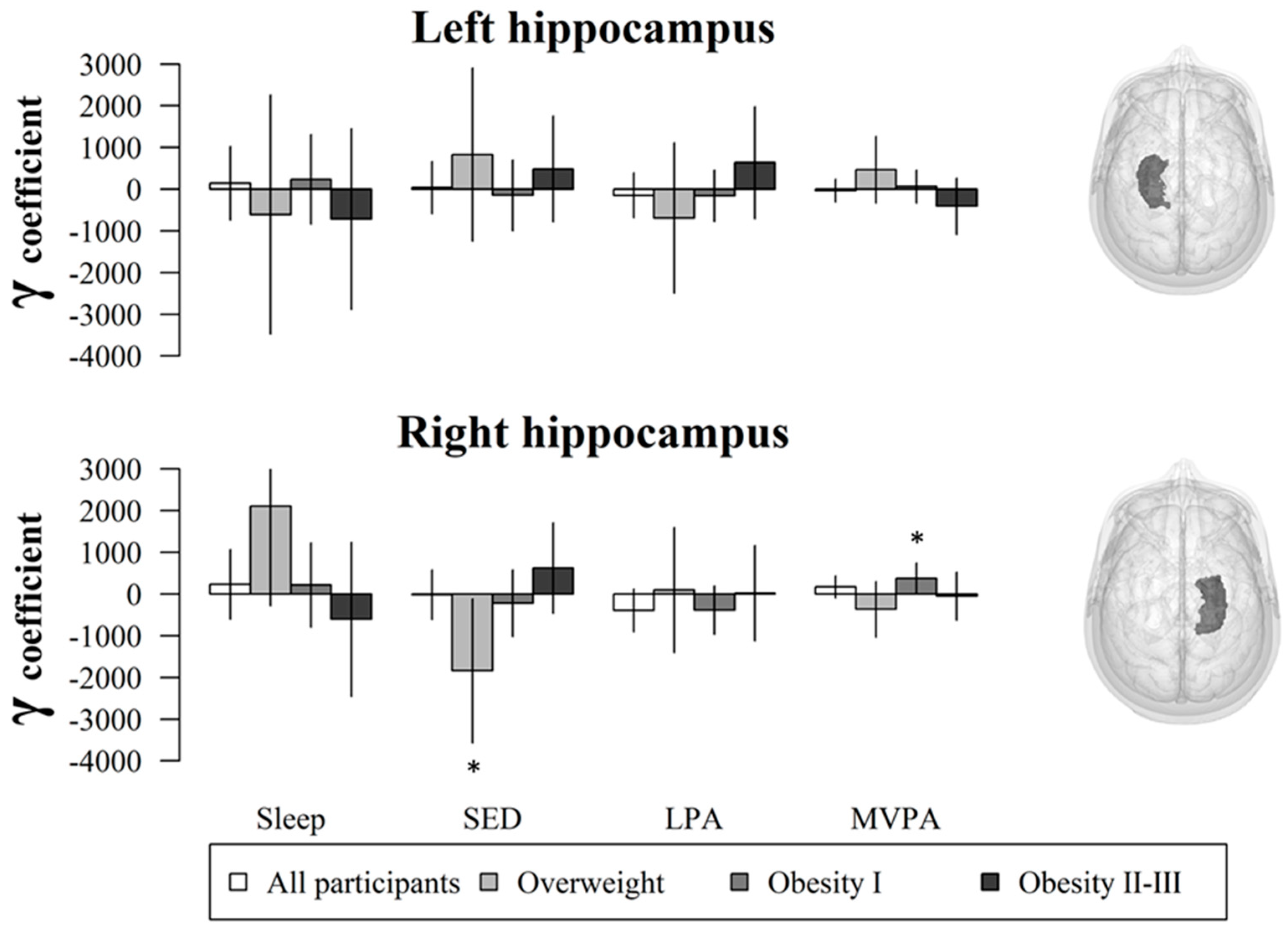
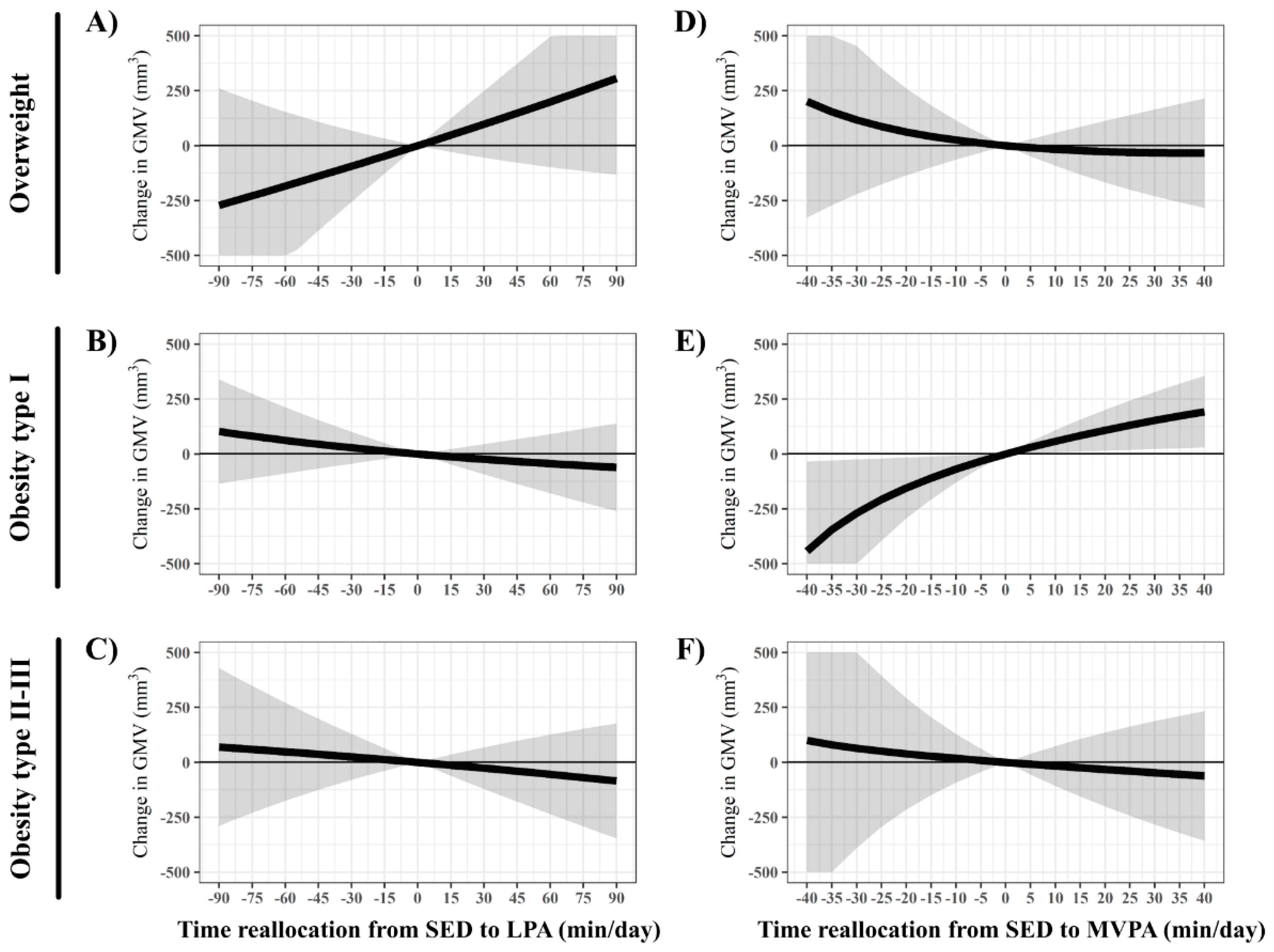
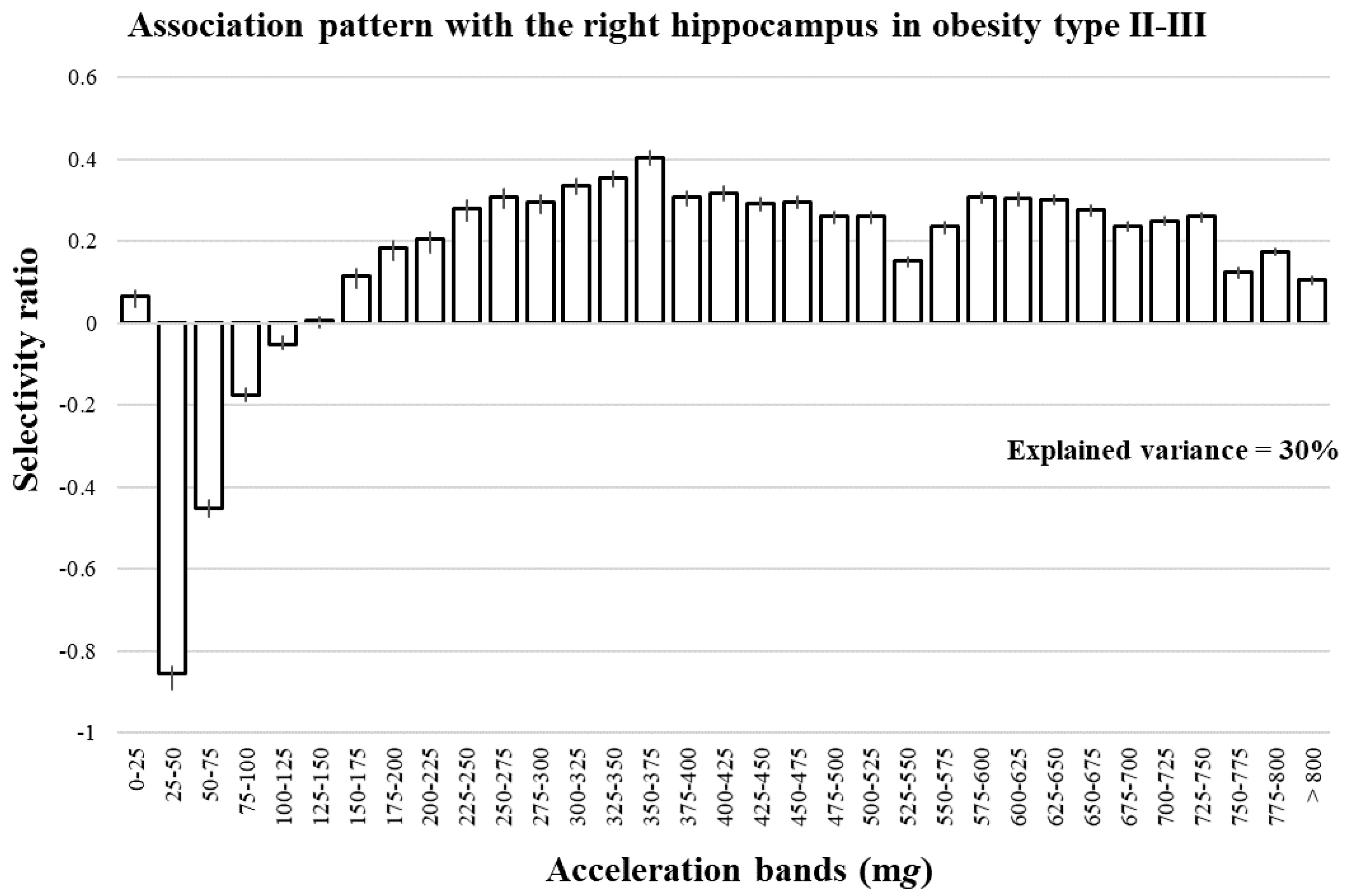
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Compositional Data Transformation
Appendix B
Interpretation of Selectivity Ratio in Multivariate Pattern Analysis
References
- 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Cohen, N.; Eichenbaum, H. Memory, Amnesia and the Hippocampal System; MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Davachi, L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006, 16, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Hillman, C.H.; Cohen, N.J. Aerobic fitness enhances relational memory in preadolescent children: The FITKids randomized control trial. Hippocampus 2012, 22, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Cornejo, I.; Cadenas-Sanchez, C.; Contreras-Rodriguez, O.; Verdejo-Roman, J.; Mora-Gonzalez, J.; Migueles, J.H.; Henriksson, P.; Davis, C.L.; Verdejo-Garcia, A.; Catena, A.; et al. A whole brain volumetric approach in overweight/obese children: Examining the association with different physical fitness components and academic performance. The ActiveBrains project. Neuroimage 2017, 159, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.S.; Hinds, J.W. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science 1977, 197, 1092–1094. [Google Scholar] [CrossRef]
- Neve, R.L.; Finch, E.A.; Bird, E.D.; Benowitz, L.I. Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3638–3642. [Google Scholar] [CrossRef]
- Kandola, A.; Hendrikse, J.; Lucassen, P.J.; Yücel, M. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: Practical implications for mental health treatment. Front. Hum. Neurosci. 2016, 10, 373–398. [Google Scholar] [CrossRef]
- Jaroudi, W.; Garami, J.; Garrido, S.; Hornberger, M.; Keri, S.; Moustafa, A.A. Factors underlying cognitive decline in old age and Alzheimer’s disease: The role of the hippocampus. Rev. Neurosci. 2017, 28, 705–714. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical Activity, Fitness, Cognitive Function, and Academic Achievement in Children: A Systematic Review. Med. Sci. Sports Exerc. 2016, 48, 1197–1222. [Google Scholar] [CrossRef]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef]
- WHO. Global Recommendations on Physical Activity for Health; WHO: Geneva, Switzerland, 2010; ISBN 978-92-4-159-997-9. [Google Scholar]
- Ruotsalainen, I.; Renvall, V.; Gorbach, T.; Syväoja, H.J.; Tammelin, T.H.; Karvanen, J.; Parviainen, T. Aerobic fitness, but not physical activity, is associated with grey matter volume in adolescents. Behav. Brain Res. 2019, 362, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Herting, M.M.; Keenan, M.F.; Nagel, B.J. Aerobic Fitness Linked to Cortical Brain Development in Adolescent Males: Preliminary Findings Suggest a Possible Role of BDNF Genotype. Front. Hum. Neurosci. 2016, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Sallis, J.F. Self-report measures of children’s physical activity. J. Sch. Health 1991, 61, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Aminian, S.; et al. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Migueles, J.H.; Cadenas-Sanchez, C.; Ekelund, U.; Delisle Nyström, C.; Mora-Gonzalez, J.; Löf, M.; Labayen, I.; Ruiz, J.R.; Ortega, F.B. Accelerometer Data Collection and Processing Criteria to Assess Physical Activity and Other Outcomes: A Systematic Review and Practical Considerations. Sport. Med. 2017, 47, 1821–1845. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Palarea-Albaladejo, J.; Dontje, M.L.; Skelton, D.A. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: A novel compositional data analysis approach. PLoS ONE 2015, 10, e0139984. [Google Scholar] [CrossRef]
- Dumuid, D.; Stanford, T.E.; Martin-Fernández, J.A.; Pedišić, Ž.; Maher, C.A.; Lewis, L.K.; Hron, K.; Katzmarzyk, P.T.; Chaput, J.P.; Fogelholm, M.; et al. Compositional data analysis for physical activity, sedentary time and sleep research. Stat. Methods Med. Res. 2018, 27, 3726–3738. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- bin Shahril, M.R.; bte Ahmad, A.; bte Zainuddin, L.R.; bte Ismail, K.F.; Aung, M.M.T. Association between physical activity and health-related quality of life in children: A cross-sectional study. Health Qual. Life Outcomes 2016, 14, 71. [Google Scholar]
- Ou, X.; Andres, A.; Pivik, R.T.; Cleves, M.A.; Badger, T.M. Brain gray and white matter differences in healthy normal weight and obese children. J. Magn. Reson. Imaging 2015, 42, 1205–1213. [Google Scholar] [CrossRef]
- Cadenas-Sánchez, C.; Mora-González, J.; Migueles, J.H.; Martín-Matillas, M.; Gómez-Vida, J.; Escolano-Margarit, M.V.; Maldonado, J.; Enriquez, G.M.; Pastor-Villaescusa, B.; de Teresa, C.; et al. An exercise-based randomized controlled trial on brain, cognition, physical health and mental health in overweight/obese children (ActiveBrains project): Rationale, design and methods. Contemp. Clin. Trials 2016, 47, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Massa, G. Defining morbid obesity in children based on BMI 40 at age 18 using the extended international (IOTF) cut-offs. Pediatr. Obes. 2014, 9, e94–e98. [Google Scholar] [CrossRef] [PubMed]
- Migueles, J.H.; Cadenas-Sanchez, C.; Rowlands, A.V.; Henriksson, P.; Shiroma, E.J.; Acosta, F.M.; Rodriguez-Ayllon, M.; Esteban-Cornejo, I.; Plaza-Florido, A.; Gil-Cosano, J.J.; et al. Comparability of accelerometer signal aggregation metrics across placements and dominant wrist cut points for the assessment of physical activity in adults. Sci. Rep. 2019, 9, 18235. [Google Scholar] [CrossRef]
- Migueles, J.H.; Cadenas-Sanchez, C.; Tudor-Locke, C.; Löf, M.; Esteban-Cornejo, I.; Molina-Garcia, P.; Mora-Gonzalez, J.; Rodriguez-Ayllon, M.; Garcia-Marmol, E.; Ekelund, U.; et al. Comparability of published cut-points for the assessment of physical activity: Implications for data harmonization. Scand. J. Med. Sci. Sport. 2019, 29, 566–574. [Google Scholar] [CrossRef]
- Migueles, J.H.; Rowlands, A.V.; Huber, F.; Sabia, S.; van Hees, V.T. GGIR: A Research Community—Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes From Multi-Day Raw Accelerometer Data. J. Meas. Phys. Behav. 2019, 2, 188–196. [Google Scholar] [CrossRef]
- Van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef]
- Van Hees, V.T.; Sabia, S.; Anderson, K.N.; Denton, S.J.; Oliver, J.; Catt, M.; Abell, J.G.; Kivimäki, M.; Trenell, M.I.; Singh-Manoux, A. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS ONE 2015, 10, e0142533. [Google Scholar] [CrossRef]
- Van Hees, V.T.; Sabia, S.; Jones, S.E.; Wood, A.R.; Anderson, K.N.; Kivimäki, M.; Frayling, T.M.; Pack, A.I.; Bucan, M.; Trenell, M.I.; et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Hildebrand, M.; Hansen, B.H.; van Hees, V.T.; Ekelund, U.; Van Hees, V.T.; Ekelund, U. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand. J. Med. Sci. Sport. 2017, 27, 1814–1823. [Google Scholar] [CrossRef]
- Hildebrand, M.; van Hees, V.T.; Hansen, B.H.; Ekelund, U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011, 56, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef]
- Moore, S.A.; McKay, H.A.; Macdonald, H.; Nettlefold, L.; Baxter-Jones, A.D.G.; Cameron, N.; Brasher, P.M.A. Enhancing a somatic maturity prediction model. Med. Sci. Sports Exerc. 2015, 47, 1755–1764. [Google Scholar] [CrossRef]
- Crova, C.; Struzzolino, I.; Marchetti, R.; Masci, I.; Vannozzi, G.; Forte, R.; Pesce, C. Cognitively challenging physical activity benefits executive function in overweight children. J. Sports Sci. 2014, 32, 201–211. [Google Scholar] [CrossRef]
- Kvalheim, O.M.; Karstang, T.V. Interpretation of latent-variable regression models. Chemom. Intell. Lab. Syst. 1989, 7, 39–51. [Google Scholar] [CrossRef]
- Aadland, E.; Kvalheim, O.M.; Anderssen, S.A.; Resaland, G.K.; Andersen, L.B. The multivariate physical activity signature associated with metabolic health in children. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 77. [Google Scholar] [CrossRef]
- Xu, Q.-S.; Liang, Y.-Z. Monte Carlo cross validation. Chemom. Intell. Lab. Syst. 2001, 56, 1–11. [Google Scholar] [CrossRef]
- Rajalahti, T.; Kvalheim, O.M. Multivariate data analysis in pharmaceutics: A tutorial review. Int. J. Pharm. 2011, 417, 280–290. [Google Scholar] [CrossRef]
- Aadland, E.; Kvalheim, O.M.; Anderssen, S.A.; Resaland, G.K.; Andersen, L.B.O. The Triaxial Physical Activity Signature Associated with Metabolic Health in Children. Med. Sci. Sports Exerc. 2019, 51, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Kuzik, N.; Carson, V.; Andersen, L.B.; Sardinha, L.B.; Grøntved, A.; Hansen, B.H.; Ekelund, U. Physical Activity and Sedentary Time Associations with Metabolic Health Across Weight Statuses in Children and Adolescents. Obesity 2017, 25, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Drollette, E.S.; Hillman, C.H. Walking effects on memory in children: Implications for individual differences in BMI. Ment. Health Phys. Act. 2020, 18, 100317. [Google Scholar] [CrossRef]
- Kang, E.B.; Koo, J.H.; Jang, Y.C.; Yang, C.H.; Lee, Y.; Cosio-Lima, L.M.; Cho, J.Y. Neuroprotective Effects of Endurance Exercise Against High-Fat Diet-Induced Hippocampal Neuroinflammation. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Do, D.; Jack, C. Modifiable factors that alter the size of the hippocampus with ageing. Nat. Rev. Neurol. 2012, 8, 189–202. [Google Scholar] [CrossRef]
- Park, H.-S.; Cho, H.-S.; Kim, T.-W. Physical exercise promotes memory capability by enhancing hippocampal mitochondrial functions and inhibiting apoptosis in obesity-induced insulin resistance by high fat diet. Metab. Brain Dis. 2018, 33, 283–292. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Mattson, M.P. Bidirectional metabolic regulation of neurocognitive function. Neurobiol. Learn. Mem. 2011, 96, 507–516. [Google Scholar] [CrossRef]
- Stranahan, A.M. Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience 2015, 309, 125–139. [Google Scholar] [CrossRef]
- Burgess, N.; Maguire, E.A.; O’Keefe, J. The human hippocampus and spatial and episodic memory. Neuron 2002, 35, 625–641. [Google Scholar] [CrossRef]
- Gold, J.J.; Trauner, D.A. Hippocampal volume and memory performance in children with perinatal stroke. Pediatr. Neurol. 2014, 50, 18–25. [Google Scholar] [CrossRef]
- Lynch, K.M.; Shi, Y.; Toga, A.W.; Clark, K.A.; Pediatric Imaging, Neurocognition and Genetics Study. Hippocampal Shape Maturation in Childhood and Adolescence. Cereb. Cortex 2019, 29, 3651–3665. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, M.H.; Migueles, J.H.; Cadenas-Sanchez, C.; Henriksson, P.; Mora-Gonzalez, J.; Henriksson, H.; Labayen, I.; Löf, M.; Esteban-Cornejo, I.; Ortega, F.B. Hip and wrist accelerometers showed consistent associations with fitness and fatness in children aged 8–12 years. Acta Paediatr. 2019. [Google Scholar] [CrossRef] [PubMed]
- McGregor, D.; Palarea-Albaladejo, J.; Dall, P.; Hron, K.; Chastin, S. Cox regression survival analysis with compositional covariates: Application to modelling mortality risk from 24-h physical activity patterns. Stat. Methods Med. Res. 2019. [Google Scholar] [CrossRef]
- Pedišić, Ž.; Dumuid, D.; Olds, T.S. Integrating sleep, sedentary behaviour, and physical activity research in the emerging field of time-use epidemiology: Definitions, concepts, statistical methods, theoretical framework, and future directions. Kinesiology 2017, 49, 252–269. [Google Scholar]

| All (n = 93, 37 girls) | Overweight (n = 23, 9 girls) | Obesity I (n = 41, 15 girls) | Obesity II–III (n = 29, 13 girls) | |
|---|---|---|---|---|
| Age (years) | 10.01 (1.12) | 10.13 (1.08) | 10.29 (1.04) | 9.51 (1.14) |
| PHV (years) | –2.31 (0.97) | –2.36 (1.04) | –2.1 (0.93) | –2.58 (0.91) |
| Weight (kg) | 55.67 (10.69) | 46.32 (7.30) | 56.92 (9.63) | 61.74 (9.31) |
| Height (cm) | 143.95 (8.10) | 142.16 (8.80) | 146.59 (7.78) | 141.84 (7.08) |
| BMI (kg/m2) | 26.74 (3.63) | 22.64 (1.41) | 26.26 (2.06) | 30.68 (2.36) |
| Total brain volume (mm3) | 1202.07 (106.67) | 1210.02 (99.41) | 1221.03 (94.93) | 1169.54 (122.50) |
| Parental university level, % | ||||
| Neither parent | 68 | 57 | 59 | 90 |
| One parent | 16 | 17 | 22 | 7 |
| Both parents | 16 | 26 | 19 | 3 |
| Gray matter volume | ||||
| L hippocampus (mm3) | 3468.73 (371.48) | 3387.17 (348.96) | 3572.49 (346.4) | 3386.71 (397.67) |
| R hippocampus (mm3) | 3597.99 (382.49) | 3568.46 (420.35) | 3709.9 (354.77) | 3463.19 (352.41) |
| Physical activity | ||||
| SED (min/day) | 561.39 (60.85) | 534.25 (71.12) | 559.35 (50.59) | 585.78 (57.52) |
| LPA (min/day) | 275.36 (39.75) | 277.85 (40.42) | 273.16 (43.31) | 276.49 (34.83) |
| MVPA (min/day) | 54.61 (20.91) | 61.76 (26.79) | 53.84 (19.66) | 50.05 (16) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migueles, J.H.; Cadenas-Sanchez, C.; Esteban-Cornejo, I.; Torres-Lopez, L.V.; Aadland, E.; Chastin, S.F.; Erickson, K.I.; Catena, A.; Ortega, F.B. Associations of Objectively-Assessed Physical Activity and Sedentary Time with Hippocampal Gray Matter Volume in Children with Overweight/Obesity. J. Clin. Med. 2020, 9, 1080. https://doi.org/10.3390/jcm9041080
Migueles JH, Cadenas-Sanchez C, Esteban-Cornejo I, Torres-Lopez LV, Aadland E, Chastin SF, Erickson KI, Catena A, Ortega FB. Associations of Objectively-Assessed Physical Activity and Sedentary Time with Hippocampal Gray Matter Volume in Children with Overweight/Obesity. Journal of Clinical Medicine. 2020; 9(4):1080. https://doi.org/10.3390/jcm9041080
Chicago/Turabian StyleMigueles, Jairo H., Cristina Cadenas-Sanchez, Irene Esteban-Cornejo, Lucia V. Torres-Lopez, Eivind Aadland, Sébastien F. Chastin, Kirk I. Erickson, Andres Catena, and Francisco B. Ortega. 2020. "Associations of Objectively-Assessed Physical Activity and Sedentary Time with Hippocampal Gray Matter Volume in Children with Overweight/Obesity" Journal of Clinical Medicine 9, no. 4: 1080. https://doi.org/10.3390/jcm9041080
APA StyleMigueles, J. H., Cadenas-Sanchez, C., Esteban-Cornejo, I., Torres-Lopez, L. V., Aadland, E., Chastin, S. F., Erickson, K. I., Catena, A., & Ortega, F. B. (2020). Associations of Objectively-Assessed Physical Activity and Sedentary Time with Hippocampal Gray Matter Volume in Children with Overweight/Obesity. Journal of Clinical Medicine, 9(4), 1080. https://doi.org/10.3390/jcm9041080








