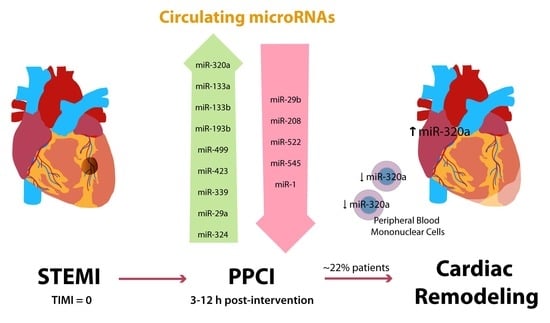
Circulating miR-320a as a Predictive Biomarker for Left Ventricular Remodelling in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Blood Extraction
2.2. Human Samples
2.3. RNA Isolation and miRNA Analysis
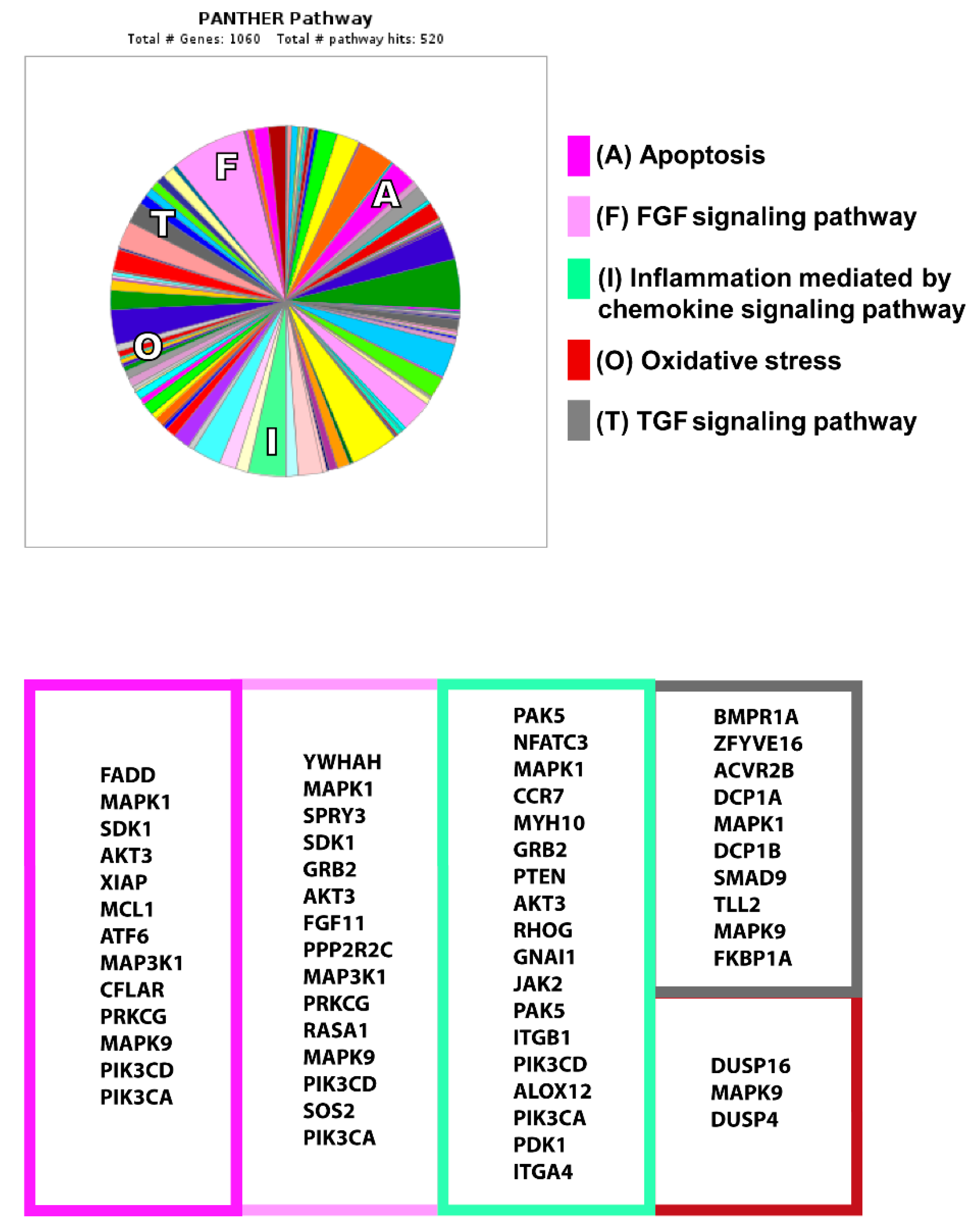
2.4. In Silico miRNA Studies
2.5. Statistical Analysis
3. Results
3.1. Analysis of the Clinical Data of the Subjects
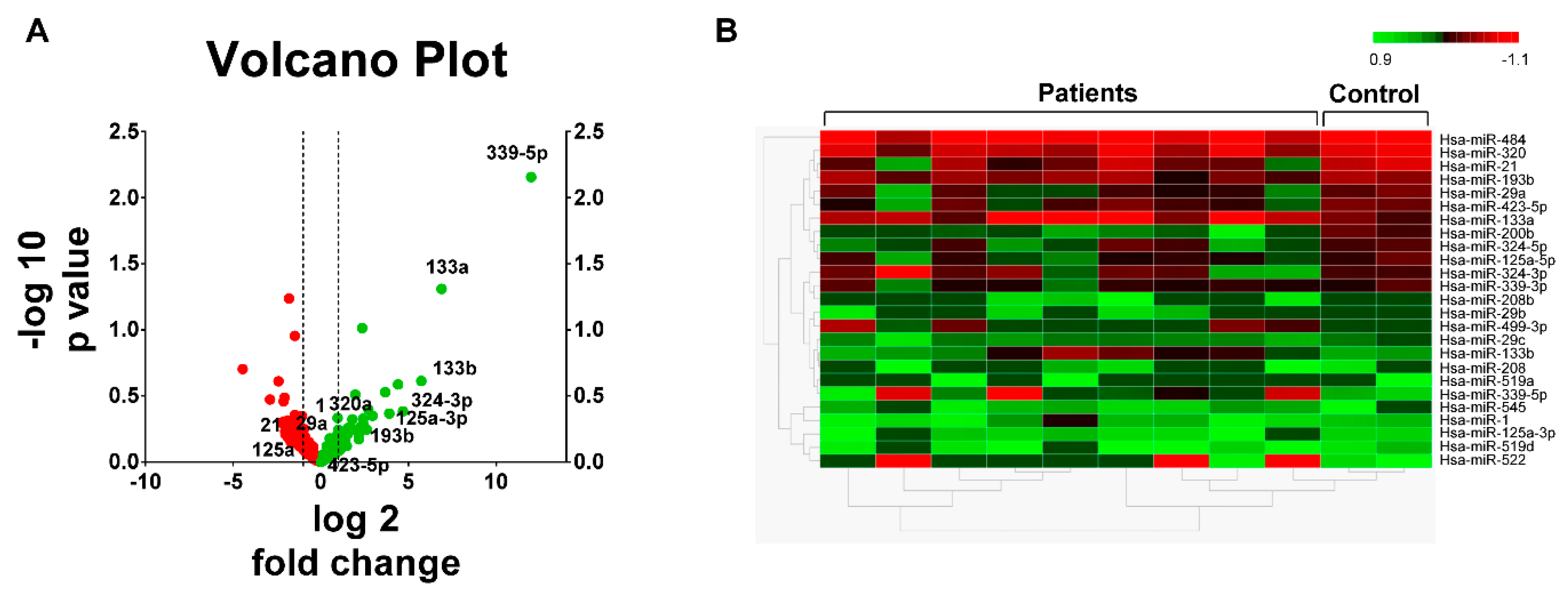
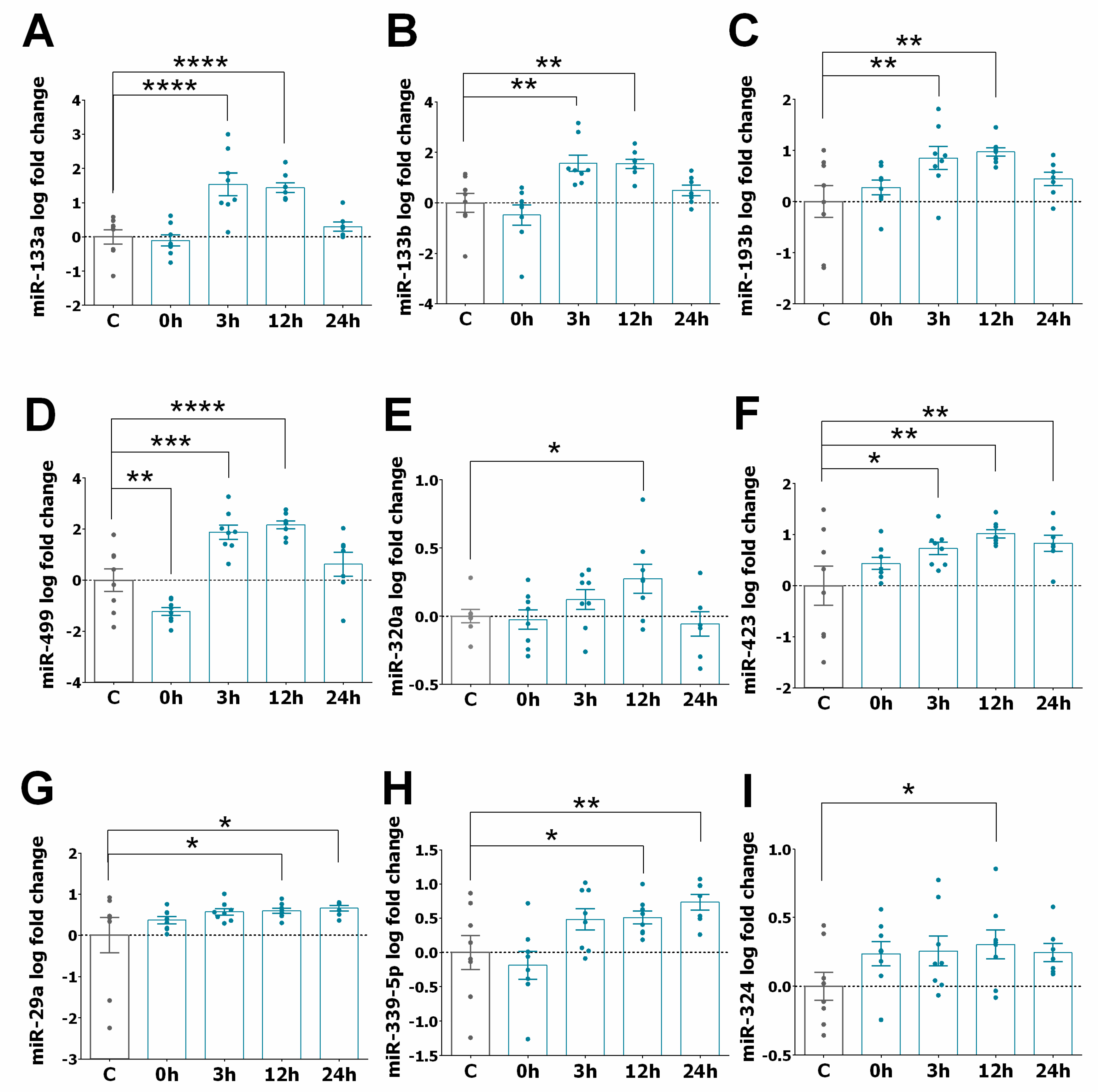
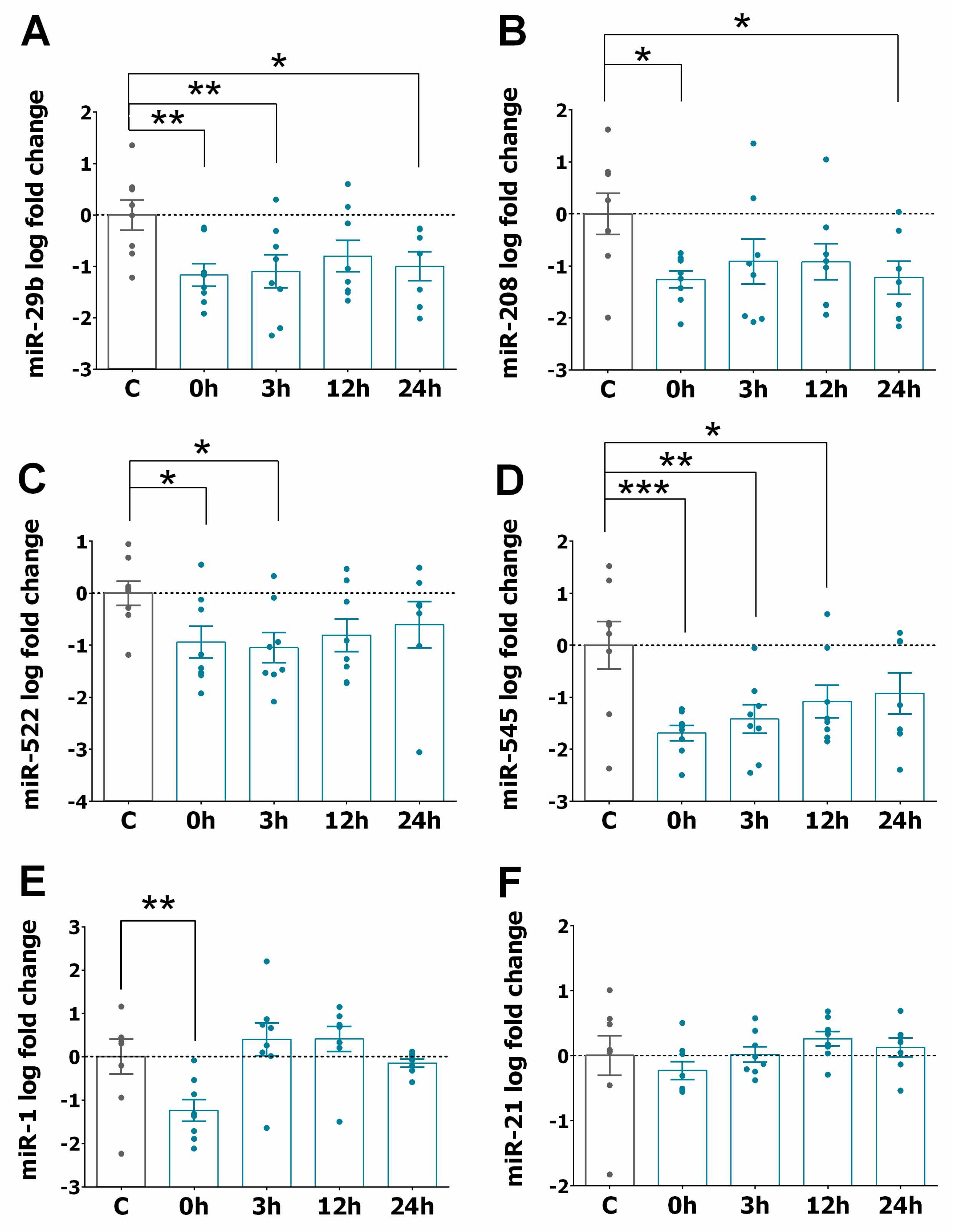
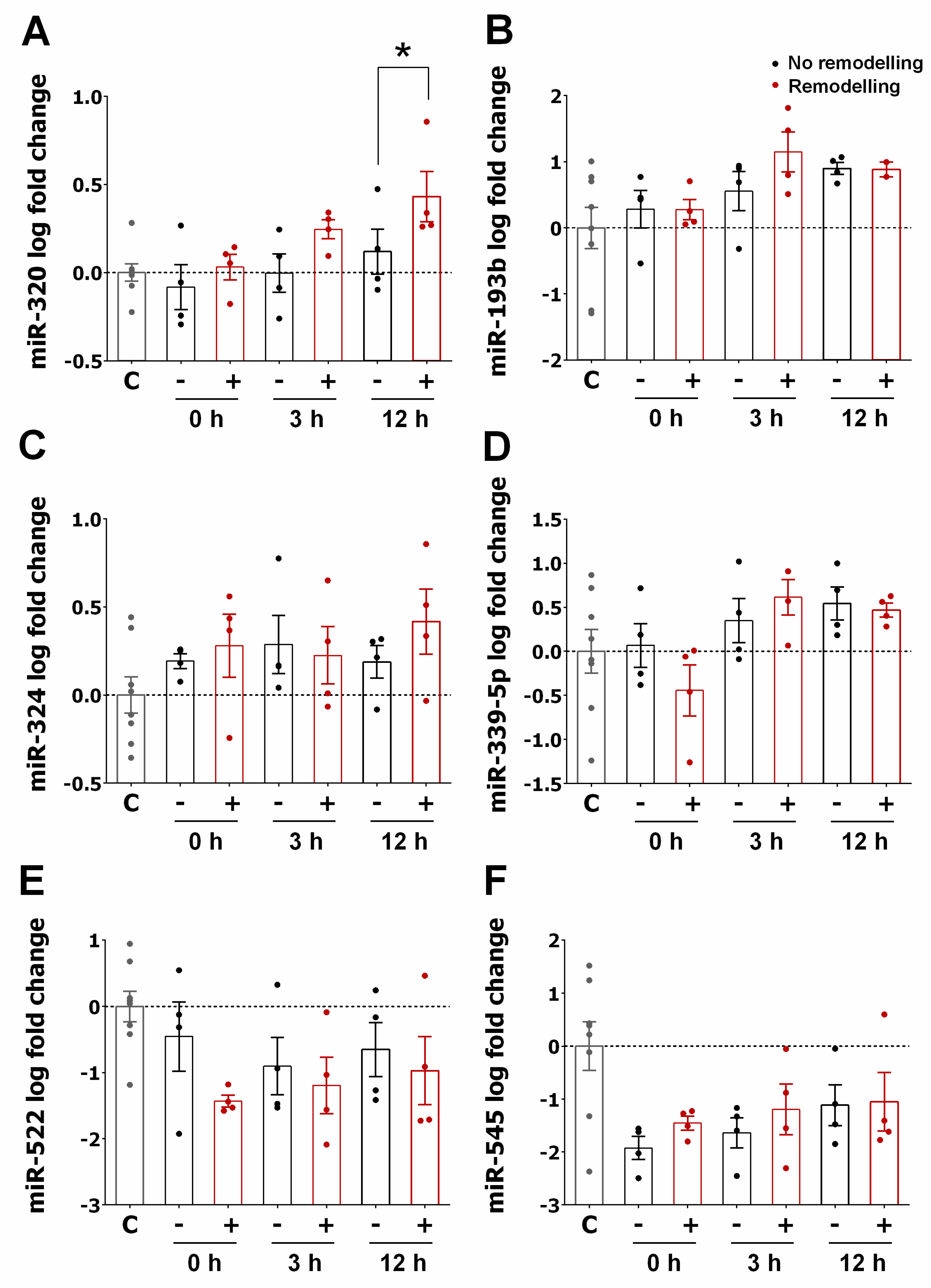
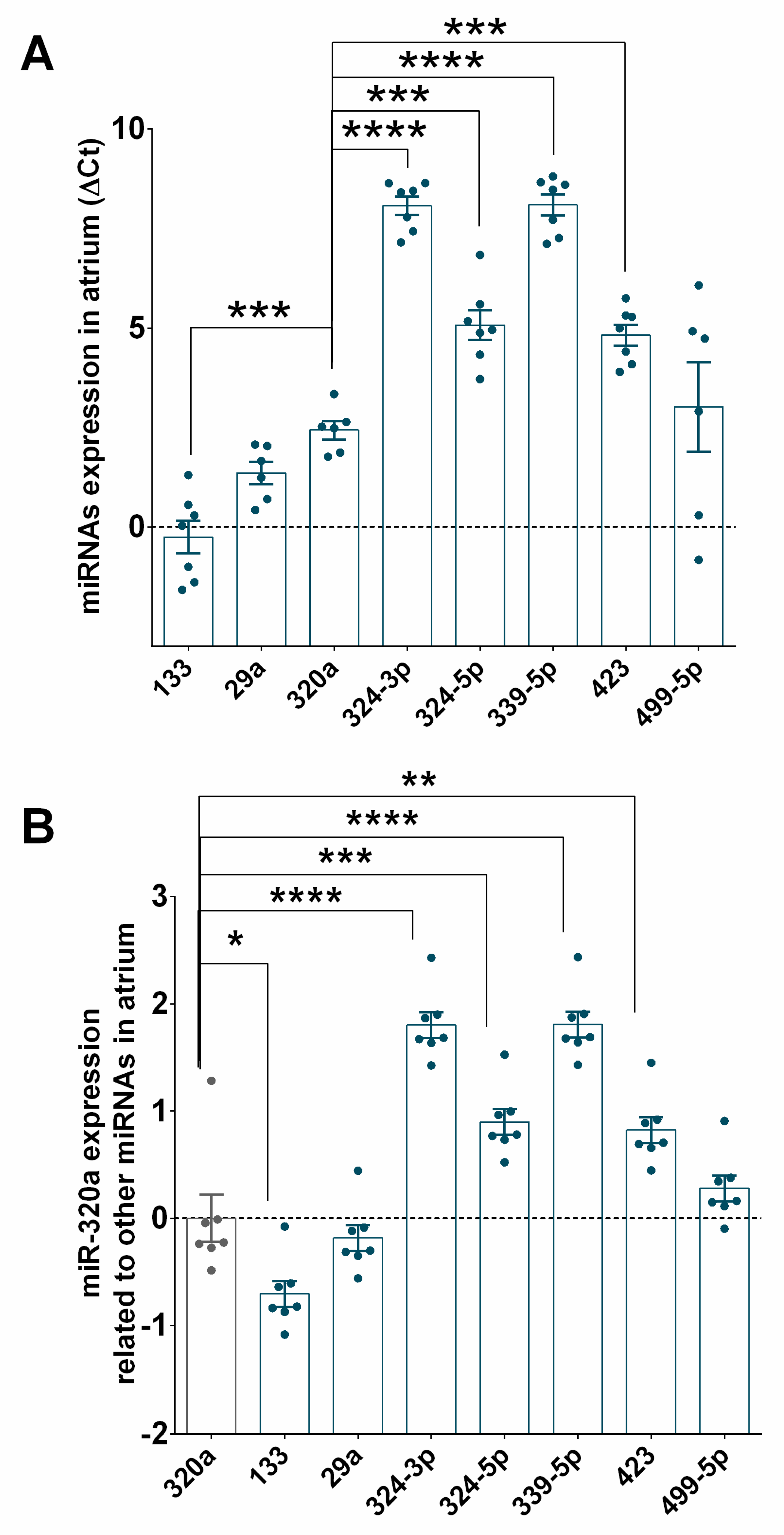
3.2. miRNA Expression Profiles in STEMI
3.3. miRNA Expression Pattern in STEMI with LVAR
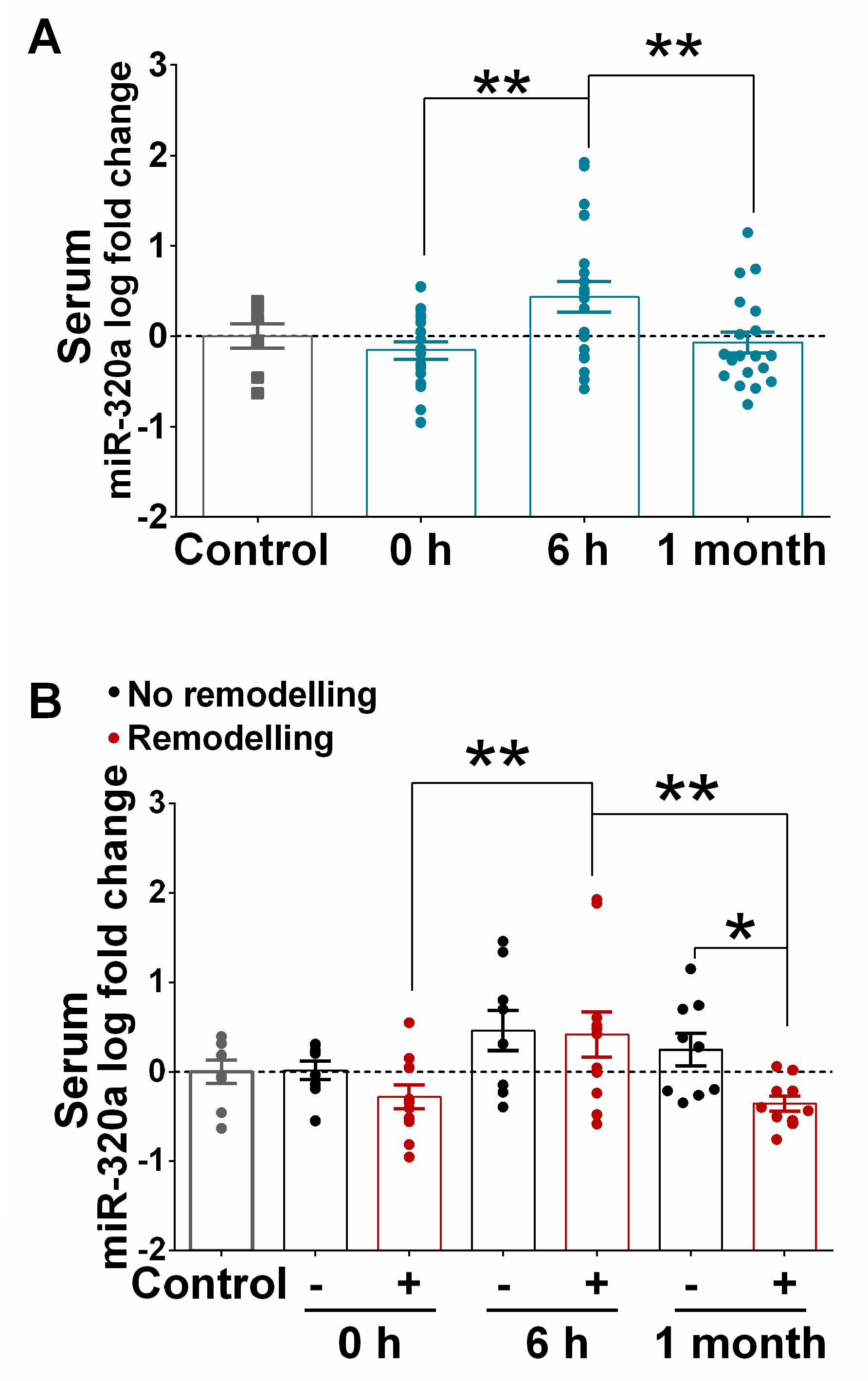
3.4. Correlation of Serum Levels of miR-320a with LVAR
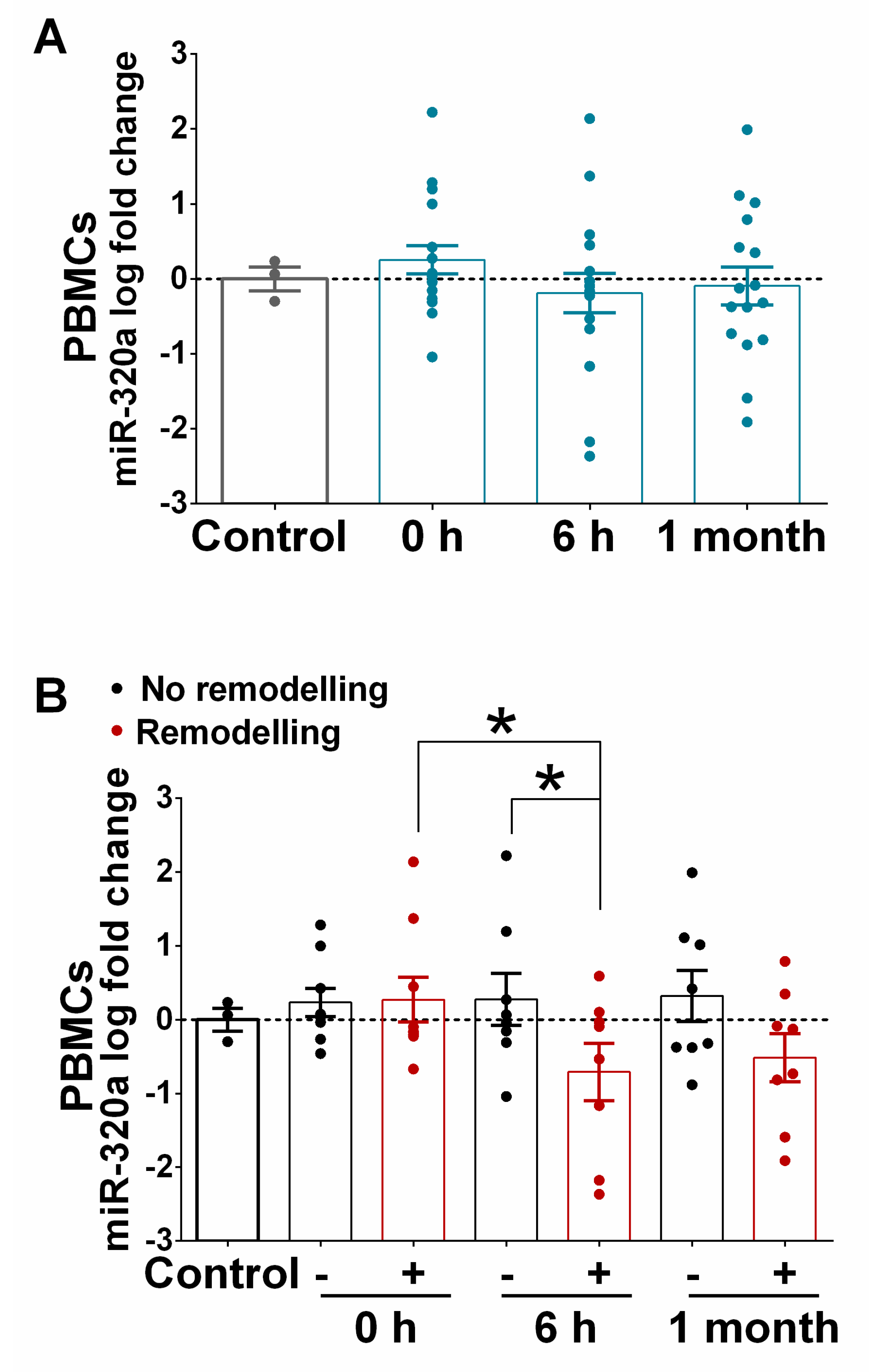
3.5. Expression of miR-320a in PBMCs Isolated from STEMI and in the Myocardium of Ischaemic Heart Failure Patients
3.6. Analysis of miR-320a Target Genes
4. Discussion
5. Conclusions
Study Limitations and Clinical Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Vernon, S.T.; Coffey, S.; D’Souza, M.; Chow, C.K.; Kilian, J.; Hyun, K.; Shaw, J.A.; Adams, M.; Roberts-Thomson, P.; Brieger, D.; et al. ST-Segment-Elevation Myocardial Infarction (STEMI) Patients Without Standard Modifiable Cardiovascular Risk Factors-How Common Are They, and What Are Their Outcomes? J. Am. Heart Assoc. 2019, 8, e013296. [Google Scholar] [CrossRef] [PubMed]
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-balloon time and mortality among patients undergoing primary PCI. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Gershlick, T.; Witzenbichler, B.; Guagliumi, G.; Fahy, M.; Dangas, G.; Mehran, R.; Stone, G.W. Incidence and predictors of heart failure following percutaneous coronary intervention in ST-segment elevation myocardial infarction: The HORIZONS-AMI trial. Am. Heart J. 2011, 162, 663–670. [Google Scholar] [CrossRef]
- Caccioppo, A.; Franchin, L.; Grosso, A.; Angelini, F.; D’Ascenzo, F.; Brizzi, M.F. Ischemia Reperfusion Injury: Mechanisms of Damage/Protection and Novel Strategies for Cardiac Recovery/Regeneration. Int. J. Mol. Sci. 2019, 20, 5024. [Google Scholar] [CrossRef]
- Danchin, N.; Popovic, B.; Puymirat, E.; Goldstein, P.; Belle, L.; Cayla, G.; Roubille, F.; Lemesle, G.; Ferrières, J.; Schiele, F.; et al. Five-year outcomes following timely primary percutaneous intervention, late primary percutaneous intervention, or a pharmaco-invasive strategy in ST-segment elevation myocardial infarction: The FAST-MI programme. Eur. Heart J. 2019, 14, 858–866. [Google Scholar] [CrossRef]
- Thrane, P.G.; Kristensen, S.D.; Olesen, K.K.W.; Mortensen, L.S.; Bøtker, H.E.; Thuesen, L.; Hansen, H.S.; Abildgaard, U.; Engstrøm, T.; Andersen, H.R.; et al. 16-year follow-up of the Danish Acute Myocardial Infarction 2 (DANAMI-2) trial: Primary percutaneous coronary intervention vs. fibrinolysis in ST-segment elevation myocardial infarction. Eur. Heart J. 2019, 14, 847–854. [Google Scholar] [CrossRef]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef]
- Sager, H.B.; Kessler, T.; Schunkert, H. Monocytes and macrophages in cardiac injury and repair. J. Thorac. Dis. 2017, 9, S30–S35. [Google Scholar] [CrossRef]
- Dutta, P.; Nahrendorf, M. Monocytes in myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Li, J. Inflammation and inflammatory cells in myocardial infarction and reperfusion injury: A double-edged sword. Clin. Med. Insights Cardiol. 2016, 10, 79–84. [Google Scholar] [CrossRef]
- Fertin, M.; Dubois, E.; Belliard, A.; Amouyel, P.; Pinet, F.; Bauters, C. Usefulness of circulating biomarkers for the prediction of left ventricular remodeling after myocardial infarction. Am. J. Cardiol. 2012, 110, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Ng, L.L. Biomarkers in acute myocardial infarction. BMC Med. 2010, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. MiRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges review-article. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Luo, C.; Wen, Y.; Liu, R.; Sun, H.; Tang, L. MicroRNAs in acute pancreatitis: From pathogenesis to novel diagnosis and therapy. J. Cell. Physiol. 2019, 235, 1948–1961. [Google Scholar] [CrossRef]
- Shoeibi, S. Diagnostic and theranostic microRNAs in the pathogenesis of atherosclerosis. Acta Physiol. 2019, 288, e13353. [Google Scholar] [CrossRef]
- Smani, T.; Mayoral-González, I.; Galeano-Otero, I.; Gallardo-Castillo, I.; Rosado, J.A.; Ordoñez, A.; Hmadcha, A. Chapter 15: Non-coding RNAs and Ischemic Cardiovascular Diseases. Non-coding RNAs in Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 39, 2704–2716. [Google Scholar]
- Economou, E.K.; Oikonomou, E.; Siasos, G.; Papageorgiou, N.; Tsalamandris, S.; Mourouzis, K.; Papaioanou, S.; Tousoulis, D. The role of microRNAs in coronary artery disease: From pathophysiology to diagnosis and treatment. Atherosclerosis 2015, 241, 624–633. [Google Scholar] [CrossRef]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 2014, 124, 2136–2146. [Google Scholar] [CrossRef]
- Thum, T.; Condorelli, G. Long Noncoding RNAs and MicroRNAs in Cardiovascular Pathophysiology. Circ. Res. 2015, 116, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Perez-Polo, J.R.; Qian, J.; Birnbaum, Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol. Genomics 2011, 43, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.; Latronico, M.V.G.; Cavarretta, E. microRNAs in Cardiovascular Diseases. J. Am. Coll. Cardiol. 2014, 63, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Cediel, G.; Bär, C.; Núñez, J.; Revuelta-Lopez, E.; Gavara, J.; Ríos-Navarro, C.; Llorente-Cortes, V.; Bodí, V.; Thum, T.; et al. Circulating miR-1254 predicts ventricular remodeling in patients with ST-Segment-Elevation Myocardial Infarction: A cardiovascular magnetic resonance study. Sci. Rep. 2018, 8, 15115. [Google Scholar] [CrossRef]
- MacIejak, A.; Kostarska-Srokosz, E.; Gierlak, W.; Dluzniewski, M.; Kuch, M.; Marchel, M.; Opolski, G.; Kiliszek, M.; Matlak, K.; Dobrzycki, S.; et al. Circulating MIR-30a-5p as a prognostic biomarker of left ventricular dysfunction after acute myocardial infarction. Sci. Rep. 2018, 8, 9883. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s guidelines and standards committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Ai, J.; Zhang, R.; Li, Y.; Pu, J.; Lu, Y.; Jiao, J.; Li, K.; Yu, B.; Li, Z.; Wang, R.; et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem. Biophys. Res. Commun. 2010, 391, 73–77. [Google Scholar] [CrossRef]
- Wong, L.L.; Armugam, A.; Sepramaniam, S.; Karolina, D.S.; Lim, K.Y.; Lim, J.Y.; Chong, J.P.C.; Ng, J.Y.X.; Chen, Y.-T.; Chan, M.M.Y.; et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur. J. Heart Fail. 2015, 17, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Zhou, M.; He, J.; Meng, W.; Ma, X.; Dong, S.; Meng, X.; Zhao, X.; Wang, X.; He, F. Circulating miR-208b and miR-34a are associated with left ventricular remodeling after acute myocardial infarction. Int. J. Mol. Sci. 2014, 15, 5774–5788. [Google Scholar] [CrossRef] [PubMed]
- Nabiałek, E.; Wańha, W.; Kula, D.; Jadczyk, T.; Krajewska, M.; Kowalówka, A.; Dworowy, S.; Hrycek, E.; Włudarczyk, W.; Parma, Z.; et al. Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in acute myocardial infarction and stable coronary heart disease. Minerva Cardioangiol. 2013, 61, 627–637. [Google Scholar]
- Ji, Q.; Jiang, Q.; Yan, W.; Li, X.; Zhang, Y.; Meng, P.; Shao, M.; Chen, L.; Zhu, H.; Tian, N. Expression of circulating microRNAs in patients with ST segment elevation acute myocardial infarction. Minerva Cardioangiol. 2015, 63, 397–402. [Google Scholar] [PubMed]
- Arbab-Zadeh, A.; Nakano, M.; Virmani, R.; Fuster, V. Acute coronary events. Circulation 2012, 125, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Pagidipati, N.J.; Gaziano, T.A. Estimating deaths from cardiovascular disease: A review of global methodologies of mortality measurement. Circulation 2013, 127, 749–756. [Google Scholar] [CrossRef]
- Cha, M.J.; Lee, J.H.; Jung, H.N.; Kim, Y.; Choe, Y.H.; Kim, S.M. Cardiac magnetic resonance-tissue tracking for the early prediction of adverse left ventricular remodeling after ST-segment elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2019, 35, 2095–2102. [Google Scholar] [CrossRef]
- Cuenin, L.; Lamoureux, S.; Schaaf, M.; Bochaton, T.; Monassier, J.P.; Claeys, M.J.; Rioufol, G.; Finet, G.; Garcia-Dorado, D.; Angoulvant, D.; et al. Incidence and significance of spontaneous ST segment re-elevation after reperfused anterior acute myocardial infarction―Relationship with infarct size, adverse remodeling, and events at 1 year. Circ. J. 2018, 82, 1379–1386. [Google Scholar] [CrossRef]
- Colpaert, R.M.W.; Calore, M. MicroRNAs in Cardiac Diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef]
- Devaux, Y.; Vausort, M.; McCann, G.P.; Zangrando, J.; Kelly, D.; Razvi, N.; Zhang, L.; Ng, L.L.; Wagner, D.R.; Squire, I.B. MicroRNA-150: A novel marker of left ventricular remodeling after acute myocardial infarction. Circ. Cardiovasc. Genet. 2013, 6, 290–298. [Google Scholar] [CrossRef]
- He, F.; Lv, P.; Zhao, X.; Wang, X.; Ma, X.; Meng, W.; Meng, X.; Dong, S. Predictive value of circulating miR-328 and miR-134 for acute myocardial infarction. Mol. Cell. Biochem. 2014, 394, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Lima, J.; Mohammed, A.; Cormack, S.; Jones, S.; Ali, A.; Panahi, P.; Barter, M.; Bagnall, A.; Ali, S.; Young, D.; et al. Kinetics Analysis of Circulating MicroRNAs Unveils Markers of Failed Myocardial Reperfusion. Clin. Chem. 2020, 66, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Ye, Z.; Sun, Y.; Yang, H.; Li, L. Relationship between circulating miRNA-30e and no-reflow phenomenon in STEMI patients undergoing primary coronary intervention. Scand. J. Clin. Lab. Invest. 2018, 78, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Kong, S.W.; Lu, J.; Bisping, E.; Zhang, H.; Allen, P.D.; Golub, T.R.; Pieske, B.; Pu, W.T. Altered microRNA expression in human heart disease. Physiol. Genom. 2007, 31, 367–373. [Google Scholar] [CrossRef]
- Davidson, S.M.; Andreadou, I.; Barile, L.; Birnbaum, Y.; Cabrera-Fuentes, H.A.; Cohen, M.V.; Downey, J.M.; Girao, H.; Pagliaro, P.; Penna, C.; et al. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovasc. Res. 2019, 115, 1156–1166. [Google Scholar] [CrossRef]
- Iaconetti, C.; Sorrentino, S.; De Rosa, S.; Indolfi, C. Exosomal miRNAs in heart disease. Physiology 2016, 31, 16–24. [Google Scholar] [CrossRef]
- Quesenberry, P.J.; Aliotta, J.; Deregibus, M.C.; Camussi, G. Role of extracellular RNA-carrying vesicles in cell differentiation and reprogramming. Stem Cell Res. Ther. 2015, 6, 153. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Sánchez-Madrid, F. Intercellular communication: Diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef]
- Wang, X.H.; Qian, R.Z.; Zhang, W.; Chen, S.F.; Jin, H.M.; Hu, R.M. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 181–188. [Google Scholar] [CrossRef]
- Ren, X.-P.; Wu, J.; Wang, X.; Sartor, M.A.; Jones, K.; Qian, J.; Nicolaou, P.; Pritchard, T.J.; Fan, G.-C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef]
- Song, C.-L.; Liu, B.; Diao, H.-Y.; Shi, Y.-F.; Li, Y.-X.; Zhang, J.-C.; Lu, Y.; Wang, G.; Liu, J.; Yu, Y.-P.; et al. The Protective Effect of MicroRNA-320 on Left Ventricular Remodeling after Myocardial Ischemia-Reperfusion Injury in the Rat Model. Int. J. Mol. Sci. 2014, 15, 17442–17456. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Galuppo, P.; Wolf, C.; Fiedler, J.; Kneitz, S.; Van Laake, L.W.; Doevendans, P.A.; Mummery, C.L.; Borlak, J.; Haverich, A.; et al. MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation 2007, 116, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, J.; Zhao, Y.; Zhang, X.; Dai, B.; Zhan, J.; Yin, Z.; Nie, X.; Fu, X.-D.; Chen, C.; et al. Nuclear miR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ. Res. 2019, 125, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Song, C.L.; Liu, B.; Diao, H.Y.; Shi, Y.F.; Zhang, J.C.; Li, Y.X.; Liu, N.; Yu, Y.P.; Wang, G.; Wang, J.P.; et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget 2016, 7, 39740–39757. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Q.; Jiang, H.; Lu, Z.B. MiR-320 regulates cardiomyocyte apoptosis induced by ischemia–reperfusion injury by targeting AKIP1. Cell. Mol. Biol. Lett. 2018, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Frangogiannis, N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic to Transl. Sci. 2019, 4, 449–467. [Google Scholar] [CrossRef]
- Itoh, N.; Ohta, H.; Nakayama, Y.; Konishi, M. Roles of FGF signals in heart development, health, and disease. Front. Cell Dev. Biol. 2016, 4, 110. [Google Scholar] [CrossRef]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid. Med. Cell. Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef]
- Eefting, F.; Rensing, B.; Wigman, J.; Pannekoek, W.J.; Liu, W.M.; Cramer, M.J.; Lips, D.J.; Doevendans, P.A. Role of apoptosis in reperfusion injury. Cardiovasc. Res. 2004, 61, 414–426. [Google Scholar] [CrossRef]

| Controls (n = 14) | Patients (n = 42) | P Value | |
|---|---|---|---|
| Age (years) | 62.1 ± 11.6 | 58.4 ± 11.3 | 0.287 |
| Male sex | 7 (50.0%) | 37 (88.1%) | 0.002 * |
| Arterial hypertension | 7 (50.0%) | 21 (50.0%) | 1.000 |
| Smoking | 7 (50.0%) | 17 (40.5%) | 0.541 |
| Dyslipidaemia | 8 (57.1%) | 19 (45.2%) | 0.449 |
| Type 2 diabetes mellitus (%) | 4 (28.6%) | 10 (23.8%) | 0.727 |
| LVEDV (ml/m2) $ | 50 ± 10.9 | 58.1 ± 12.7 | 0.162 |
| Creatine kinase (mg/dL) $ | 96.4 ± 60.1 | 2502 ± 2290.6 | >0.001 * |
| Troponin T (ng/mL) $ | 7.3 ± 4.9 | 6097.6 ± 5526.9 | 0.003 * |
| STEMI Patients (n = 39) | No remodelling (n = 25) | Remodelling (n = 14) | P Value | |
|---|---|---|---|---|
| Age (years) | 57.8 ± 10.4 | 57.2 ± 10.6 | 59.1 ± 10.3 | 0.596 |
| Male sex | 35 (89.7%) | 23 (92.0%) | 12 (85.7%) | 0.547 |
| Arterial hypertension | 18 (46.2%) | 10 (40.0%) | 8 (57.1%) | 0.316 |
| Smoking | 16 (41.0%) | 11 (44.0%) | 5 (35.7%) | 0.625 |
| Dyslipidaemia | 17 (45.2%) | 12 (48.0%) | 5 (35.7%) | 0.471 |
| Type 2 diabetes mellitus | 9 (23.1%) | 6 (24.0%) | 3 (21.4%) | 0.860 |
| LVEDV (ml/m2) $ | 57.9 ± 12.8 | 58.9 ± 12.4 | 55.9 ± 13.6 | 0.487 |
| LVEDV (ml/m2) $$$ | 67.8 ± 26.0 | 56.9 ± 11.2 | 88.8 ± 33.4 | >0.001 * |
| Creatine kinase (mg/dL) $ | 3213.8 ±2209.0 | 2447.8 ± 1795.6 | 4526.9 ± 2289.9 | 0.004 * |
| Troponin T (ng/mL) $ | 5806.6 ± 5404.1 | 4853.3 ± 4414.5 | 7508.9 ± 6672.3 | 0.143 |
| Pro-BNP (pg/mL) $$ | 1172.9 ± 710.7 | 1029.2 ± 896.2 | 1388.5 ± 270.4 | 0.466 |
| Factor | OR | 95% CI | P Value |
|---|---|---|---|
| miR-320a & | 0.005 | 0.000–0.892 | 0.045 * |
| Age | 1.075 | 0.929–1.243 | 0.330 |
| Sex | <0.001 | - | 0.999 |
| Creatine Kinase | 1.001 | 1.000–1.002 | 0.189 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeano-Otero, I.; Del Toro, R.; Guisado, A.; Díaz, I.; Mayoral-González, I.; Guerrero-Márquez, F.; Gutiérrez-Carretero, E.; Casquero-Domínguez, S.; Díaz-de la Llera, L.; Barón-Esquivias, G.; et al. Circulating miR-320a as a Predictive Biomarker for Left Ventricular Remodelling in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention. J. Clin. Med. 2020, 9, 1051. https://doi.org/10.3390/jcm9041051
Galeano-Otero I, Del Toro R, Guisado A, Díaz I, Mayoral-González I, Guerrero-Márquez F, Gutiérrez-Carretero E, Casquero-Domínguez S, Díaz-de la Llera L, Barón-Esquivias G, et al. Circulating miR-320a as a Predictive Biomarker for Left Ventricular Remodelling in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2020; 9(4):1051. https://doi.org/10.3390/jcm9041051
Chicago/Turabian StyleGaleano-Otero, Isabel, Raquel Del Toro, Agustín Guisado, Ignacio Díaz, Isabel Mayoral-González, Francisco Guerrero-Márquez, Encarnación Gutiérrez-Carretero, Sara Casquero-Domínguez, Luis Díaz-de la Llera, Gonzalo Barón-Esquivias, and et al. 2020. "Circulating miR-320a as a Predictive Biomarker for Left Ventricular Remodelling in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention" Journal of Clinical Medicine 9, no. 4: 1051. https://doi.org/10.3390/jcm9041051
APA StyleGaleano-Otero, I., Del Toro, R., Guisado, A., Díaz, I., Mayoral-González, I., Guerrero-Márquez, F., Gutiérrez-Carretero, E., Casquero-Domínguez, S., Díaz-de la Llera, L., Barón-Esquivias, G., Jiménez-Navarro, M., Smani, T., & Ordóñez-Fernández, A. (2020). Circulating miR-320a as a Predictive Biomarker for Left Ventricular Remodelling in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine, 9(4), 1051. https://doi.org/10.3390/jcm9041051