A Pilot Study Evaluating the Effects of 670 nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Intervention
2.3. Procedure
2.4. Assessments
2.5. Imaging
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Particiapant Characteristics
3.2. Study Outcomes
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.3. Effect of 670 nm Light Therapy Between Groups
3.4. Compliance
3.5. Adverse Events
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye 2001, 15, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Ramrattan, R.S. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2857–2864. [Google Scholar]
- Ko, F. Associations with retinal pigment epithelium thickness measures in a large cohort: Results from the UK biobank. Ophthalmology 2017, 124, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L. Clinical classification of age-related macular degeneration. Ophthalmology. 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S. Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv. Ophthalmol. 2016, 61, 521–537. [Google Scholar] [CrossRef]
- Owsley, C. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4776–4789. [Google Scholar] [CrossRef]
- Jackson, G.R.; Owsley, C.; Curcio, C.A. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res. Rev. 2002, 1, 381–396. [Google Scholar] [CrossRef]
- Tan, R.; Guymer, R.H.; Luu, C.D. Subretinal Drusenoid Deposits and the loss of rod function in intermediate age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4154–4161. [Google Scholar] [CrossRef]
- Flynn, O.J.; Cukras, C.A.; Jeffrey, B.G. Characterization of rod function phenotypes across a range of age-related macular degeneration severities and subretinal drusenoid deposits. Investig. Ophthalmol. Vis. Sci. 2018, 59, 241–2421. [Google Scholar] [CrossRef]
- Stone, J. The locations of mitochondria in mammalian photoreceptors: Relation to retinal vasculature. Brain Res. 2008, 1189, 58–69. [Google Scholar] [CrossRef]
- Kokkinopoulos, I. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol. Aging 2013, 34, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Gkotsi, D. Recharging mitochondrial batteries in old eyes. Near infra-red increases ATP. Exp. Eye Res. 2014, 122, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kam, J.H. Fundamental differences in patterns of retinal ageing between primates and mice. Sci. Rep. 2019, 9, 12574. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.; Demidova, T. Mechanisms of low level light therapy. Proc. SPIE 2006, 6140, 1–12. [Google Scholar]
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Karu, T.I. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J. Photochem. Photobiol. B 2005, 81, 98–106. [Google Scholar] [CrossRef]
- Sivapathasuntharam, C. Aging retinal function is improved by near infrared light (670 nm) that is associated with corrected mitochondrial decline. Neurobiol. Aging 2017, 52, 66–70. [Google Scholar] [CrossRef]
- El Massri, N. Photobiomodulation reduces gliosis in the basal ganglia of aged mice. Neurobiol. Aging 2018, 66, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tang, J. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: In vivo and in vitro. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3681–3690. [Google Scholar] [CrossRef]
- Ivandic, B.T.; Ivandic, T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed. Laser Surg. 2008, 26, 241–245. [Google Scholar] [CrossRef]
- Merry, G.F. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017, 95, e270–e277. [Google Scholar] [CrossRef] [PubMed]
- Sivapathasuntharam, C. Improving mitochondrial function significantly reduces the rate of age related photoreceptor loss. Exp. Eye Res. 2019, 185, 107691. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, D.M.; Mitrofanis, J.; Stone, J. Targeting the body to protect the brain: Inducing neuroprotection with remotely-applied near infrared light. Neural. Regen. Res. 2015, 10, 349–351. [Google Scholar] [CrossRef]
- Calaza, K.C.; Kam, J.H.; Hogg, C.; Jeffery, G. Mitochondrial decline precedes phenotype development in the complement factor H mouse model of retinal degeneration but can be corrected by near infrared light. Neurobiol. Aging 2015, 36, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
| Healthy Ageing (n = 12) | iAMD with no SDD (n = 15) | iAMD with SDD (n = 8) | |
|---|---|---|---|
| Mean Age (± SD) | 69.9 (± 2.5) | 69.4 (± 7.2) | 69.3 (± 6.8) |
| Ethnicity (n, %) | |||
| Asian | 1 (8%) | 1 (7%) | 0 (0%) |
| Caucasian | 11 (92%) | 15 (93%) | 8 (100%) |
| Gender balance | |||
| n (% female) | 7 (58%) | 10 (67%) | 5 (63%) |
| Mean BCVA | 87.1 ± 6.5 | 84.8 ± 7.6 | 81.6 ± 3.7 |
| (ETDRS letters ± SD) | |||
| Smoking status | |||
| Current or former smoker (%) | 33% | 60% | 25% |
| Diabetes history (%) | 0% | 0% | 0% |
| Hypertension history (%) | 33% | 20% | 38% |
| Hyperlipedemia history (%) | 50% | 26% | 13% |
| AREDS or other eye supplements | 0% | 60% | 63% |
| Outcomes | Healthy Ageing Mean (SD); n | iAMD with no SDD Mean (SD); n | iAMD with SDD Mean (SD); n | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Months | p Value | Baseline | 12 Months | p Value | Baseline | 12 Months | p Value | |
| BCVA (ETDRS letters) | 87.1 (6.5); 12 | 85.7 (6.4); 12 | 0.356 | 84.8 (7.6); 15 | 85.7 (6.6); 15 | 0.456 | 81.6 (3.7); 8 | 83.0 (3.9); 8 | 0.211 |
| LLVA (ETDRS letters) | 73.5 (6.0); 12 | 70.4 (6.2); 12 | 0.182 | 70.8 (8.1); 15 | 67.2 (9.1); 15 | 0.005 | 68.1 (6.3); 8 | 65.9 (8.7); 8 | 0.188 |
| LLD (ETDRS letters) | 13.6 (3.7); 12 | 15.3 (4.3); 12 | 0.363 | 14.0 (5.5); 15 | 18.5 (6.3); 15 | 0.006 | 13.5 (5.9); 8 | 17.1 (8.3); 8 | 0.050 |
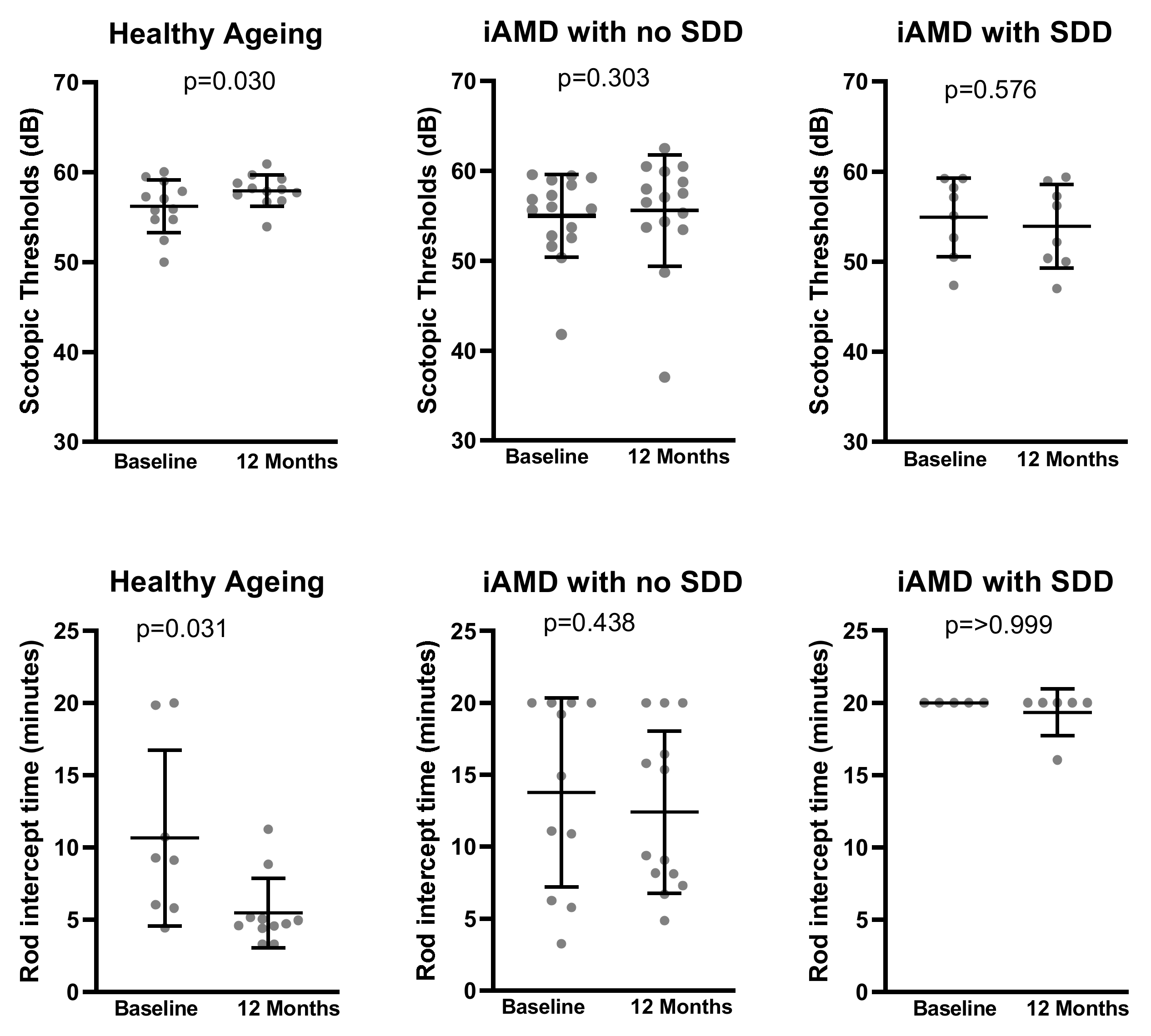
| Scotopic Thresholds (dB) | 56.18 (2.94); 12 | 57.96 (1.75); 12 | 0.030 | 54.69 (4.59); 15 | 55.58 (6.20); 15 | 0.303 | 54.92 (4.39); 8 | 53.93 (4.64); 8 | 0.576 |
| RIT (minutes) | 9.35 (5.21); 7 | 5.59 (2.58); 7 | 0.031 | 14.92 (6.12); 8 | 13.24 (5.17); 8 | 0.438 | 20.00 (0.00); 5 | 19.21 (1.77); 5 | >0.999 |
| Photopic 28.3Hz flicker ERG amplitude (µV) | 14.21 (5.92); 11 | 11.00 (4.21); 11 | 0.020 | 16.82 (7.74); 12 | 14.54 (6.30); 12 | 0.583 | 14.59 (4.67); 7 | 12.43 (5.88); 7 | 0.443 |
| Photopic 28.3Hz flicker ERG timing (ms) | 27.80 (1.28); 11 | 28.05 (0.82); 11 | 0.338 | 28.31 (1.34); 12 | 28.88 (1.28); 12 | 0.192 | 28.13 (1.23); 7 | 29.43 (1.14); 7 | 0.038 |
| BCVA (ETDRS Letters) | Mean (SD); n | Change from Baseline Mean (SE) | Adjusted Difference Between Groups (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy ageing | iAMD with no SDD | iAMD with SDD | Healthy ageing | iAMD with no SDD | iAMD with SDD | iAMD with no SDD vs HA | p-value | iAMD- with SDD vs HA | p-value | |
| Baseline | 87.1 (6.5); 12 | 84.8 (7.6); 15 | 81.6 (3.7); 8 | - | - | - | - | - | - | - |
| 1 Month | 87.4 (7.2); 12 | 85.9 (6.4); 15 | 84.1 (4.5); 8 | 0.33 (1.05) | 1.13 (0.93) | 2.50 (1.28) | 0.80 (−1.97–3.58) | 0.569 | 2.17 (−1.10–5.43) | 0.192 |
| 3 Months | 86.2 (8.8); 12 | 86.0 (5.5); 15 | 83.1 (5.6); 7 | −0.92 (1.05) | 1.20 (0.93) | 1.42 (1.34) | 2.12 (−0.66–4.89) | 0.134 | 2.34 (−1.02–5.70) | 0.170 |
| 6 Months | 86.5 (6.3); 10 | 84.8 (7.3); 13 | 84.2 (3.7); 6 | 0.57 (1.11) | 0.89 (0.98) | 1.40 (1.41) | 0.32 (−2.58–3.22) | 0.829 | 0.84 (−2.69–4.37) | 0.640 |
| 12 Months | 85.7 (6.4); 12 | 85.7 (6.6); 15 | 83.0 (3.9); 8 | −1.42 (1.05) | 0.87 (0.93) | 1.38 (1.28) | 2.28 (−0.47–5.03) | 0.104 | 2.79 (−0.47–6.05) | 0.092 |
| LLVA (ETDRS Letters) | Mean (SD); n | Change from baseline Mean (SE) | Adjusted difference between groups (95% CI) | |||||||
| Healthy ageing | iAMD with no SDD | iAMD with SDD | Healthy ageing | iAMD with no SDD | iAMD with SDD | iAMD with no SDD vs HA | p-value | iAMD- with SDD vs HA | p-value | |
| Baseline | 73.5 (6.0); 12 | 70.8 (8.1); 15 | 68.1 (6.3); 8 | - | - | - | - | - | - | |
| 1 Month | 71.7 (7.0); 12 | 68.6 (8.2); 15 | 68.9 (6.5); 8 | −1.83 (1.50) | −2.20 (1.34) | 0.75 (1.84) | −0.37 (−4.35–3.62) | 0.856 | 2.58 (−2.12–7.28) | 0.279 |
| 3 Months | 74.3 (7.2); 12 | 70.3 (8.0); 15 | 69.4 (7.7); 7 | 0.75 (1.50) | −0.53 (1.34) | 0.90 (1.92) | −1.28 (−5.27–2.70) | 0.525 | 0.15 (−4.69–4.98) | 0.950 |
| 6 Months | 75.0 (6.1); 10 | 69.5 (8.9); 13 | 69.7 (8.4); 6 | 2.25 (1.59) | −0.05 (1.40) | 2.05 (2.02) | −2.30 (−6.50–1.90) | 0.281 | −0.20 (−5.29–4.89) | 0.939 |
| 12 Months | 70.4 (6.2); 12 | 67.2 (9.0); 15 | 65.9 (8.7); 8 | −3.08 (1.50) | −3.60 (1.34) | −2.25 (1.84) | −0.52 (−4.50–3.47) | 0.798 | 0.83 (−3.87–5.53) | 0.726 |
| LLD (ETDRS Letters) | Mean (SD); n | Change from baseline Mean (SE) | Adjusted difference between groups (95% CI) | |||||||
| Healthy ageing | iAMD with no SDD | iAMD with SDD | Healthy ageing | iAMD with no SDD | iAMD with SDD | iAMD with no SDD vs HA | p-value | iAMD with SDD vs HA | P-value | |
| Baseline | 13.6 (3.7); 12 | 14.0 (5.5); 15 | 13.5 (5.9); 8 | - | - | - | - | - | - | |
| 1 Month | 15.6 (5.8); 12 | 17.3 (7.1); 15 | 15.3 (5.0); 8 | 2.17 (1.57) | 3.33 (1.40) | 1.75 (1.92) | 1.17 (−2.99–5.32) | 0.580 | −0.42 (−5.32–4.49) | 0.867 |
| 3 Months | 11.9 (5.7); 12 | 15.7 (7.0); 15 | 13.7 (5.6); 7 | −1.67 (1.57) | 1.73 (1.40) | 2.13 (1.92) | 3.40 (−0.76–7.56) | 0.108 | 3.79 (−1.11–8.69) | 0.128 |
| 6 Months | 11.5 (3.9); 10 | 15.3 (7.3); 13 | 14.5 (8.9); 6 | −1.74 (1.66) | 0.94 (1.46) | −0.62 (2.10) | 2.68 (−1.70–7.06) | 0.229 | 1.12 (−4.19–6.42) | 0.678 |
| 12 Months | 15.3 (4.3); 12 | 18.5 (6.3); 15 | 17.1 (8.3); 8 | 1.67 (1.57) | 4.47 (1.40) | 3.63 (1.92) | 2.80 (−1.36–6.96) | 0.185 | 1.96 (−2.94–6.86) | 0.431 |
| Rod-Intercept Time (RIT, minutes) | Mean (SD); n | Change from Baseline Mean (SE) | Adjusted Difference Between Groups (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy ageing | iAMD with no SDD | iAMD with SDD | Healthy ageing | iAMD with no SDD | iAMD with SDD | iAMD with no SDD vs HA | p-value | iAMD with SDD vs HA | p-value | |
| Baseline | 10.67 (6.09); 8 | 14.52 (6.41); 10 | 20.00 (0.00); 5 | - | - | - | - | - | - | - |
| 1 Month | 9.06 (5.33); 8 | 14.19 (6.08); 12 | 17.29 (4.36); 5 | −1.89 (1.72) | 0.93 (1.55) | −0.42 (2.45) | 2.82 (−1.78–7.42) | 0.227 | 1.47 (−4.47–7.40) | 0.625 |
| 3 Months | 5.83 (3.33); 11 | 12.51 (6.89); 15 | 19.92 (0.22); 7 | −5.61 (1.74) | −0.62 (1.50) | −0.27 (2.40) | 4.98 (0.43–9.54) | 0.032 | 5.33 (−0.55–11.21) | 0.075 |
| 6 Months | 5.39 (2.60); 10 | 13.27 (6.42); 13 | 17.21 (4.36); 5 | −6.37 (1.77) | −0.44 (1.57) | −3.93 (2.45) | 5.92 (1.24–10.61) | 0.014 | 2.44 (−3.56–8.43) | 0.422 |
| 12 Months | 5.48 (2.40); 11 | 12.40 (5.64); 13 | 19.34 (1.61); 6 | −5.80 (1.74) | −1.20 (1.57) | −0.46 (2.40) | 4.60 (−0.04–9.25) | 0.052 | 5.34 (−0.54–11.23) | 0.075 |
| Scotopic Thresholds (dB) | Mean (SD); n | Change from baseline Mean (SE) | Adjusted difference between groups (95% CI) | |||||||
| Healthy ageing | iAMD with no SDD | iAMD with SDD | Healthy ageing | iAMD with no SDD | iAMD with SDD | iAMD with no SDD vs HA | p-value | iAMD with SDD vs HA | p-value | |
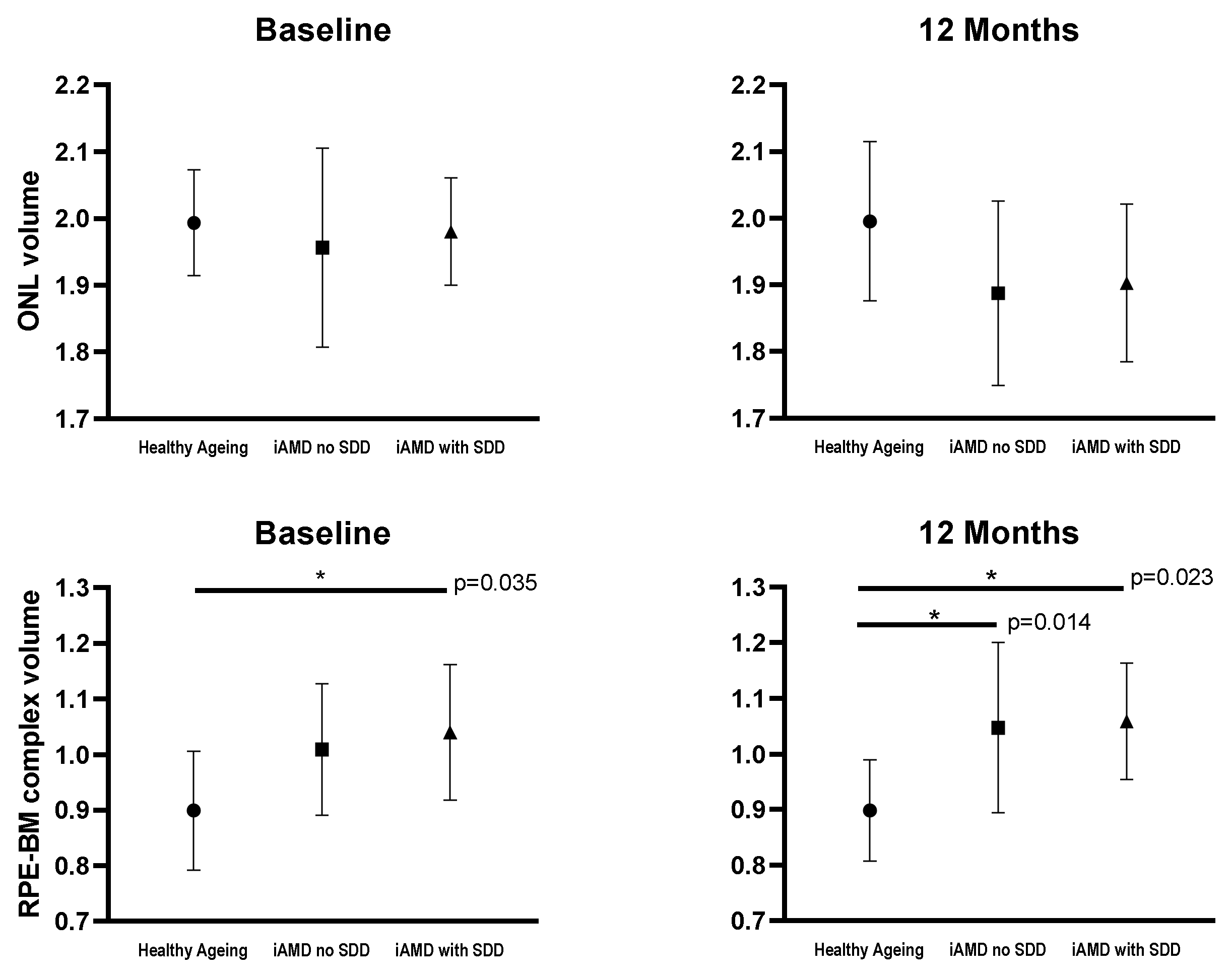
| Baseline | 56.18 (2.94); 12 | 54.70 (4.60); 15 | 54.92 (4.40); 8 | - | - | - | - | - | - | |
| 1 Month | 55.27 (3.23); 12 | 56.05 (4.01); 15 | 54.85 (5.42); 8 | −0.91 (1.09) | 1.36 (0.98) | −0.07 (1.34) | 2.27 (−0.63–5.18) | 0.124 | 0.84 (−2.58–4.27) | 0.628 |
| 3 Months | 57.47 (2.32); 12 | 57.46 (3.10); 15 | 55.58 (4.15); 7 | 1.29 (1.09) | 2.77 (0.98) | 0.83 (1.40) | 1.48 (−1.42–4.39) | 0.314 | −0.46 (−3.97–3.06) | 0.797 |
| 6 Months | 57.42 (1.93); 10 | 56.11 (3.73); 13 | 54.84 (3.39); 6 | 1.21 (1.16) | 1.89 (1.02) | 0.09 (1.47) | 0.67 (−2.39–3.73) | 0.664 | −1.12 (−4.82–2.58) | 0.550 |
| 12 Months | 57.95 (1.75); 12 | 55.58 (6.20); 15 | 53.93 (4.64); 8 | 1.77 (1.09) | 0.89 (0.98) | −0.99 (1.34) | −0.87 (−3.78–2.03) | 0.553 | −2.76 (−6.18–0.67) | 0.114 |
| Photopic Flicker Timing (ms) | Mean (SD); n | Change from Baseline Mean (SE) | Adjusted Difference between Groups (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy ageing | iAMD with no SDD | iAMD with SDD | Healthy ageing | iAMD with no SDD | iAMD with SDD | iAMD with no SDD vs HA | p-value | iAMDwith SDD vs HA | p-value | |
| Baseline | 27.80 (1.28); 11 | 28.33 (1.29); 13 | 28.20 (1.16); 8 | - | - | - | - | - | - | - |
| 1 Month | 27.89 (1.00); 11 | 28.44 (0.92); 14 | 28.74 (1.37); 7 | 0.03 (0.29) | −0.20 (0.27) | 0.13 (0.35) | −0.22 (−1.01–0.56) | 0.569 | 0.10 (−0.80–1.00) | 0.827 |
| 3 Months | 27.63 (1.50); 12 | 28.22 (0.78); 14 | 28.70 (0.95); 7 | −0.30 (0.28) | −0.20 (0.26) | −0.09 (0.35) | 0.09 (−0.67–0.86) | 0.805 | 0.20 (−0.69–1.09) | 0.649 |
| 6 Months | 27.81 (0.97); 10 | 28.59 (1.09); 12 | 28.23 (0.99); 6 | −0.20 (0.30) | 0.22 (0.27) | −0.16 (0.37) | 0.42 (−0.38–1.22) | 0.302 | 0.04 (−0.90–0.98) | 0.937 |
| 12 Months | 27.99 (0.82); 12 | 29.01 (1.22); 14 | 29.43 (1.14); 7 | 0.06 (0.28) | 0.55 (0.26) | 0.86 (0.35) | 0.48 (−0.28–1.24) | 0.210 | 0.80 (−0.09–1.69) | 0.077 |
| Photopic flicker Amplitude (µV) | Mean (SD); n | Change from baseline Mean (SE) | Adjusted difference between groups (95% CI) | |||||||
| Healthy ageing | iAMD with no SDD | iAMD with SDD | Healthy ageing | iAMD with no SDD | iAMD with SDD | iAMD with no SDD vs HA | p-value | iAMD with SDD vs HA | P-value | |
| Baseline | 14.21 (5.92); 11 | 16.75 (7.41); 13 | 14.24 (4.44); 8 | - | - | - | - | - | - | - |
| 1 Month | 15.04 (7.86); 11 | 15.64 (7.36); 14 | 17.59 (4.77); 7 | 1.18 (1.65) | −0.08 (1.53) | 2.64 (1.99) | −1.26 (−5.72–3.10) | 0.575 | 1.46 (−3.66–6.59) | 0.573 |
| 3 Months | 12.39 (7.74); 12 | 15.42 (5.47); 14 | 14.16 (3.64); 7 | −1.61 (1.60) | 0.72 (1.49) | −0.19 (1.99) | 2.33 (−2.01–6.68) | 0.289 | 1.42 (−3.65–6.49) | 0.580 |
| 6 Months | 12.77 (4.84); 10 | 14.63 (5.66); 12 | 14.40 (5.98); 6 | −0.29 (1.70) | −0.90 (1.55) | −0.29 (2.10) | −0.61 (−5.17–3.95) | 0.791 | 0.00 (−5.35–5.35) | 1.000 |
| 12 Months | 10.88 (4.03); 12 | 14.32 (5.91); 14 | 12.43 (5.88); 7 | −3.12 (1.60) | −1.21 (1.50) | −1.87 (2.00) | 1.91 (−2.43–6.26) | 0.385 | 1.25 (−3.82–6.33) | 0.626 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grewal, M.K.; Sivapathasuntharam, C.; Chandra, S.; Gurudas, S.; Chong, V.; Bird, A.; Jeffery, G.; Sivaprasad, S. A Pilot Study Evaluating the Effects of 670 nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration. J. Clin. Med. 2020, 9, 1001. https://doi.org/10.3390/jcm9041001
Grewal MK, Sivapathasuntharam C, Chandra S, Gurudas S, Chong V, Bird A, Jeffery G, Sivaprasad S. A Pilot Study Evaluating the Effects of 670 nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration. Journal of Clinical Medicine. 2020; 9(4):1001. https://doi.org/10.3390/jcm9041001
Chicago/Turabian StyleGrewal, Manjot K., Chrishne Sivapathasuntharam, Shruti Chandra, Sarega Gurudas, Victor Chong, Alan Bird, Glen Jeffery, and Sobha Sivaprasad. 2020. "A Pilot Study Evaluating the Effects of 670 nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration" Journal of Clinical Medicine 9, no. 4: 1001. https://doi.org/10.3390/jcm9041001
APA StyleGrewal, M. K., Sivapathasuntharam, C., Chandra, S., Gurudas, S., Chong, V., Bird, A., Jeffery, G., & Sivaprasad, S. (2020). A Pilot Study Evaluating the Effects of 670 nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration. Journal of Clinical Medicine, 9(4), 1001. https://doi.org/10.3390/jcm9041001






