A Systematic Review of the Effectiveness of Non-Invasive Brain Stimulation Techniques to Reduce Violence Proneness by Interfering in Anger and Irritability
Abstract
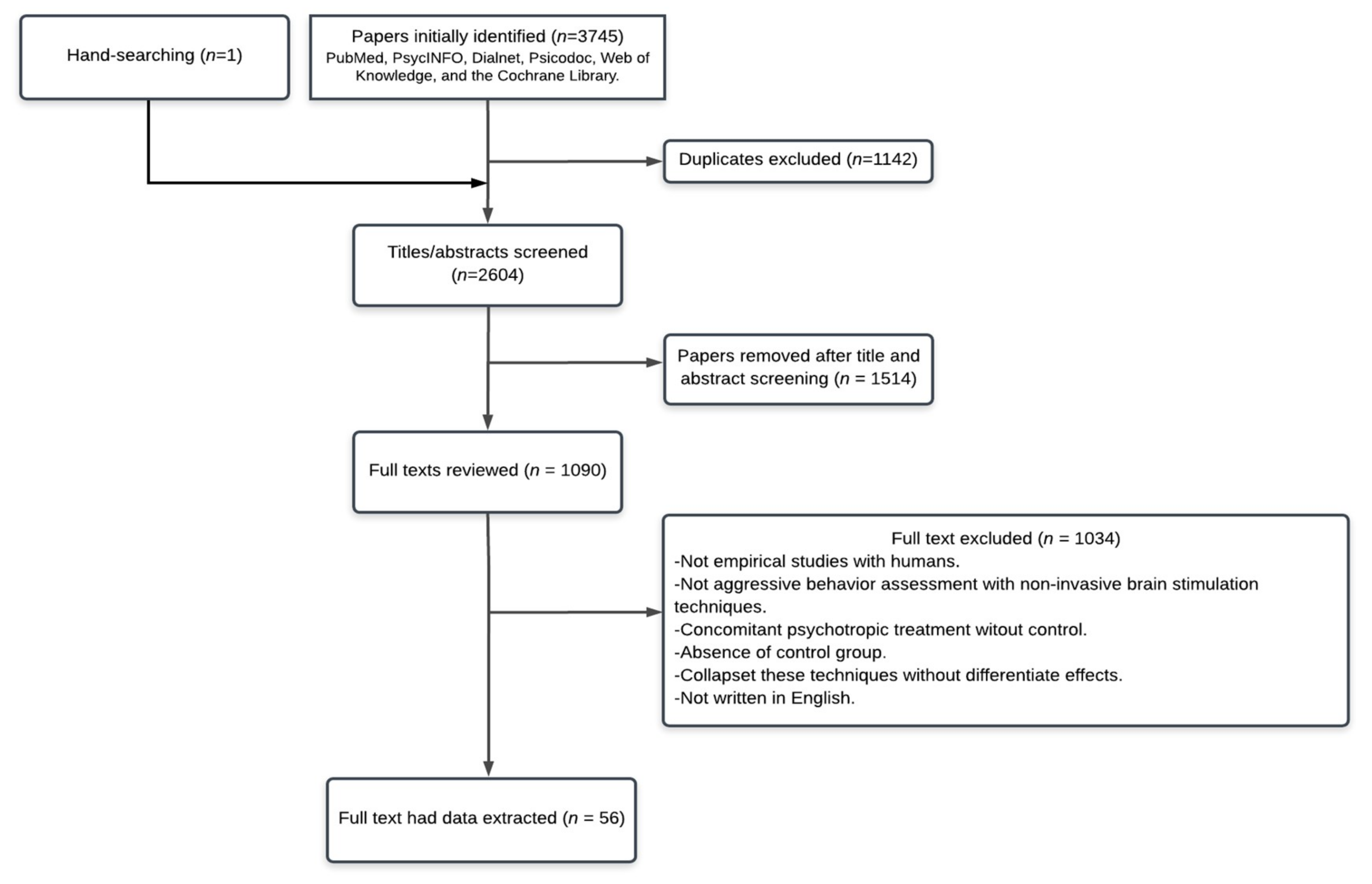
1. Introduction
2. Search Strategy
3. Results
3.1. Normative Individuals
3.2. Self-Reports
3.3. Laboratory Task
3.4. Violent Population
3.5. Pathological Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glenn, A.L.; Raine, A. Neurocriminology: Implications for the punishment, prediction and prevention of criminal behaviour. Nat. Rev. Neurosci. 2014, 15, 54–63. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J. A review of effective interventions for reducing aggression and violence. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2577–2597. [Google Scholar] [CrossRef] [PubMed]
- Moya-Albiol, L.; Sariñana-González, P.; Vitoria-Estruch, S.; Romero-Martínez, Á. La neurocriminología como disciplina aplicada emergente. Vox Juris 2017, 33, 6. [Google Scholar] [CrossRef]
- Brunner, F.; Neumann, I.; Yoon, D.; Rettenberger, M.; Stück, E.; Briken, P. Determinants of dropout from correctional offender treatment. Front. Psychiatry 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martínez, Á.; González, M.; Lila, M.; Gracia, E.; Martí-Bonmatí, L.; Alberich-Bayarri, Á.; Maldonado-Puig, R.; Ten-Esteve, A.; Moya-Albiol, L. The brain resting-state functional connectivity underlying violence proneness: Is it a reliable marker for neurocriminology? A systematic review. Behav. Sci. 2019, 9, 11. [Google Scholar] [CrossRef]
- Humble, F.; Berk, M. Pharmacological management of aggression and violence. Hum. Psychopharmacol. Clin. Exp. 2003, 18, 423–436. [Google Scholar] [CrossRef]
- Cascade, E.; Kalali, A.H.; Kennedy, S.H. Real-world data on SSRI antidepressant side effects. Psychiatry 2009, 6, 16. [Google Scholar]
- Ferguson, J.M. SSRI antidepressant medications: Adverse effects and tolerability. Primary Care Companion J. Clin. Psychiatry 2001, 3, 22. [Google Scholar] [CrossRef]
- Stamp, F. Psychiatric Treatment of Violent Offenders in Prison. Available online: https://aic.gov.au/sites/default/files/publications/proceedings/downloads/19-stamp.pdf (accessed on 28 January 2020).
- Chung, S.W.; Hoy, K.E.; Fitzgerald, P.B. Theta-burst stimulation: A new form of TMS treatment for depression? Depress. Anxiety 2015, 32, 182–192. [Google Scholar] [CrossRef]
- Cirillo, P.; Gold, A.K.; Nardi, A.E.; Ornelas, A.C.; Nierenberg, A.A.; Camprodon, J.; Kinrys, G. Transcranial magnetic stimulation in anxiety and trauma-related disorders: A systematic review and meta-analysis. Brain Behav. 2019, 9, e01284. [Google Scholar] [CrossRef]
- Cocchi, L.; Zalesky, A.; Nott, Z.; Whybird, G.; Fitzgerald, P.B.; Breakspear, M. Transcranial magnetic stimulation in obsessive-compulsive disorder: A focus on network mechanisms and state dependence. NeuroImage Clin. 2018, 19, 661–674. [Google Scholar] [CrossRef]
- Kirsch, D.L.; Nichols, F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatric Clin. 2013, 36, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.; George, M.S.; Grammer, G.; Janicak, P.G.; Pascual-Leone, A.; Wirecki, T.S. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016, 9, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.N.; Lloyd, S.W.; Lux, L.; Gartlehner, G.; Hansen, R.A.; Brode, S.; Jonas, D.E.; Swinson Evans, T.; Viswanathan, M.; Lohr, K.N. Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. J. Clin Psychiatry 2014, 75, 477–489. [Google Scholar] [CrossRef]
- Philip, N.S.; Nelson, B.G.; Frohlich, F.; Lim, K.O.; Widge, A.S.; Carpenter, L.L. Low-intensity transcranial current stimulation in psychiatry. Am. J. Psychiatry 2017, 174, 628–639. [Google Scholar] [CrossRef]
- Hoogendam, J.M.; Ramakers, G.M.; Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Kirimoto, H.; Tamaki, H.; Otsuru, N.; Yamashiro, K.; Onishi, H.; Nojima, I.; Oliviero, A. Transcranial static magnetic field stimulation over the primary motor cortex induces plastic changes in cortical nociceptive processing. Front. Hum. Neurosci. 2018, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Suppa, A.; Huang, Y.Z.; Funke, K.; Ridding, M.C.; Cheeran, B.; Di Lazzaro, V.; Ziemann, U.; Rothwell, J.C. Ten years of theta burst stimulation in humans: Established knowledge, unknowns and prospects. Brain Stimul. 2016, 9, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Sigelman, J. State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. J. Personality Soc. Psychol. 2001, 80, 797. [Google Scholar] [CrossRef]
- Depue, R.A.; Iacono, W.G. Neurobehavioral aspects of affective disorders. Annu. Rev. Psychol. 1989, 40, 457–492. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martínez, Á.; Hidalgo-Moreno, G.; Moya-Albiol, L. Neuropsychological consequences of chronic stress: The case of informal caregivers. Aging Ment. Health 2018, 1, 13. [Google Scholar] [CrossRef]
- Schutter, D.J.; van Honk, J.; d’Alfonso, A.A.; Postma, A.; de Haan, E.H. Effects of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. Neuroreport 2001, 12, 445–447. [Google Scholar] [CrossRef]
- Grisaru, N.; Bruno, R.; Pridmore, S. Effect on the emotions of healthy individuals of slow repetitive transcranial magnetic stimulation applied to the prefrontal cortex. J. ECT 2001, 17, 184–189. [Google Scholar] [CrossRef]
- Jenkins, J.; Shajahan, P.M.; Lappin, J.M.; Ebmeier, K.P. Right and left prefrontal transcranial magnetic stimulation at 1 Hz does not affect mood in healthy volunteers. BMC Psychiatry 2002, 2, 1. [Google Scholar] [CrossRef]
- Schutter, D.J.; van Honk, J. Increased positive emotional memory after repetitive transcranial magnetic stimulation over the orbitofrontal cortex. J. Psychiatry Neurosci. 2006, 31, 101. [Google Scholar]
- Hofman, D.; Schutter, D.J. Inside the wire: Aggression and functional interhemispheric connectivity in the human brain. Psychophysiology 2009, 46, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.; Lenz, B.; Friedrich, K.; Dygon, D.; Richter-Schmidinger, T.; Jacobi, A.; Mueller, S.E.; Maihöfner, C.; Sperling, W.; Kornhuber, J. Repetitive transcranial magnetic stimulation influences mood in healthy male volunteers. J. Psychiatry Res. 2011, 45, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; Leyman, L.; De Raedt, R.; Vanderhasselt, M.A.; D’haenen, H. Lack of impact of repetitive high frequency transcranial magnetic stimulation on mood in healthy female subjects. J. Affect. Disord. 2006, 90, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; Leyman, L.; De Raedt, R.; Vanderhasselt, M.A.; D’haenen, H. Left and right high frequency repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex does not affect mood in female volunteers. Clin. Neurophysiol. 2008, 119, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Koenigs, M.; Ukueberuwa, D.; Campion, P.; Grafman, J.; Wassermann, E. Bilateral frontal transcranial direct current stimulation: Failure to replicate classic findings in healthy subjects. Clin. Neurophysiol. 2009, 120, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Leyman, L.; De Raedt, R.; Vanderhasselt, M.A.; Baeken, C. Influence of high-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex on the inhibition of emotional information in healthy volunteers. Psychol. Med. 2009, 39, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; De Raedt, R.; Van Schuerbeek, P.; Vanderhasselt, M.A.; De Mey, J.; Bossuyt, A.; Luypaert, R. Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav. Brain Res. 2010, 214, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; Vanderhasselt, M.A.; De Raedt, R. Baseline ‘state anxiety’influences HPA-axis sensitivity to one sham-controlled HF-rTMS session applied to the right dorsolateral prefrontal cortex. Psychoneuroendocrinology 2011, 36, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; Schrijvers, D.L.; Sabbe, B.G.C.; Vanderhasselt, M.A.; De Raedt, R. Impact of one HF-rTMS session on fine motor function in right-handed healthy female subjects: A comparison of stimulation over the left versus the right dorsolateral prefrontal cortex. Neuropsychobiology 2012, 65, 96–102. [Google Scholar] [CrossRef]
- Baumgartner, T.; Schiller, B.; Rieskamp, J.; Gianotti, L.R.; Knoch, D. Diminishing parochialism in intergroup conflict by disrupting the right temporo-parietal junction. Soc. Cogn. Affect. Neurosci. 2013, 9, 653–660. [Google Scholar] [CrossRef]
- Baeken, C.; Vanderhasselt, M.A.; Remue, J.; Rossi, V.; Schiettecatte, J.; Anckaert, E.; De Raedt, R. One left dorsolateral prefrontal cortical HF-rTMS session attenuates HPA-system sensitivity to critical feedback in healthy females. Neuropsychologia 2014, 57, 112–121. [Google Scholar] [CrossRef]
- Moulier, V.; Gaudeau-Bosma, C.; Isaac, C.; Allard, A.C.; Bouaziz, N.; Sidhoumi, D.; Braha-Zeitoun, S.; Benadhira, R.; Thomas, F.; Januel, D. Effect of repetitive transcranial magnetic stimulation on mood in healthy subjects. Socioaffective Neurosci. Psychol. 2016, 6, 29672. [Google Scholar] [CrossRef]
- Iyer, M.B.; Mattu, U.; Grafman, J.; Lomarev, M.; Sato, S.; Wassermann, E.M. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005, 64, 872–875. [Google Scholar] [CrossRef]
- Hortensius, R.; Schutter, D.J.; Harmon-Jones, E. When anger leads to aggression: Induction of relative left frontal cortical activity with transcranial direct current stimulation increases the anger–aggression relationship. Soc. Cogn. Affect. Neurosci. 2011, 7, 342–347. [Google Scholar] [CrossRef]
- Plazier, M.; Joos, K.; Vanneste SOst, J.; De Ridder, D. Bifrontal and bioccipital transcranial direct current stimulation (tDCS) does not induce mood changes in healthy volunteers: A placebo-controlled study. Brain Stimul. 2012, 5, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Yamaguchi, M.; Fujii, T.; Kitahara, Y. Mood and cognitive function following repeated transcranial direct current stimulation in healthy volunteers: A preliminary report. Neurosci. Res. 2013, 77, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.J.; Hortensius, R.; Harmon-Jones, E. When anger leads to rumination: Induction of relative right frontal cortical activity with transcranial direct current stimulation increases anger-related rumination. Psychol. Sci. 2013, 24, 475–481. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.K.; McKinly, R.A.; Goodyear, C.; Nelson, J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 2014, 7, 499–507. [Google Scholar] [CrossRef]
- Vitor-Costa, M.; Okuno, N.M.; Bortolotti, H.; Bertollo, M.; Boggio, P.S.; Fregni, F.; Altimari, L.R. Improving cycling performance: Transcranial direct current stimulation increases time to exhaustion in cycling. PLoS ONE 2015, 10, e0144916. [Google Scholar] [CrossRef]
- Riva, P.; Romero-Lauro, L.J.; DeWall, C.N.; Chester, D.S.; Bushman, B.J. Reducing aggressive responses to social exclusion using transcranial direct current stimulation. Soc. Cogn. Affect. Neurosci. 2015, 10, 352–356. [Google Scholar] [CrossRef]
- Dambacher, F.; Schuhmann, T.; Lobbestael, J.; Arntz, A.; Brugman, S.; Sack, A.T. Reducing proactive aggression through non-invasive brain stimulation. Soc. Cogn. Affect. Neurosci. 2015, 10, 1303–1309. [Google Scholar] [CrossRef]
- De Putter, L.M.; Vanderhasselt, M.A.; Baeken, C.; De Raedt, R.; Koster, E.H. Combining tDCS and working memory training to down regulate state rumination: A single-session double blind sham-controlled trial. Cogn. Ther. Res. 2015, 39, 754–765. [Google Scholar] [CrossRef]
- De Raedt, R.; Remue, J.; Loeys, T.; Hooley, J.M.; Baeken, C. The effect of transcranial direct current stimulation of the prefrontal cortex on implicit self-esteem is mediated by rumination after criticism. Behav. Res. Ther. 2017, 99, 138–146. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Nelson, J.M.; Goodyear, C. Transcranial direct current stimulation versus caffeine as a fatigue countermeasure. Brain Stimul. 2017, 10, 1070–1078. [Google Scholar] [CrossRef]
- Vanderhasselt, M.A.; Sanchez, A.; Josephy, H.; Baeken, C.; Brunoni, A.R.; De Raedt, R. Anodal tDCS over the right dorsolateral prefrontal cortex modulates cognitive processing of emotional information as a function of trait rumination in healthy volunteers. Biol. Psychol. 2017, 123, 111–118. [Google Scholar] [CrossRef]
- Choy, O.; Raine, A.; Hamilton, R.H. Stimulation of the prefrontal cortex reduces intentions to commit aggression: A randomized, double-blind, placebo-controlled, stratified, parallel-group trial. J. Neurosci. 2018, 38, 6505–6512. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, A.; Riva, P.; Lauro, L.J.R.; Bushman, B.J. Stimulating the ventrolateral prefrontal cortex (VLPFC) modulates frustration-induced aggression: A tDCS experiment. Brain Stimul. 2020, 13, 302–309. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Amo, C.; Sánchez-Martínez, G.; Torrontegi, E.; Vázquez-Carrión, J.; Montalvo, Z.; Lucia, A.; de la Villa, P. Enhancement of Mood but not Performance in Elite Athletes with Transcranial Direct-Current Stimulation. Int. J. Sports Physiol. Perform. 2019, 14, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.T.; So, W.Y. Cranial electrotherapy stimulation affects mood state but not levels of peripheral neurotrophic factors or hypothalamic-pituitary-adrenal axis regulation. Technol. Health Care 2017, 25, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Demirtas-Tatlidede, A.; Freitas, C.; Pascual-Leone, A.; Schmahmann, J.D. Modulatory effects of theta burst stimulation on cerebellar nonsomatic functions. Cerebellum 2011, 10, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, A.; Ahn, S.; Alagapan, S.; Fröhlich, F. Modulating neural oscillations by transcranial static magnetic field stimulation of the dorsolateral prefrontal cortex: A crossover, double-blind, sham-controlled pilot study. Eur. J. Neurosci. 2019, 49, 250–262. [Google Scholar] [CrossRef]
- Perach-Barzilay, N.; Tauber, A.; Klein, E.; Chistyakov, A.; Ne’eman, R.; Shamay-Tsoory, S.G. Asymmetry in the dorsolateral prefrontal cortex and aggressive behavior: A continuous theta-burst magnetic stimulation study. Soc. Neurosci. 2013, 8, 178–188. [Google Scholar] [CrossRef]
- De Dreu, C.K.; Kret, M.E.; Sligte, I.G. Modulating prefrontal control in humans reveals distinct pathways to competitive success and collective waste. Soc. Cogn. Affect. Neurosci. 2016, 11, 1236–1244. [Google Scholar] [CrossRef][Green Version]
- Dambacher, F.; Schuhmann, T.; Lobbestael, J.; Arntz, A.; Brugman, S.; Sack, A.T. No effects of bilateral tDCS over inferior frontal gyrus on response inhibition and aggression. PLoS ONE 2015, 10, e0132170. [Google Scholar] [CrossRef]
- Riva, P.; Gabbiadini, A.; Lauro, L.J.R.; Andrighetto, L.; Volpato, C.; Bushman, B.J. Neuromodulation can reduce aggressive behavior elicited by violent video games. Cogn. Affect. Behav. Neurosci. 2017, 17, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Gilam, G.; Abend, R.; Gurevitch, G.; Erdman, A.; Baker, H.; Ben-Zion, Z.; Hendler, T. Attenuating anger and aggression with neuromodulation of the vmPFC: A simultaneous tDCS-fMRI study. Cortex 2018, 109, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y. Right ventrolateral prefrontal cortex involvement in proactive and reactive aggression: A transcranial direct current stimulation study. NeuroReport 2018, 29, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Dedoncker, J.; Vanderhasselt, M.A.; Remue, J.; De Witte, S.; Wu, G.R.; Hooley, J.M.; De Raedt, R.; Baeken, C. Prefrontal TDCS attenuates medial prefrontal connectivity upon being criticized in individuals scoring high on perceived criticism. Brain Imaging Behav. 2019, 13, 1060–1070. [Google Scholar] [CrossRef]
- Molero-Chamizo, A.; Riquel, R.M.; Moriana, J.A.; Nitsche, M.A.; Rivera-Urbina, G.N. Bilateral prefrontal cortex anodal tDCS effects on self-reported aggressiveness in imprisoned violent offenders. Neuroscience 2019, 397, 31–40. [Google Scholar] [CrossRef]
- Baruth, J.M.; Casanova, M.F.; El-Baz, A.; Horrell, T.; Mathai, G.; Sears, L.; Sokhadze, E. Low-frequency repetitive transcranial magnetic stimulation modulates evoked-gamma frequency oscillations in autism spectrum disorder. J. Neurother. 2010, 14, 179–194. [Google Scholar] [CrossRef]
- Casanova, M.F.; Baruth, J.M.; El-Baz, A.; Tasman, A.; Sears, L.; Sokhadze, E. Repetitive transcanial magnetic stimulation (RTMS) modulates event-related potential (ERP) indices of attention in autism. Transl. Neurosci. 2012, 3, 170–180. [Google Scholar] [CrossRef]
- Casanova, M.F.; Hensley, M.K.; Sokhadze, E.M.; El-Baz, A.S.; Wang, Y.; Li, X.; Sears, L. Effects of weekly low-frequency rTMS on autonomic measures in children with autism spectrum disorder. Front. Hum. Neurosci. 2014, 8, 851. [Google Scholar] [CrossRef]
- Sokhadze, E.M.; El-Baz, A.S.; Sears, L.L.; Opris, I.; Casanova, M.F. rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Front. Syst. Neurosci. 2014, 8, 134. [Google Scholar] [CrossRef]
- Wang, Y.; Hensley, M.K.; Tasman, A.; Sears, L.; Casanova, M.F.; Sokhadze, E.M. Heart rate variability and skin conductance during repetitive TMS course in children with autism. Appl. Psychophysiol. Biofeedback 2016, 41, 47–60. [Google Scholar] [CrossRef]
- Baeken, C.; De Raedt, R.; Leyman, L.; Schiettecatte, J.; Kaufman, L.; Poppe, K.; Vanderhasselt, M.A.; Anckaert, E.; Bossuyt, A. The impact of one HF-rTMS session on mood and salivary cortisol in treatment resistant unipolar melancholic depressed patients. J. Affect. Disord. 2009, 113, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fregni, F.; Brody, A.L.; Rahman, A.S. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front. Psychiatry 2013, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.; Magnavita, G.M.; Allegro, J.V.B.N.; Neto, C.E.B.P.; Lucena, R.D.C.S.; Fregni, F. Feasibility of transcranial direct current stimulation use in children aged 5 to 12 years. J. Child. Neurol. 2014, 29, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.B.; Tiberi, A.; Marshall, J. The use of cranial electrotherapy stimulation in the treatment of closed-head-injured patients. Brain Injury 1994, 8, 357–361. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, W.; Liu, X.; Xu, Q.; Tang, L.; Wu, S. Adjunctive treatment with high frequency repetitive transcranial magnetic stimulation for the behavioral and psychological symptoms of patients with Alzheimer’s disease: A randomized, double-blind, sham-controlled study. Shanghai Arch. Psychiatry 2015, 27, 280. [Google Scholar]
- Sun, W.; Mao, W.; Meng, X.; Wang, D.; Qiao, L.; Tao, W.; Li, L.; Jia, X.; Han, C.; Fu, M.; et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: A controlled clinical study. Epilepsia 2012, 53, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Hansbauer, M.; Wobrock, T.; Kunze, B.; Langguth, B.; Landgrebe, M.; Eichhammer, P.; Frank, E.; Cordes, J.; Wölwer, W.; Winterer, G.; et al. Efficacy of high-frequency repetitive transcranial magnetic stimulation on PANSS factors in schizophrenia with predominant negative symptoms–Results from an exploratory re-analysis. Psychiatry Res. 2018, 263, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.G.; Croxson, P.L. Behavioral control by the orbital prefrontal cortex: Reversal of fortune. Nat. Neurosci. 2013, 16, 984. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Raine, A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res. Neuroimaging 2009, 174, 81–88. [Google Scholar] [CrossRef]
- Batchelor, H.K.; Marriott, J.F. Paediatric pharmacokinetics: Key considerations. Br. J. Clin. Pharmacol. 2015, 79, 395–404. [Google Scholar] [CrossRef]
| Authors | Sample | Age, Gender and Handedness | Brain Structures | Brain Stimulation | Research Design | Main Results (Anger) |
|---|---|---|---|---|---|---|
| Normative and healthy sample | ||||||
| Self-reports | ||||||
| Schutter et al., [24] | 12 | 28.4 ± 8.90 | Right DLPFC | rTMS 1 Hz 20 min | Single blind, sham-controlled | STAS, absence differences |
| 67% men and 33% women | Single session | |||||
| Right-handed | ||||||
| Grisaru et al., [25] | 18 | 40.5 ± 11.60 | Left and right PFC | rTMS 1 Hz | Randomized, sham-controlled | VAS, absence differences |
| 39% men and 61% women | Four days | |||||
| Right-handed | ||||||
| Jenkins et al., [26] | 19 | 24.6 ± 5.30 | Left and right DLPFC | rTMS 1 Hz Two sessions spaced 2 weeks | Pseudo-randomization | POMS, absence differences |
| 47% men and 53% women | ||||||
| - | ||||||
| Schutter et al., [27] | 12 | From 18 to 25 | Left OFC | rTMS 1 Hz 20 min | Double-blind, sham-controlled | POMS and VAS, absence differences |
| 50% men and 50% women | Single session | |||||
| Right-handed | ||||||
| Hofman et al., [28] | 20 | 21.0 ± 1.81 | Right frontal cortex | rTMS 0.18 ± 0.02 Hz Single session | Pre-post design | Higher left-to-right transcallosal inhibition associated higher AQ score |
| 10% men and 90% women | ||||||
| Right-handed | ||||||
| Schaller et al., [29] | 38 | 24.0 ± 2.77 | Left DLPFC | rTMS | Double-blind, sham-controlled | BDI, decrease in irritability |
| 100% men | 25 Hz | VAS, absence differences | ||||
| Right-handed | 9 sessions/consec. days | |||||
| Baeken et al., [30] | 28 | 24.68 ± 5.85 | Left DLPFC | rTMS 10 Hz 20 min Single session | Single-blind, sham-controlled | POMS and VAS, absence differences |
| 100% women | ||||||
| Right-handed | ||||||
| Baeken et al., [31] | 27 | 25.2 ± 5.00 | Left DLPFC | rTMS 10 Hz 20 min Single session | Single-blind, sham-controlled | POMS and VAS, absence differences |
| 100% women | ||||||
| Right-handed | ||||||
| Koenigs et al., [32] | 21 | 25.6 ± 5.8 | Bilateral frontal cortex | tDCS anodal and cathodal 2.5 mA 35 min | Double-blind, sham-controlled | POMS, absence differences |
| 57% men and 43% women | Single session | |||||
| - | ||||||
| Leyman et al., [33] | 18 | 21.1 ± 1.45 | Left and right DLPFC | rTMS 10 Hz Two sessions, spaced 1 week | Single-blind, sham-controlled | VAS, absence differences |
| 100% women | ||||||
| Right-handed | ||||||
| Baeken et al., [34] | 20 | 23.30 ± 2.94 | Left and right DLPFC | rTMS 10 Hz 20 min | Single-blind, randomized | POMS, absence differences |
| 100% women | Single session | |||||
| - | ||||||
| Baeken et al., [35] | 24 | 22.29 ± 2.58 | Right DLPFC | rTMS 10 Hz 20 min | Single-blind, sham-controlled | Self-reported anger, absence differences |
| 100% women | Single session | |||||
| Right-handed | ||||||
| Baeken et al., [36] | 36 | 21.20 ± 1.44 | Right and left DLPFC | rTMS 10 Hz 20 min | Single-blind, randomized, sham-controlled | Self-reported anger, absence differences |
| 100% women | Single session | |||||
| Right-handed | ||||||
| Baumgartner et al., [37] | 36 | 24.3 ± 4.2 | Left temporo-parietal junction | rTMS 1 Hz 20 min Single session | Randomized, sham-controlled | Self-reported anger |
| 100% men | ||||||
| Right-handed | ||||||
| Baeken et al., [38] | 30 | 1.53 ± 2.85 | Left DLPFC | rTMS 20 Hz 20 min Single session | Single-blind, sham-controlled | Self-reported anger, not mediate changes |
| 100% women | ||||||
| Right-handed | ||||||
| Moulier et al., [39] | 20 | 33.7 ± 12.2 | Left DLPFC | rTMS 10 Hz 10 sessions/15 min/2 weeks | Double blind, Sham-controlled | VAS, absence differences |
| 60% men and 40% women | ||||||
| Right-handed | ||||||
| Iyer et al., [40] | 103 | 37.5 ± 12.9 | Left PFC | tDCS anodal and cathodal 1–2 mA 20 min | Single-blind, sham-controlled | VAS, absence differences |
| 46% men and 54% women | Single session | |||||
| Right-handed | ||||||
| Hortensius et al., [41] | 80 | - | Frontal cortex | tDCS 2 mA 15 min Single session | Double blind, randomized, sham-controlled | Self-reported anger, absence differences |
| 50% men and 50% women Right-handed | ||||||
| Plazier et al., [42] | 17 | 21.47 ± 0.91 | Right (anodal) and left (cathodal) DLPFC and occipital | tDCS 1.5 mA 20 min | Double blind, randomized, sham-controlled | Self-reported anger, absence differences |
| 100% men | Single session | |||||
| - | ||||||
| Motohashi et al., [43] | 12 | 22 ± 2.2 | Left DLPFC | tDCS 1 mA 4-daily 20 min | Single-blind, sham-controlled | POMS, absence differences |
| 100% men | Four days | |||||
| 83% right-handed | ||||||
| Kelley et al., [44] | 90 | - | Left and right PFC | tDCS 2 mA 15 min Single session | Double-blind, sham-controlled | Self-reported anger, absence differences |
| 33% men and 67% women | ||||||
| Right-handed | ||||||
| McIntire et al., [45] | 30 | 29.3 ± 3.4 | DLPFC | tDCS (anodal) + caffeine 2 mA 30 min | Randomized, sham-controlled | POMS and VAS, absence differences |
| 73% men and 26% women | Single session | |||||
| Right-handed | ||||||
| Vitor-Costa et al., [46] | 11 | 26 ± 4 | Primary motor cortex | tDCS 2 mA 30 min Three days, spaced 48 h | Single-blind, sham-controlled | Self-reported anger, not mediate changes |
| 100% men | ||||||
| - | ||||||
| Riva et al., [47] | 80 | 23.06 ± 4.36 | Right VLPFC | tDCS 1.5 mA 20 min Single session | Randomized, sham-controlled | STAS, absence differences |
| 21% men and 79% women | ||||||
| - | ||||||
| Dambacher et al., [48] | 64 | 21.89 ± 3.26 | Inferior frontal cortex | tDCS 1–2 mA 21.75 min | Randomized, sham-controlled | RPQ, absence differences |
| 61% men and 39% women | Single session | |||||
| - | ||||||
| De Putter et al., [49] | 66 | 23.09 ± 5.03 | DLPFC | tDCS 2 mA 25 min | Double blind, Sham-controlled | POMS, absence differences |
| 20% men and 80% women | Single session | |||||
| - | ||||||
| De Raedt et al., [50] | 32 | 22.6 ± 2.3 | DLPFC | tDCS (anodal) 1.5 mA 20 min | Single-blind, sham-controlled | STAS, absence differences |
| 100% women | Single session | |||||
| Right-handed | ||||||
| McIntire et al., [51] | 50 | 27 ± 5 | Left (anodal) and right (cathodal) DLPFC | tDCS + caffeine 2 mA | Random, sham-controlled | POMS, absence differences |
| 72% men and 28% women | 36 h | |||||
| - | ||||||
| Vanderhasselt et al., [52] | 35 | 23.40 ± 4.43 | Right DLPFC | tDCS (anodal) 2 mA 20 min | Single-blind, sham-controlled | VAS, absence differences |
| 31% men and 69% women | Single session | |||||
| Right-handed | ||||||
| Choy et al., [53] | 81 | 20 years | Bilateral DLPFC | tDCS (anodal) 2 mA 20 min | Double-Blind, Placebo-Controlled, Stratified, Parallel-Group Trial | Increases activation PFC less desire to commit physical and sexual assault (hypothetical vignettes/scenarios) |
| 44% men and 56% women | Two sessions | |||||
| - | ||||||
| Gallucci et al., [54] | 90 | 22.27 ± 2.46 | VLPFC | tDCS (anodal) 1.5 mA 20 min | Double blind, randomized placebo-controlled design; sham-controlled | STAS, absence differences |
| 50% men and 50% women | Single session | |||||
| - | ||||||
| Valenzuela et al., [55] | 8 | 27 ± 2 | Left primary motor cortex | tDCS (anodal) 2 mA 20 min | Double-blind, cross-over, sham-controlled | BMS, absence differences |
| 100% males | Single session | |||||
| - | ||||||
| Roh et al., [56] | 50 | 54.8 ± 2.8 | Earlobes of patients | CES 0.5 Hz 20 min 3 times/week; 8 weeks | Cross-over, sham-controlled | POMS, absence differences |
| 100% women | ||||||
| - | ||||||
| Demirtas-Tatlidede et al., [57] | 12 | 28.8 ± 9.94 | Vermis and cerebellar hemispheres | iTBS 10 burst/session Three sessions | Randomized | POMS, absence differences |
| 50% men and 50% women | ||||||
| Right-handed | ||||||
| Sheffield et al., [58] | 24 | 26.54 ± 12.28 | Left or right PFC (frontal alpha asymmetry) | tSMS Single session | Double-blind, sham-controlled | AQ, absence relationship cortical changes and AQ score |
| 54% men and 46% women | ||||||
| Right-handed | ||||||
| Laboratory tasks | ||||||
| Perach-Barzilay et al., [59] | 16 | 28 ± 4.68 | Left DLPFC | cTBS 5 Hz/50 bursts Single session | Randomized placebo-controlled design; sham-controlled | SOP, stimulation left DLPFC increased reactive and proactive aggression |
| 88% men and 12% women Right-handed | ||||||
| De Dreu et al., [60] | 18 | 25.16 ± 2.00 | Right inferior frontal gyrus | TBS Three sessions | Double-blind, sham-controlled | TAP, High activation entailed less aggression |
| 100% men | ||||||
| - | ||||||
| Hortensius et al., [41] | 80 | - | Frontal cortex | tDCS 2 mA 15 min Single session | Double blind, randomized placebo-controlled design; sham-controlled | TAP, left frontal activity entailed high aggression after provocation |
| 50% men and 50% women Right-handed | ||||||
| Riva et al., [47] | 80 | 23.06 ± 4.36 | Right VLPFC | tDCS 1.5 mA 20 min Single session | Randomized, sham-controlled | TAP, Anodal stimulation right VLPFC entailed less aggression in socially excluded participants after videogame exposure |
| 21% men and 79% women | ||||||
| - | ||||||
| Dambacher et al., [48] | 64 | 21.89 ± 3.26 | Bilateral inferior frontal cortex | tDCS 1.5 mA 21.75 min Single session | Randomized, sham-controlled | TAP, absence differences |
| 61% men and 39% women | ||||||
| - | ||||||
| Dambacher et al., [61] | 43 | 22.14 ± 2.00 | Right DLPFC | tDCS 2 mA (20 phases) 750 s | Randomized placebo-controlled design sham-controlled | TAP, right hemispheric dominance reduced proactive aggression in men |
| 47% men and 53% women | Single session | |||||
| - | ||||||
| Riva et al., [62] | 79 | 21.73 ± 2.38 | Right VLPFC | tDCS (anodal) 1.5 mA 20 min | Randomized placebo-controlled design; sham-controlled | TAP, Lower levels of aggressive behaviour |
| 52% men and 48% women | Single session | |||||
| - | ||||||
| Gilam et al., [63] | 25 | 26.16 ± 3.63 | Bilateral VMPFC | tDCS (anodal) | Double-blind, sham-controlled | Increased activation entailed less self-reported anger after provocation |
| 40% men and 60% women | 1.2 mA 22 min Two sessions | |||||
| - | ||||||
| Chen et al., [64] | 32 | 20–22 years | Right VLPFC | tDCS 2 mA 20 s | Randomized, sham-controlled | TAP, Reduction in proactive and reactive aggression |
| 50% men and 50% women | Single session | |||||
| - | ||||||
| Gallucci et al., [54] | 90 | 22.27 ± 2.46 | Left VLPFC | tDCS (anodal) 1.5 mA 20 min | Double-blind, randomized sham-controlled | Left VLPFC increased aggression. |
| 50% men and 50% women | Single session | Males were more aggressive than females | ||||
| - | ||||||
| Dedoncker et al., [65] | 41 | 22.9 ± 2.61 | Left DLPFC | tDCS (anodal) 1.5 mA 20 min | Randomized sham-controlled | VAS, absence changes |
| 100% females | Single session | |||||
| Right-handed | ||||||
| Violent individuals (inmates) | ||||||
| Molero-Chamizo et al., [66] | 41 | 36.2 ± 12.3 | Bilateral PFC | tDCS (anodal) | Single-blind, sham-controlled | AQ, murders experienced reductions in the physical and verbal aggression |
| 100% men | 1.5 mA 15 min | |||||
| - | 3 sessions/consec. days | |||||
| Pathological conditions | ||||||
| Autism spectrum disorders | ||||||
| Baruth et al., [67] | 25 | 13.9 ± 5.3 | Bilateral DLPFC | rTMS | Randomized-controlled (waiting list) | ABC, reductions in irritability |
| 84% men and 16% women | 1 Hz | |||||
| - | 12 sessions 30 min | |||||
| 12 sessions/weeks | ||||||
| Casanova et al., [68] | 45 | 13.0 ± 2.7 | Bilateral DLPFC | rTMS | Randomized-controlled (waiting list) | ABC, reductions in irritability |
| 87% men and 13% women | 1 Hz 30 min | |||||
| - | 12 sessions/weeks | |||||
| Casanova et al., [69] | 18 | 13.1 ± 2.2 | Bilateral DLPFC | rTMS | Pre-post design | ABC, reductions in irritability |
| 78% men and 12% women | 0.5 Hz 30 min | |||||
| - | 18 weeks/sessions | |||||
| Sokhadze et al., [70] | 54 | 14.5 ± 2.9 | Bilateral DLPFC | rTMS | Randomized-controlled (waiting list) | ABC, reductions in irritability |
| 81% men and 19% women | 1 Hz 30 min | |||||
| - | 18 weeks/sessions | |||||
| Wang et al., [71] | 33 | 12.9 ± 3.8 | Bilateral DLPFC | rTMS | Pre-post design | ABC, reductions in irritability |
| 84% men and 16% women | 0.5 Hz 30 min | |||||
| - | 12 weeks/sessions | |||||
| Unipolar depressed patients of the melancholic subtype (free drugs) | ||||||
| Baeken et al., [72] | 20 | 44.3 ± 10.6 | Left DLPFC | rTMS active | Single-blind, sham-controlled | POMS, absence differences |
| 35% men and 65% female | 10 Hz 20 min | |||||
| Right-handed | Single session | |||||
| Abstinent smokers | ||||||
| Xu et al., [73] | 24 | 45 ± 7.6 | Left DLPFC (anodal) and right supraorbital area (cathodal) | tDCS (anodal) 2 mA 20 min | Single-blind, sham-controlled | POMS, absence differences |
| 87% men and 13% women | Two sessions | |||||
| - | ||||||
| Language disorders | ||||||
| Andrade et al., [74] | 14 | From 5 to 12 | Anode (Broca area (mid-left inferior frontal gyrus) and cathode right supraorbital area. | tDCS | Pre-post design | 35.7% increased irritability: severe (14.3%), moderate (14.3%) and mild (7.1%). |
| 71% men and 29% women | 10 sessions, 2 days interval | |||||
| - | ||||||
| Closed-head injury | ||||||
| Smith et al., [75] | 21 | - | Earlobes of patients | CES | Double-blind, sham-controlled | POMS, reductions anger |
| 100 Hz | ||||||
| 4 days/week for 3 weeks | ||||||
| Alzheimer | ||||||
| Wu et al., [76] | 52 | From 70 to 80 | Left DLPFC | rTMS + low dose risperidone | Double-blind, sham-controlled | BEHAVE-AD, reductions aggressiveness |
| 40% men and 60% women | 20 Hz | |||||
| - | 5 sessions/week for 4 weeks | |||||
| Refractory partial epilepsy | ||||||
| Sun et al., [77] | 60 | 21 years (average) | Epileptogenic | rTMS + antiepileptic drugs (unchanged dose) | Single-blind, sham-controlled | SCL-90-R, absence changes |
| 68% men and 32% women | focus | 0.5 Hz | ||||
| - | Daily for 3 weeks | |||||
| Schizophrenia | ||||||
| Hansbauer et al., [78] | 146 | 36 years (average) | Left DLPFC | rTMS | Double-blind, sham-controlled | PANSS, absence changes |
| 75% men and 25% women | 10 Hz | |||||
| 82% righ-handed | 5 sessions/week for 3 weeks | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Martínez, Á.; Bressanutti, S.; Moya-Albiol, L. A Systematic Review of the Effectiveness of Non-Invasive Brain Stimulation Techniques to Reduce Violence Proneness by Interfering in Anger and Irritability. J. Clin. Med. 2020, 9, 882. https://doi.org/10.3390/jcm9030882
Romero-Martínez Á, Bressanutti S, Moya-Albiol L. A Systematic Review of the Effectiveness of Non-Invasive Brain Stimulation Techniques to Reduce Violence Proneness by Interfering in Anger and Irritability. Journal of Clinical Medicine. 2020; 9(3):882. https://doi.org/10.3390/jcm9030882
Chicago/Turabian StyleRomero-Martínez, Ángel, Sara Bressanutti, and Luis Moya-Albiol. 2020. "A Systematic Review of the Effectiveness of Non-Invasive Brain Stimulation Techniques to Reduce Violence Proneness by Interfering in Anger and Irritability" Journal of Clinical Medicine 9, no. 3: 882. https://doi.org/10.3390/jcm9030882
APA StyleRomero-Martínez, Á., Bressanutti, S., & Moya-Albiol, L. (2020). A Systematic Review of the Effectiveness of Non-Invasive Brain Stimulation Techniques to Reduce Violence Proneness by Interfering in Anger and Irritability. Journal of Clinical Medicine, 9(3), 882. https://doi.org/10.3390/jcm9030882





