High-Frequency Near-Infrared Diode Laser Irradiation Attenuates IL-1β-Induced Expression of Inflammatory Cytokines and Matrix Metalloproteinases in Human Primary Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Laser Irradiation
2.3. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.4. Two-Color Western Blot Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analysis
3. Results
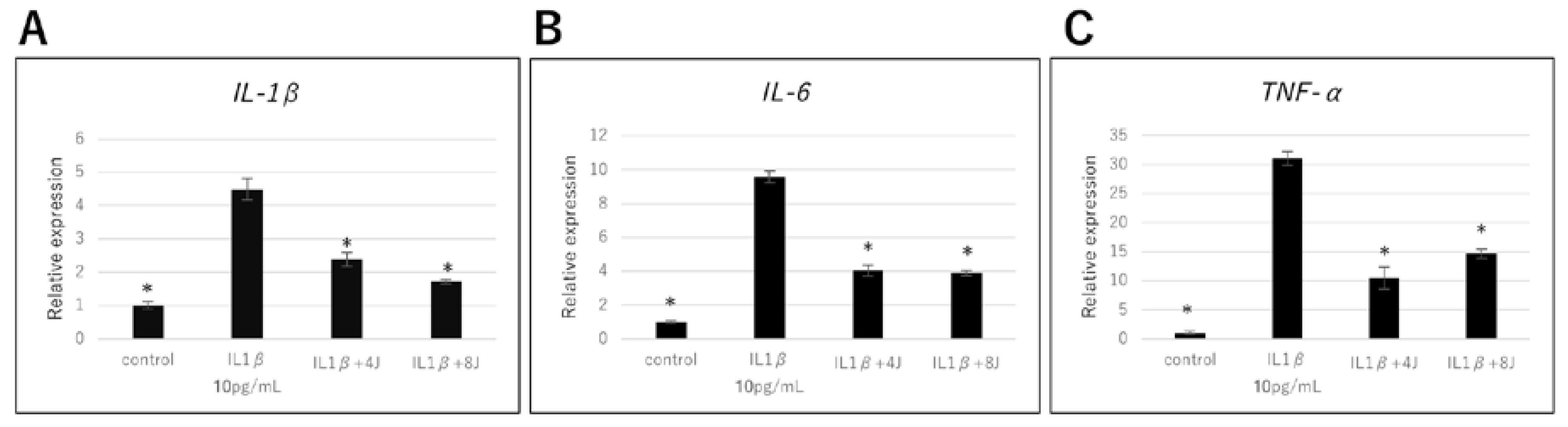
3.1. Effect of High-Frequency Near-Infrared Diode Laser Irradiation on Gene Expression of Inflammatory Cytokines in NHAC-Kn Cells
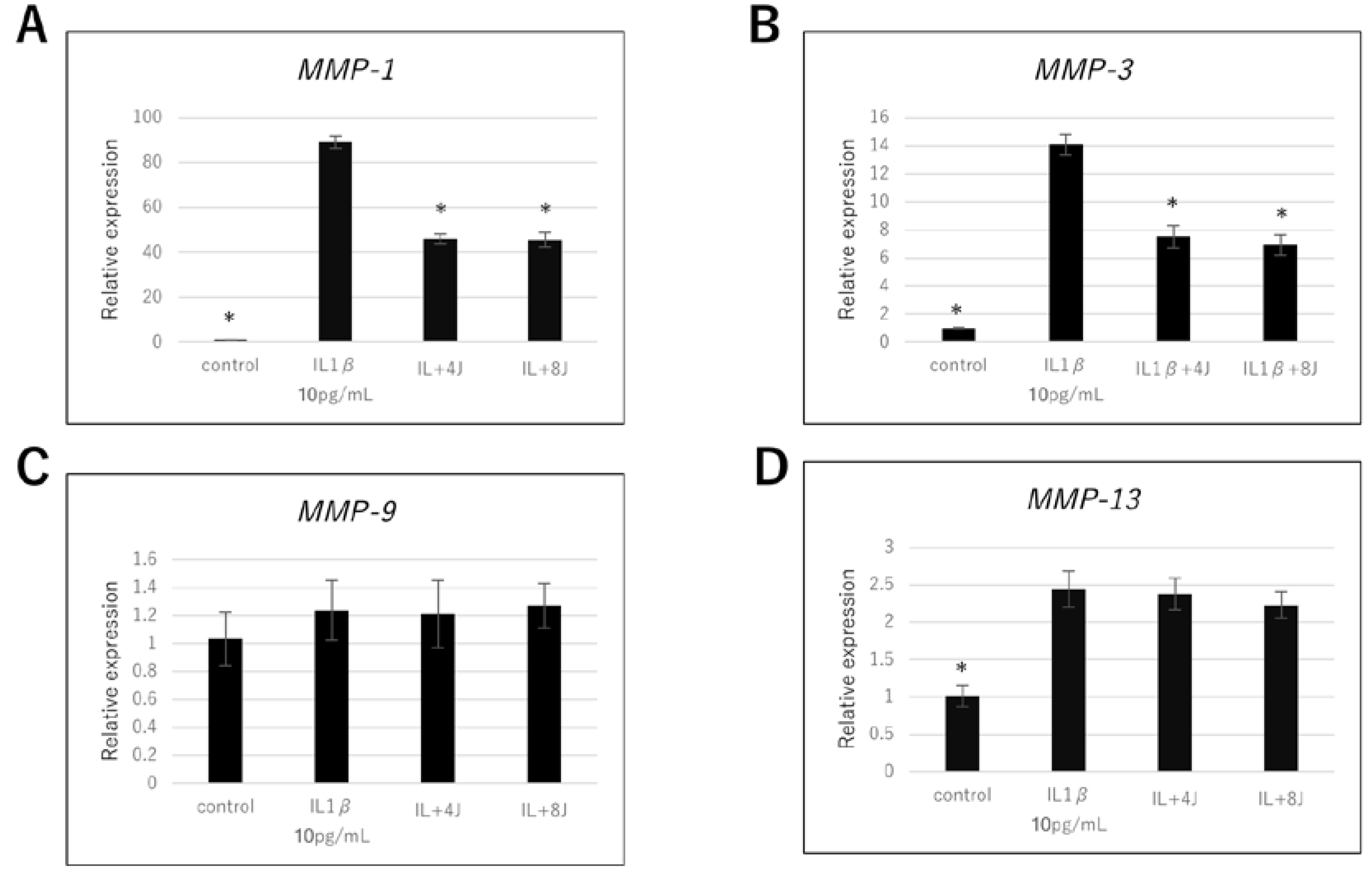
3.2. Effect of High-Frequency Near-Infrared Diode Laser Irradiation on Gene Expression of MMPs in NHAC-Kn Cells
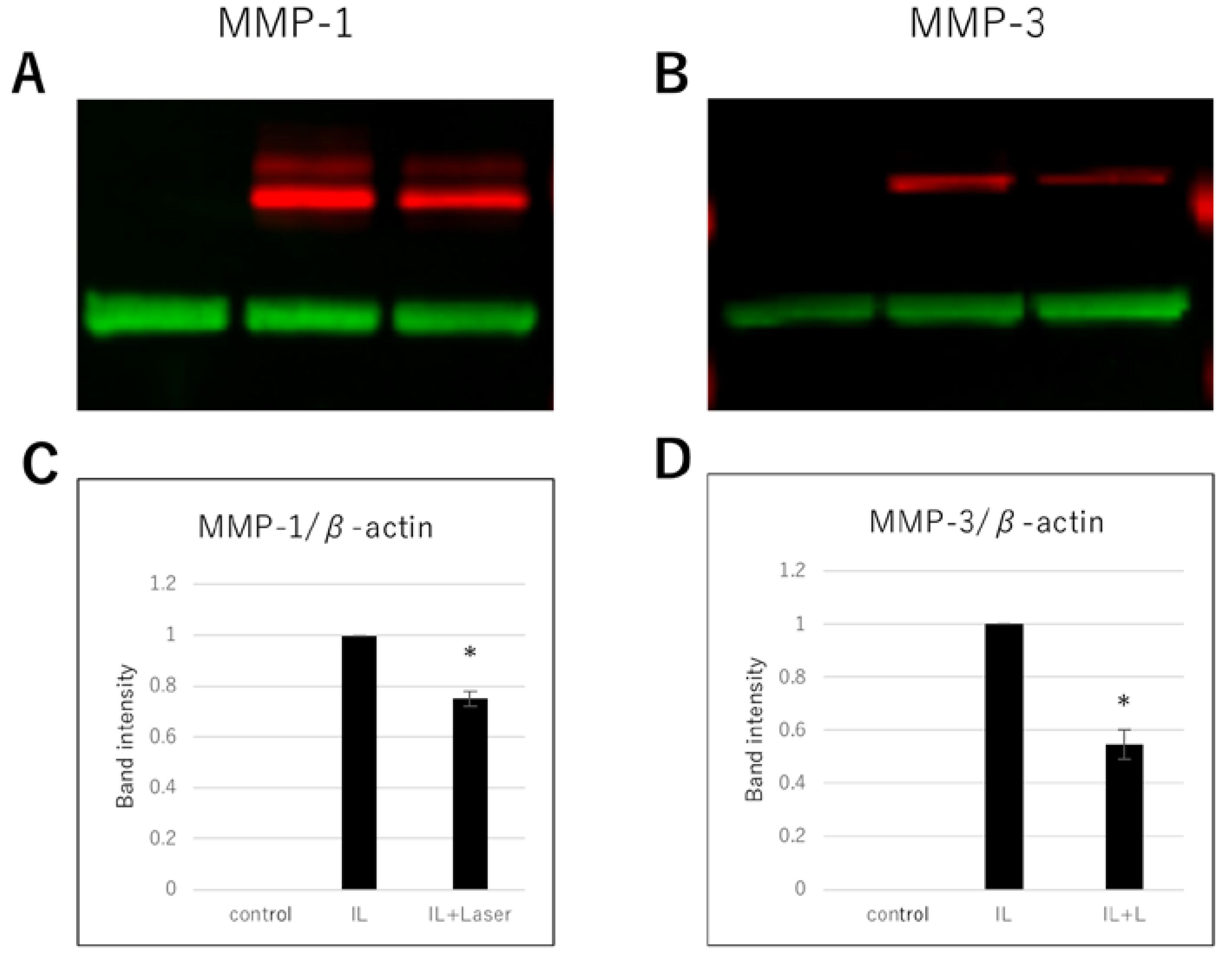
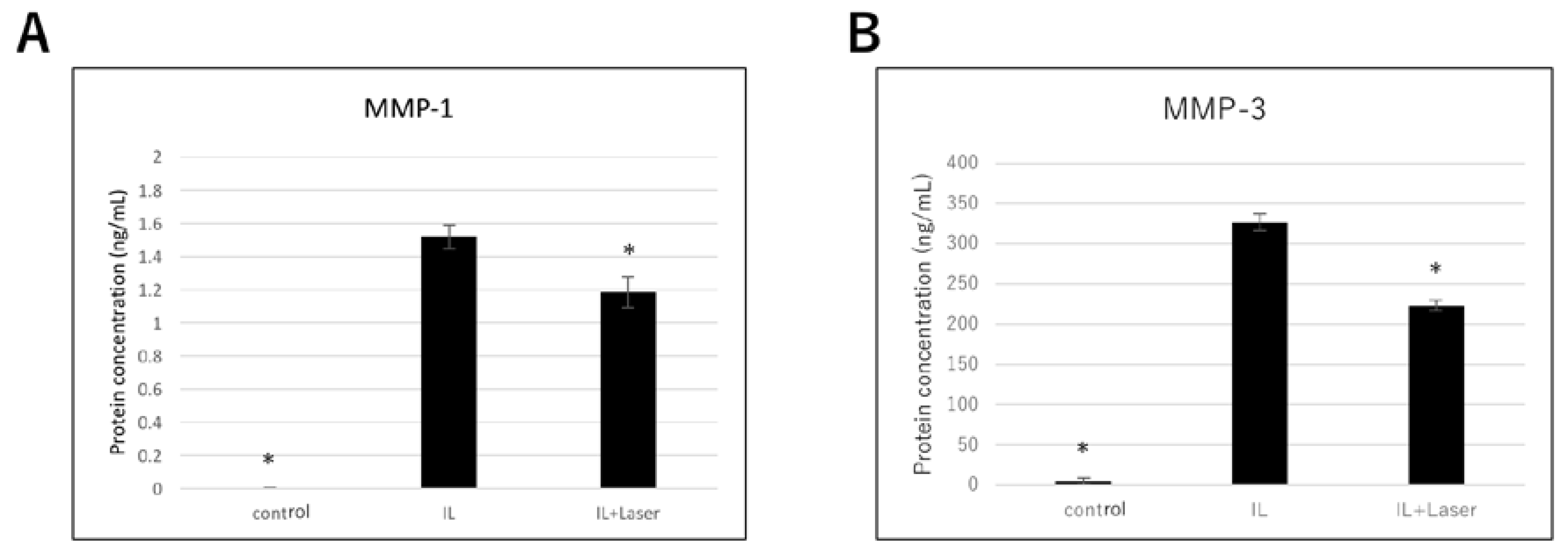
3.3. Effect of High-Frequency Near-Infrared Diode Laser Irradiation on Protein Expression of MMPs in NHAC-Kn Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messier, S.P.; Callahan, L.F.; Beavers, D.P.; Queen, K.; Mihalko, S.L.; Miller, G.D.; Losina, E.; Katz, J.N.; Loeser, R.F.; Quandt, S.A.; et al. Weight-loss and exercise for communities with arthritis in North Carolina (we-can): Design and rationale of a pragmatic, assessor-blinded, randomized controlled trial. BMC Musculoskelet. Disord. 2017, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Jerosch, J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: Outlook on other nutrient partners especially omega-3 fatty acids. Int. J. Rheumatol. 2011, 2011, 969012. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.; Assis, L.; Criniti, C.; Fernandes, D.; Tim, C.; Renno, A.C.M. Chondroitin sulfate and glucosamine sulfate associated to photobiomoulation prevents degenerative morphological changes in an experimental model of osteoarthritis in rats. Lasers Med. Sci. 2018, 33, 549–557. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, W.B. The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z. Rheumatol. 1999, 58, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Shlopov, B.V.; Lie, W.R.; Mainardi, C.L.; Cole, A.A.; Chubinskaya, S.; Hasty, K.A. Osteoarthritic lesions: Involvement of three different collagenases. Arthritis Rheum. 1997, 40, 2065–2074. [Google Scholar] [CrossRef]
- Loeser, R.F. Molecular mechanisms of cartilage destruction in osteoarthritis. J. Musculoskelet. Nueronal Interact. 2008, 8, 303–306. [Google Scholar]
- Loeser, R.F. Molecular mechanisms of cartilage destruction: Mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006, 54, 1357–1360. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Cimmino, M.A.; Scarpa, R.; Caporali, R.; Parazzini, F.; Zaninelli, A.; Atzeni, F.; Canesi, B. Osteoarthritis: An overview of the disease and its treatment strategies. Semin. Arthritis Rheum. 2005, 35, 1–10. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Roos, E.M. Clinical update: Treating osteoarthritis. Lancet 2007, 370, 2082–2084. [Google Scholar] [CrossRef]
- Ohno, S.; Schmid, T.; Tanne, Y.; Kamiya, T.; Honda, K.; Ohno-Nakahara, M.; Swentko, N.; Desai, T.A.; Tanne, K.; Knudson, C.B.; et al. Expression of superficial zone protein in mandibular condyle cartilage. Osteoarthr. Cartil. 2006, 14, 807–813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamiya, T.; Tanimoto, K.; Tanne, Y.; Lin, Y.Y.; Kunimatsu, R.; Yoshioka, M.; Tanaka, N.; Tanaka, E.; Tanne, K. Effects of mechanical stimuli on the synthesis of superficial zone protein in chondrocytes. J. Biomed. Mater. Res. Part A 2010, 92, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Asakawa-Tanne, Y.; Su, S.; Kunimatsu, R.; Hirose, N.; Mitsuyoshi, T.; Okamoto, Y.; Tanaka, E.; Tanne, K.; Tanimoto, K. Effects of enzymatic degradation after loading in temporomandibular joint. J. Dent. Res. 2015, 94, 337–343. [Google Scholar] [CrossRef]
- Tanne, K. Degenerative changes of articular cartilage in association with mechanical stimuli. Jpn. Dent. Sci. Rev. 2008, 44, 38–47. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef]
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000, 43, 1905–1915. [Google Scholar] [CrossRef]
- Brown, G.A. AAOS clinical practice guideline: Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd edition. J. Am. Acad. Orthop. Surg. 2013, 21, 577–579. [Google Scholar] [CrossRef]
- Su, S.C.; Tanimoto, K.; Tanne, Y.; Kunimatsu, R.; Hirose, N.; Mitsuyoshi, T.; Okamoto, Y.; Tanne, K. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthr. Cartil. 2014, 22, 845–851. [Google Scholar] [CrossRef]
- Yanoshita, M.; Hirose, N.; Okamoto, Y.; Sumi, C.; Takano, M.; Nishiyama, S.; Asakawa-Tanne, Y.; Horie, K.; Onishi, A.; Yamauchi, Y.; et al. Cyclic tensile strain upregulates pro-inflammatory cytokine expression via FAK-MAPK signaling in chondrocytes. Inflammation 2018, 41, 1621–1630. [Google Scholar] [CrossRef]
- Sumi, C.; Hirose, N.; Yanoshita, M.; Takano, M.; Nishiyama, S.; Okamoto, Y.; Asakawa, Y.; Tanimoto, K. Semaphorin 3A inhibits inflammation in chondrocytes under excessive mechanical stress. Mediat. Inflamm. 2018, 8, 5703651. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.A.G.; Hernandez-Diaz, S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res. 2001, 3, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Meek, I.L.; Van de Laar, M.A.; Vonkeman, H.E. Non-steroidal anti-inflammatory drugs: An overview of cardiovascular risks. Pharmaceuticals 2010, 3, 2146–2162. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Li, H.; Yang, T.; Deng, Z.H.; Yang, Y.; Zhang, Y.; Ding, X.; Lei, G.H. Effectiveness of continuous and pulsed ultrasound for the management of knee osteoarthritis: A systematic review and network meta-analysis. Osteoarthr. Cartil. 2014, 22, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.D.O.; Pompeo, K.D.; Brodt, G.A.; Baroni, B.M.; da Silva Junior, D.P.; Vaz, M.A. Effects of neuromuscular electrical stimulation and low-level laser therapy on the muscle architecture and functional capacity in elderly patients with knee osteoarthritis: A randomized controlled trial. Clin. Rehabil. 2015, 29, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Uthman, O.A.; van der Windt, D.A.; Jordan, J.L.; Dziedzic, K.S.; Healey, E.L.; Peat, G.M.; Foster, N.E. Exercise for lower limb osteoarthritis: Systematic review incorporating trial sequential analysis and network meta-analysis. Br. J. Sports Med. 2014, 48, 1579. [Google Scholar] [CrossRef]
- Tenti, S.; Cheleschi, S.; Galeazzi, M.; Fioravanti, A. Spa therapy: Can be a valid option for treating knee osteoarthritis? Int. J. Biometeorol. 2015, 59, 1133–1143. [Google Scholar] [CrossRef]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Johnson, M.I.; Lopes-Martins, R.A.; Bogen, B.; Chow, R.; Ljunggren, A.E. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet. Disord. 2007, 8, 51. [Google Scholar] [CrossRef]
- Baltzer, A.W.; Ostapczuk, M.S.; Stosch, D. Positive effects of low level laser therapy (LLLT) on Bouchard’s and Heberden’s osteoarthritis. Lasers Surg. Med. 2016, 48, 498–504. [Google Scholar] [CrossRef]
- Langella, L.G.; Casalechi, H.L.; Tomazoni, S.S.; Johnson, D.S.; Albertini, R.; Pallotta, R.C.; Marcos, R.L.; de Carvalho, P.T.C.; Leal-Junior, E.C.P. Photobiomodulation therapy (PBMT) on acute pain and inflammation in patients who underwent total hip arthroplasty-a randomized, triple-blind, placebo-controlled clinical trial. Lasers Med. Sci. 2018, 33, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.T.; Huang, Y.Y.; Sharma, S.K.; Kurup, D.B.; De Taboada, L.; Carroll, J.D.; Hamblin, M.R. Effect of pulsing in low-level light therapy. Lasers Surg. Med. 2010, 42, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Barolet, D.; Duplay, P.; Jacomy, H.; Auclair, M. Importance of pulsing illumination parameters in low-level-light therapy. J. Biomed. Opt. 2010, 15, 048005. [Google Scholar] [CrossRef] [PubMed]
- Al-Watban, F.A.; Zhang, X.Y. The comparison of effects between pulsed and CW lasers on wound healing. J. Clin. Laser Med. Surg. 2004, 22, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dai, T.; Hamblin, M.R. Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med. Sci. 2014, 29, 257–265. [Google Scholar] [CrossRef]
- Joensen, J.; Ovsthus, K.; Reed, R.K.; Hummelsund, S.; Iversen, V.V.; Lopes-Martins, R.A.; Bjordal, J.M. Skin penetration time-profiles for continuous 810 nm and superpulsed 904 nm lasers in a rat model. Photomed. Laser Surg. 2012, 30, 688–694. [Google Scholar] [CrossRef]
- Kymplova, J.L.; Navratil, L.; Knizek, J. Contribution of phototherapy to the treatment of episiotomies. J. Clin. Laser Med. Surg. 2003, 21, 35–39. [Google Scholar] [CrossRef]
- Baroni, B.M.; Leal Junior, E.C.; De Marchi, T.; Lopes, A.L.; Salvador, M.; Vaz, M.A. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur. J. Appl. Physiol. 2010, 110, 789–796. [Google Scholar] [CrossRef]
- Keshri, G.K.; Gupta, A.; Yadav, A.; Sharma, S.K.; Singh, S.B. Photobiomodulation with pulsed and continuous wave near-infrared laser (810 nm, Al-Ga-As) augments dermal wound healing in immunosuppressed rats. PLoS ONE 2016, 18, e0166705. [Google Scholar] [CrossRef]
- Mikami, R.; Mizutani, K.; Aoki, A.; Tamura, Y.; Aoki, K.; Izumi, Y. Low-level ultrahigh-frequency and ultrashort-pulse blue laser irradiation enhances osteoblast extracellular calcification by upregulating proliferation and differentiation via transient receptor potential vanilloid 1. Lasers Surg. Med. 2018, 50, 340–352. [Google Scholar] [CrossRef]
- Ejiri, K.; Aoki, A.; Yamaguchi, Y.; Ohshima, M.; Izumi, Y. High-frequency low-level diode laser irradiation promotes proliferation and migration of primary cultured human gingival epithelial cells. Lasers Med. Sci. 2014, 29, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, R.; Gunji, H.; Tsuka, Y.; Yoshimi, Y.; Awada, T.; Sumi, K.; Nakajima, K.; Kimura, A.; Hiraki, T.; Abe, T.; et al. Effects of high-frequency near-infrared diode laser irradiation on the proliferation and migration of mouse calvarial osteoblasts. Lasers Med. Sci. 2018, 33, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Aoki, A.; Mizutani, K.; Lin, T.; Komaki, M.; Shibata, S.; Izumi, Y. High-frequency pulsed low-level diode laser therapy accelerates wound healing of tooth extraction socket: An in vivo study. Lasers Surg. Med. 2016, 48, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Marini, I.; Gatto, M.R.; Bonetti, G.A. Effects of superpulsed low-level laser therapy on temporomandibular joint pain. Clin. J. Pain 2010, 26, 611–616. [Google Scholar] [CrossRef]
- Kheshie, A.R.; Alayat, M.S.; Ali, M.M. High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: A randomized controlled trial. Lasers Med. Sci. 2014, 29, 1371–1376. [Google Scholar] [CrossRef]
- Gunji, H.; Kunimatsu, R.; Tsuka, Y.; Yoshimi, Y.; Sumi, K.; Awada, T.; Nakajima, K.; Kimura, A.; Hiraki, T.; Hirose, N.; et al. Effect of high-frequency near-infrared diode laser irradiation on periodontal tissues during experimental tooth movement in rats. Lasers Surg. Med. 2018, 50, 772–780. [Google Scholar] [CrossRef]
- Bortone, F.; Santos, H.A.; Albertini, R.; Pesquero, J.B.; Costa, M.S.; Silva, J.A., Jr. Low level laser therapy modulates kinin receptors mRNA expression in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation. Int. Immunopharmacol. 2008, 8, 206–210. [Google Scholar] [CrossRef]
- Correa, F.; Martins, R.A.B.L.; Correa, J.C.; Iversen, V.V.; Joenson, J.; Bjordal, J.M. Low-level laser therapy (GaAs lambda = 904 nm) reduces inflammatory cell migration in mice with lipopolysaccharide-induced peritonitis. Photomed. Laser Surg. 2007, 25, 245–249. [Google Scholar] [CrossRef]
- Pires, D.; Xavier, M.; Araujo, T.; Silva, J.A., Jr.; Aimbire, F.; Albertini, R. Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med. Sci. 2011, 26, 85–94. [Google Scholar] [CrossRef]
- Boschi, E.S.; Leite, C.E.; Saciura, V.C.; Caberlon, E.; Lunardelli, A.; Bitencourt, S.; Melo, D.A.; Oliveira, J.R. Anti-inflammatory effects of low-level laser therapy (660 nm) in the early phase in carrageenan-induced pleurisy in rat. Lasers Surg. Med. 2008, 40, 500–508. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, C.H.; Wang, C.Z.; Ho, M.L.; Yeh, M.L.; Wang, Y.H. Low-power laser irradiation suppresses inflammatory response of human adipose-derived stem cells by modulating intracellular cyclic AMP level and NF-kappaB activity. PLoS ONE 2013, 8, e54067. [Google Scholar] [CrossRef] [PubMed]
- Citalingam, K.; Zareen, S.; Shaari, K.; Ahmad, S. Effects of Payena dasyphylla (Miq.) on hyaluronidase enzyme activity and metalloproteinases protein expressions in interleukin-1β stimulated human chondrocytes cells. BMC Complement. Altern. Med. 2013, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Fu, Y.; Zhuang, Y.; Zhang, K.; Lu, D. LncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci. Rep. 2019, 39, BSR20191523. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; Vieira, R.; Leal-Junior, E.; dos Santos, S.; Ligeiro, A.P.; Albertini, R.; Junior, J.; de Carvalho, P. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res. Ther. 2013, 15, R116. [Google Scholar] [CrossRef]
- Dos Santos, S.A.; Alves, A.C.; Leal-Junior, E.C.; Albertini, R.; Vieira, R.P.; Ligeiro, A.P.; Junior, J.A.; de Carvalho, P.D.T. Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med. Sci. 2014, 29, 1051–1058. [Google Scholar] [CrossRef]
- Stancker, T.G.; Vieira, S.S.; Serra, A.J.; do Nascimento Lima, R.; dos Santos Feliciano, R.; Silva, J.A., Jr.; dos Santos, S.A.; dos Santos Vieira, M.A.; Simões, M.C.B.; Leal-Junior, E.C.; et al. Can photobiomodulation associated with implantation of mesenchymal adipose-derived stem cells attenuate the expression of MMPs and decrease degradation of type II collagen in an experimental model of osteoarthritis? Lasers Med. Sci. 2018, 33, 1073–1084. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Leal-Junior, E.C.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Lopes-Martins, R.Á. Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med. Sci. 2017, 32, 101–108. [Google Scholar] [CrossRef]
- Alameddine, H.S.; Morgan, J.E. Matrix metalloproteinase and tissue inhibitor of metalloproteinases in inflammation and fibrosis of skeletal muscles. J. Neuromuscul. Dis. 2016, 3, 455–473. [Google Scholar] [CrossRef]
- Yamamoto, T.; Murphy, G.; Troeberg, L. Extracellular regulation of metalloproteinases. Matrix Biol. 2015, 44, 255–263. [Google Scholar] [CrossRef]
- Naito, K.; Takahashi, M.; Kushida, K.; Suzuki, M.; Ohishi, T.; Miura, M.; Inoue, T.; Nagano, A. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: Comparison with generalized osteoarthritis. Rheumatology 1999, 38, 510–515. [Google Scholar] [CrossRef][Green Version]
- Farley, J.; Dejica, V.C.; Mort, J.S. Proteases and cartilage degradation in osteoarthritis. In Principles of Osteoarthritis—Its Definition, Character, Derivation and Modality-Related Recognition; Rothschild, B.M., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Goldring, M.B. Articular cartilage degradation in osteoarthritis. HSS J. 2012, 8, 7–9. [Google Scholar] [CrossRef]
- Dejica, V.M.; Mort, J.S.; Laverty, S.; Antoniou, J.; Zukor, D.J.; Tanzer, M.; Poole, A.R. Increased type II collagen cleavage by cathepsin K and collagenase activities with aging and osteoarthritis in human articular cartilage. Arthritis Res. Ther. 2012, 14, R113. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Kabata, T.; Hayashi, K.; Maeda, T.; Kajino, Y.; Iwai, S.; Fujita, K.; Hasegawa, K.; Inoue, D.; Sugimoto, N.; et al. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskelet. Disord. 2015, 16, 236. [Google Scholar] [CrossRef]
- Ter Huurne, M.; Schelbergen, R.; Blattes, R.; Blom, A.; de Munter, W.; Grevers, L.C.; Jeanson, J.; Noël, D.; Casteilla, L.; Jorgensen, C.; et al. Anti-inflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012, 64, 3604–3613. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.R.; Vieira, G.P.; Marques, P.P.; Oliveira, L.P.; Pimentel, E.R. Low level laser therapy accelerates the extracellular matrix reorganization of inflamed tendon. Tissue Cell 2017, 49, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.G.; Magna, H.A.; Reeves, L.M.; Lopresti-Morrow, L.L.; Yocum, S.A.; Rosner, P.J.; Geoghegan, K.F.; Hambor, J.E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996, 97, 761–768. [Google Scholar] [CrossRef]
- Singh, A.; Rayasekaran, N.; Hartenstein, B. Collagenase-3 (MMP-13) deficiency protects C57BL/6 mice from antibody-induced arthritis. Arthritis Res. Ther. 2013, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wong, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res. Ther. 2017, 19, 1–12. [Google Scholar] [CrossRef]
- Fosang, A.J.; Last, K.; Knauper, V.; Murphy, G.; Neame, P.J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13). FEBS Lett. 1996, 12, 17–20. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteinases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Bonnas, C.; Chou, J.; Werb, Z. Remodeling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.R.; Vieira, C.P.; Almeida, M.S.; Oliveira, L.P.; Aro, A.A.; Pimentel, E.R. LLLT improves tendon healing through increase of MMP activity and collagen synthesis. Laser Med. Sci. 2013, 28, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Lemos, G.A.; Rissi, R.; Pires, I.L.S.; de Oliveira, L.P.; de Aro, A.A.; Pimentel, E.R.; Palomari, E.T. Low-level laser therapy stimulates repair and reduces the extracellular matrix degradation in rats with induced arthritis in the temporomandibular joint. Lasers Med. Sci. 2016, 31, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Casalechi, H.L.; Leal-Junior, E.C.P.; Xavier, M.; Silva, J.A.; de Carvalho, P.D.T.C.; Aimbire, F.; Albertini, R. Low-level laser therapy in experimental model of collagenase-induced tendinitis in rats: Effects in acute and chronic inflammatory phases. Laser Med. Sci. 2012, 28, 989–995. [Google Scholar] [CrossRef]
- Wang, P.; Liu, C.; Yang, X.; Zhou, Y.; Wei, X.; Ji, Q.; Yang, L.; He, C. Effects of low-level laser therapy on joint pain, synovitis, anabolic, and catabolic factors in a progressive osteoarthritis rabbit model. Lasers Med. Sci. 2014, 29, 1875–1885. [Google Scholar] [CrossRef]
- Alves, A.C.; Albertini, R.; dos Santos, S.A.; Leal-Junior, E.C.; Santana, E.; Serra, A.J.; Silva, J.A., Jr.; de Carvalho, P.T.D.C. Effect of low-level laser therapy on metalloproteinase MMP-2 and MMP-9 production and percentage of collagen types I and III in a papain cartilage injury model. Lasers Med. Sci. 2014, 29, 911–919. [Google Scholar] [CrossRef]
- Yin, K.; Zhu, R.; Wang, S.; Zhao, R.C. Low level laser (LLL) attenuate LPS-induced inflammatory responses in mesenchymal stem cells via the suppression of NF-κB signaling pathway in vitro. PLoS ONE 2017, 12, e0179175. [Google Scholar] [CrossRef]
- Mengshol, J.A.; Vincenti, M.P.; Coon, C.I.; Barchowsky, A.; Brinckerhoff, C.E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000, 43, 801–811. [Google Scholar] [CrossRef]
- Zeng, L.; Rong, X.F.; Li, R.H.; Wu, X.Y. Icariin inhibits MMP1, MMP3 and MMP13 expression through MAPK pathways in IL1beta-stimulated SW1353 chondrosarcoma cells. Mol. Med. Rep. 2017, 5, 2853–2858. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D.; Gao, X.; Wu, S. Low-power laser irradiation promotes cell proliferation by activating PI3K/Akt pathway. J. Cell. Physiol. 2009, 219, 553–562. [Google Scholar] [CrossRef]
- Huang, L.; Wu, S.; Xing, D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3beta signaling pathway. J. Cell. Physiol. 2011, 226, 588–601. [Google Scholar] [CrossRef] [PubMed]
| Parameter | (Unit) | Value |
|---|---|---|
| Wavelength | (nm) | 910 |
| Operating mode | pulsed | |
| Pulse duration | (ns) | 200 |
| Frequency | (kHz) | 30 |
| Duty cycle | 0.6 | |
| Peak power | (W) | 45 |
| Average power | (W) | 0.3 |
| Average power density | (W/cm2) | 0.6 |
| Peak power density | (W/cm2) | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakata, S.; Kunimatsu, R.; Tsuka, Y.; Nakatani, A.; Hiraki, T.; Gunji, H.; Hirose, N.; Yanoshita, M.; Putranti, N.A.R.; Tanimoto, K. High-Frequency Near-Infrared Diode Laser Irradiation Attenuates IL-1β-Induced Expression of Inflammatory Cytokines and Matrix Metalloproteinases in Human Primary Chondrocytes. J. Clin. Med. 2020, 9, 881. https://doi.org/10.3390/jcm9030881
Sakata S, Kunimatsu R, Tsuka Y, Nakatani A, Hiraki T, Gunji H, Hirose N, Yanoshita M, Putranti NAR, Tanimoto K. High-Frequency Near-Infrared Diode Laser Irradiation Attenuates IL-1β-Induced Expression of Inflammatory Cytokines and Matrix Metalloproteinases in Human Primary Chondrocytes. Journal of Clinical Medicine. 2020; 9(3):881. https://doi.org/10.3390/jcm9030881
Chicago/Turabian StyleSakata, Shuzo, Ryo Kunimatsu, Yuji Tsuka, Ayaka Nakatani, Tomoka Hiraki, Hidemi Gunji, Naoto Hirose, Makoto Yanoshita, Nurul Aisyah Rizky Putranti, and Kotaro Tanimoto. 2020. "High-Frequency Near-Infrared Diode Laser Irradiation Attenuates IL-1β-Induced Expression of Inflammatory Cytokines and Matrix Metalloproteinases in Human Primary Chondrocytes" Journal of Clinical Medicine 9, no. 3: 881. https://doi.org/10.3390/jcm9030881
APA StyleSakata, S., Kunimatsu, R., Tsuka, Y., Nakatani, A., Hiraki, T., Gunji, H., Hirose, N., Yanoshita, M., Putranti, N. A. R., & Tanimoto, K. (2020). High-Frequency Near-Infrared Diode Laser Irradiation Attenuates IL-1β-Induced Expression of Inflammatory Cytokines and Matrix Metalloproteinases in Human Primary Chondrocytes. Journal of Clinical Medicine, 9(3), 881. https://doi.org/10.3390/jcm9030881





