A Novel Predictive Model for In-Hospital Mortality Based on a Combination of Multiple Blood Variables in Patients with ST-Segment-Elevation Myocardial Infarction
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Diagnosis of STEMI
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
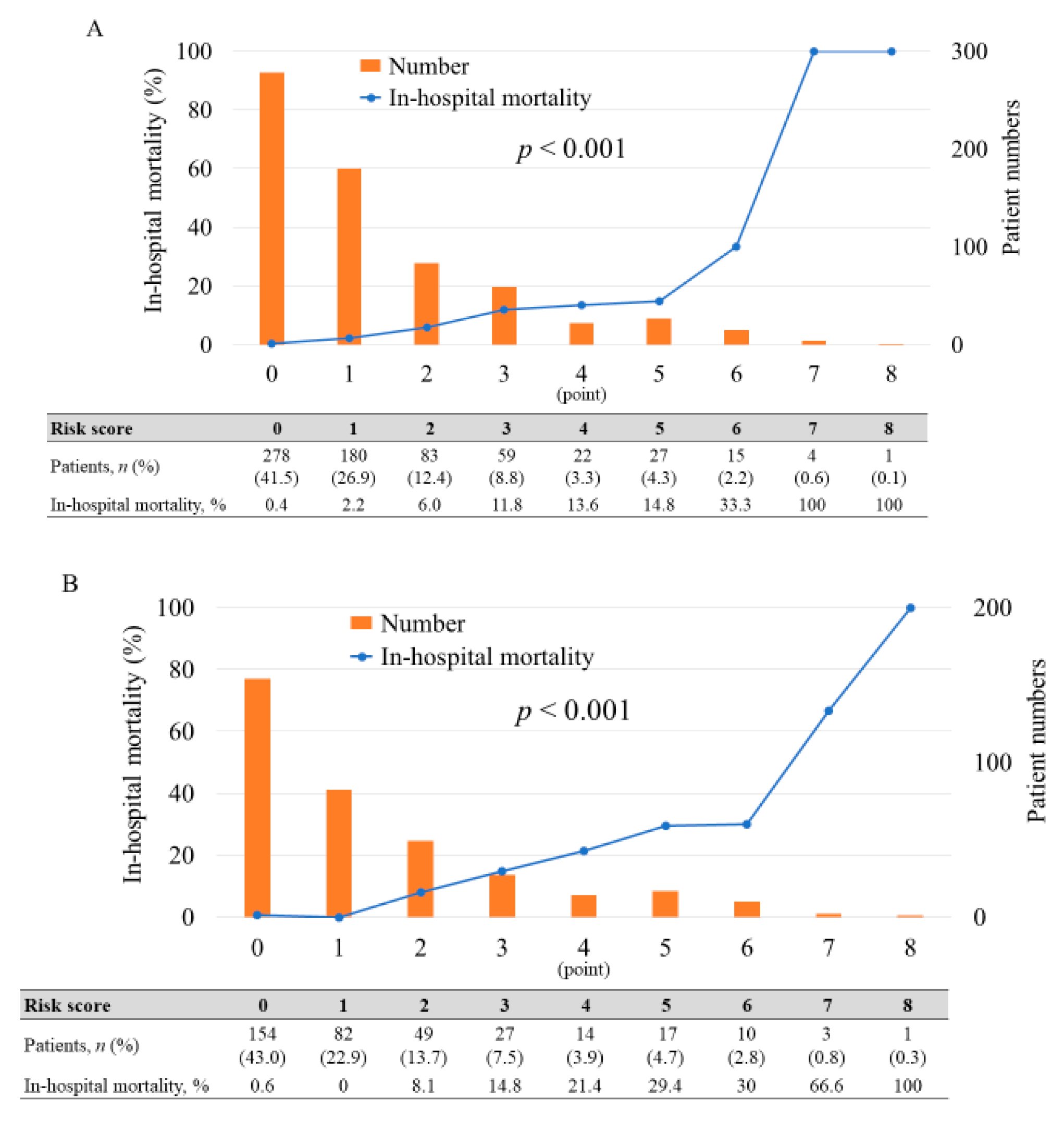
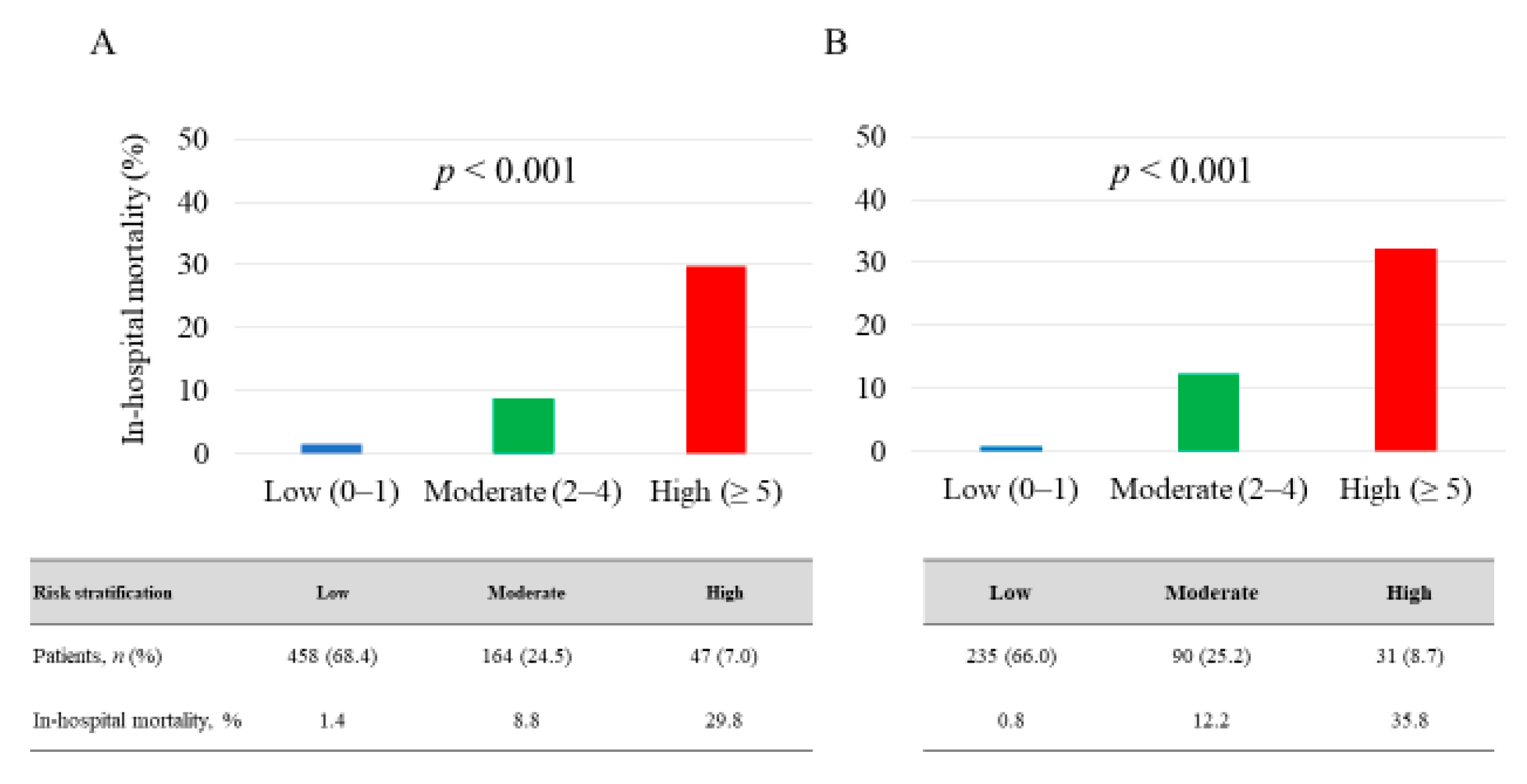
3.2. Blood Testing and Risk Stratification Model
3.3. Prediction of In-Hospital Mortality
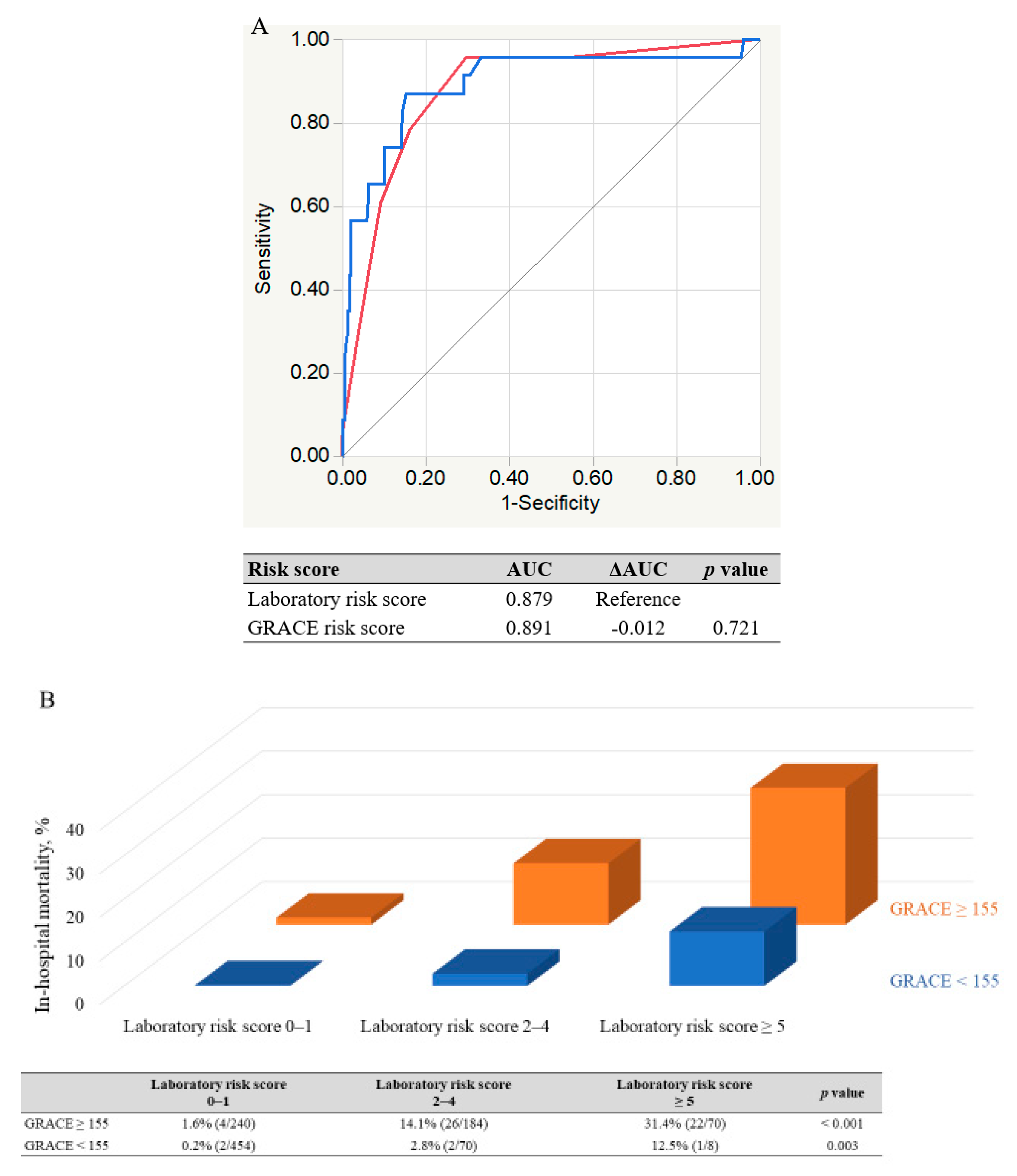
3.4. Comparison of New Risk Score with GRACE Risk Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Puymirat, E.; Simon, T.; Cayla, G.; Cottin, Y.; Elbaz, M.; Coste, P.; Lemesle, G.; Motreff, P.; Popovic, B.; Khalife, K.; et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017, 136, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Degano, I.R.; Salomaa, V.; Veronesi, G.; Ferrieres, J.; Kirchberger, I.; Laks, T.; Havulinna, A.S.; Ruidavets, J.B.; Ferrario, M.M.; Meisinger, C.; et al. Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 2015, 101, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Jacobsen, J.B.; Lash, T.L.; Botker, H.E.; Sorensen, H.T. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: A Danish nationwide cohort study. BMJ 2012, 344, e356. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hao, K.; Takahashi, J.; Miyata, S.; Shindo, T.; Nishimiya, K.; Kikuchi, Y.; Tsuburaya, R.; Matsumoto, Y.; Ito, K.; et al. Age-Specific Trends in the Incidence and In-Hospital Mortality of Acute Myocardial Infarction Over 30 Years in Japan- Report from the Miyagi AMI Registry Study. Circ. J. Off. J. Jpn. Circ. Soc. 2017, 81, 520–528. [Google Scholar] [CrossRef]
- Miyachi, H.; Takagi, A.; Miyauchi, K.; Yamasaki, M.; Tanaka, H.; Yoshikawa, M.; Saji, M.; Suzuki, M.; Yamamoto, T.; Shimizu, W.; et al. Current characteristics and management of ST elevation and non-ST elevation myocardial infarction in the Tokyo metropolitan area: From the Tokyo CCU network registered cohort. Heart Vessel. 2016, 31, 1740–1751. [Google Scholar] [CrossRef]
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-balloon time and mortality among patients undergoing primary PCI. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef]
- Shiomi, H.; Nakagawa, Y.; Morimoto, T.; Furukawa, Y.; Nakano, A.; Shirai, S.; Taniguchi, R.; Yamaji, K.; Nagao, K.; Suyama, T.; et al. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: Observational study. BMJ 2012, 344, e3257. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Morrow, D.A.; Antman, E.M.; Parsons, L.; de Lemos, J.A.; Cannon, C.P.; Giugliano, R.P.; McCabe, C.H.; Barron, H.V.; Braunwald, E. Application of the TIMI risk score for ST-elevation MI in the National Registry of Myocardial Infarction 3. JAMA 2001, 286, 1356–1359. [Google Scholar] [CrossRef]
- Komiyama, K.; Nakamura, M.; Tanabe, K.; Niikura, H.; Fujimoto, H.; Oikawa, K.; Daida, H.; Yamamoto, T.; Nagao, K.; Takayama, M. In-hospital mortality analysis of Japanese patients with acute coronary syndrome using the Tokyo CCU Network database: Applicability of the GRACE risk score. J. Cardiol. 2018, 71, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Abu-Assi, E.; Ferreira-Gonzalez, I.; Ribera, A.; Marsal, J.R.; Cascant, P.; Heras, M.; Bueno, H.; Sanchez, P.L.; Aros, F.; Marrugat, J.; et al. Do GRACE (Global Registry of Acute Coronary events) risk scores still maintain their performance for predicting mortality in the era of contemporary management of acute coronary syndromes? Am. Heart J. 2010, 160, e821–e823. [Google Scholar] [CrossRef] [PubMed]
- Wester, A.; Attar, R.; Mohammad, M.A.; Andell, P.; Hofmann, R.; Jensen, J.; Szummer, K.; Erlinge, D.; Koul, S. Impact of Baseline Anemia in Patients with Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: A Prespecified Analysis from the VALIDATE-SWEDEHEART Trial. J. Am. Heart Assoc. 2019, 8, e012741. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Fu, Q.; Tao, Z.Q.; Han, B.; Zhang, Y.G.; Huang, Y.J.; Ling, L.; Yang, X.J. Relationship between B-type natriuretic peptide and short-term prognosis in non-diabetic patients with ST-segment elevation myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 721–725. [Google Scholar] [PubMed]
- Oduncu, V.; Erkol, A.; Karabay, C.Y.; Kurt, M.; Akgun, T.; Bulut, M.; Pala, S.; Kirma, C. The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron. Artery Dis. 2013, 24, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, N.; Ishihara, M.; Noguchi, T.; Nakanishi, M.; Arakawa, T.; Asaumi, Y.; Kumasaka, L.; Kanaya, T.; Miyagi, T.; Nagai, T.; et al. Admission hyperglycemia is an independent predictor of acute kidney injury in patients with acute myocardial infarction. Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Morrow, D.A.; De Lemos, J.A.; Sabatine, M.S.; Murphy, S.A.; Rifai, N.; Gibson, C.M.; Antman, E.M.; Braunwald, E. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: An ENTIRE-TIMI-23 substudy. J. Am. Coll. Cardiol. 2004, 44, 335–339. [Google Scholar] [CrossRef]
- Kaya, M.G.; Uyarel, H.; Akpek, M.; Kalay, N.; Ergelen, M.; Ayhan, E.; Isik, T.; Cicek, G.; Elcik, D.; Sahin, O.; et al. Prognostic value of uric acid in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am. J. Cardiol. 2012, 109, 486–491. [Google Scholar] [CrossRef]
- Gibson, C.M.; Pinto, D.S.; Murphy, S.A.; Morrow, D.A.; Hobbach, H.P.; Wiviott, S.D.; Giugliano, R.P.; Cannon, C.P.; Antman, E.M.; Braunwald, E. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J. Am. Coll. Cardiol. 2003, 42, 1535–1543. [Google Scholar] [CrossRef]
- Wanamaker, B.L.; Seth, M.M.; Sukul, D.; Dixon, S.R.; Bhatt, D.L.; Madder, R.D.; Rumsfeld, J.S.; Gurm, H.S. Relationship Between Troponin on Presentation and In-Hospital Mortality in Patients With ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e013551. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. American journal of kidney diseases. Off. J. Natl. Kidney Found. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.T.; Yan, R.T.; Cantor, W.J.; Borgundvaag, B.; Cohen, E.A.; Fitchett, D.H.; Dzavik, V.; Ducas, J.; Tan, M.; Casanova, A.; et al. Relationship between risk stratification at admission and treatment effects of early invasive management following fibrinolysis: Insights from the Trial of Routine ANgioplasty and Stenting After Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI). Eur. Heart J. 2011, 32, 1994–2002. [Google Scholar] [CrossRef] [PubMed]
- Hakim, D.A.; Dangas, G.D.; Caixeta, A.; Nikolsky, E.; Lansky, A.J.; Moses, J.W.; Claessen, B.; Sanidas, E.; White, H.D.; Ohman, E.M.; et al. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: Analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Am. Heart J. 2011, 161, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Hibbert, B.; Singh, B.; Carson, K.; Premaratne, M.; Le May, M.; Chong, A.Y.; Arstall, M.; So, D. Meta-analysis of admission hyperglycaemia in acute myocardial infarction patients treated with primary angioplasty: A cause or a marker of mortality? European heart journal. Cardiovasc. Pharmacother. 2015, 1, 220–228. [Google Scholar] [CrossRef]
- Gonzalez-Pacheco, H.; Amezcua-Guerra, L.M.; Sandoval, J.; Martinez-Sanchez, C.; Ortiz-Leon, X.A.; Pena-Cabral, M.A.; Bojalil, R. Prognostic Implications of Serum Albumin Levels in Patients With Acute Coronary Syndromes. Am. J. Cardiol. 2017, 119, 951–958. [Google Scholar] [CrossRef]
- Anavekar, N.S.; McMurray, J.J.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.L.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef]
- Antman, E.M.; Tanasijevic, M.J.; Thompson, B.; Schactman, M.; McCabe, C.H.; Cannon, C.P.; Fischer, G.A.; Fung, A.Y.; Thompson, C.; Wybenga, D.; et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N. Engl. J. Med. 1996, 335, 1342–1349. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; de Lemos, J.A.; Gibson, C.M.; Murphy, S.A.; Rifai, N.; McCabe, C.; Antman, E.M.; Cannon, C.P.; Braunwald, E. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: Simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation 2002, 105, 1760–1763. [Google Scholar] [CrossRef]
- Damman, P.; Beijk, M.A.; Kuijt, W.J.; Verouden, N.J.; van Geloven, N.; Henriques, J.P.; Baan, J.; Vis, M.M.; Meuwissen, M.; van Straalen, J.P.; et al. Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 29–36. [Google Scholar] [CrossRef]
- O’Malley, R.G.; Bonaca, M.P.; Scirica, B.M.; Murphy, S.A.; Jarolim, P.; Sabatine, M.S.; Braunwald, E.; Morrow, D.A. Prognostic performance of multiple biomarkers in patients with non-ST-segment elevation acute coronary syndrome: Analysis from the MERLIN-TIMI 36 trial (Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndromes-Thrombolysis in Myocardial Infarction 36). J. Am. Coll. Cardiol. 2014, 63, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Morrow, D.A.; Cannon, C.P.; Jarolim, P.; Desai, N.R.; Sherwood, M.W.; Murphy, S.A.; Gerszten, R.E.; Sabatine, M.S. Multimarker Risk Stratification in Patients With Acute Myocardial Infarction. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Yanishi, K.; Nakamura, T.; Nakanishi, N.; Yokota, I.; Zen, K.; Yamano, T.; Shiraishi, H.; Shirayama, T.; Shiraishi, J.; Sawada, T.; et al. A Simple Risk Stratification Model for ST-Elevation Myocardial Infarction (STEMI) from the Combination of Blood Examination Variables: Acute Myocardial Infarction-Kyoto Multi-Center Risk Study Group. PLoS ONE 2016, 11, e0166391. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.A.; Antman, E.M.; Giugliano, R.P.; Cairns, R.; Charlesworth, A.; Murphy, S.A.; de Lemos, J.A.; McCabe, C.H.; Braunwald, E. A simple risk index for rapid initial triage of patients with ST-elevation myocardial infarction: An InTIME II substudy. Lancet 2001, 358, 1571–1575. [Google Scholar] [CrossRef]
- Scirica, B.M.; Kadakia, M.B.; de Lemos, J.A.; Roe, M.T.; Morrow, D.A.; Li, S.; Wiviott, S.D.; Kontos, M.C. Association between natriuretic peptides and mortality among patients admitted with myocardial infarction: A report from the ACTION Registry(R)-GWTG™. Clin. Chem. 2013, 59, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Yasue, H.; Yoshimura, M.; Ogawa, H.; Jougasaki, M.; Matsumura, T.; Mukoyama, M.; Nakao, K. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993, 88, 82–91. [Google Scholar] [CrossRef]
- Suzuki, S.; Yoshimura, M.; Nakayama, M.; Mizuno, Y.; Harada, E.; Ito, T.; Nakamura, S.; Abe, K.; Yamamuro, M.; Sakamoto, T.; et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: A long-term follow-up analysis. Circulation 2004, 110, 1387–1391. [Google Scholar] [CrossRef]
- Fox, K.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; Anderson, F.A., Jr.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef]
- Eagle, K.A.; Lim, M.J.; Dabbous, O.H.; Pieper, K.S.; Goldberg, R.J.; Van de Werf, F.; Goodman, S.G.; Granger, C.B.; Steg, P.G.; Gore, J.M.; et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004, 291, 2727–2733. [Google Scholar] [CrossRef]
- Nakatani, D.; Sakata, Y.; Suna, S.; Usami, M.; Matsumoto, S.; Shimizu, M.; Hara, M.; Uematsu, M.; Fukunami, M.; Hamasaki, T.; et al. Impact of beta blockade therapy on long-term mortality after ST-segment elevation acute myocardial infarction in the percutaneous coronary intervention era. Am. J. Cardiol. 2013, 111, 457–464. [Google Scholar] [CrossRef]
- Canto, J.G.; Rogers, W.J.; Goldberg, R.J.; Peterson, E.D.; Wenger, N.K.; Vaccarino, V.; Kiefe, C.I.; Frederick, P.D.; Sopko, G.; Zheng, Z.J. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 2012, 307, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Buchanan, G.L.; Hoye, A. Outcomes after percutaneous coronary intervention in women: Are there differences when compared with men? Interv. Cardiol. 2019, 14, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yao, J.; Xie, Y.; Luo, M. Gender-specific predictive markers of poor prognosis for patients with acute myocardial infarction during a 6-month follow-up. J. Cardiovasc. Transl. Res. 2020, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]


| Variables | Derivation Cohort (n = 669) | Validation Cohort (n = 358) | p-Value |
|---|---|---|---|
| Age, yr | 68.5 ± 12.6 | 68.9 ± 13.0 | 0.738 |
| Male, n (%) | 493 (73.7) | 257 (71.8) | 0.297 |
| Body mass index, kg/m2 | 23.8 ± 3.6 | 24.0 ± 3.9 | 0.228 |
| Systolic BP, mm Hg | 138.2 ± 33.4 | 134.9 ± 32.9 | 0.488 |
| Heart rate, beats/min | 77.0 ± 21.4 | 76.7 ± 21.1 | 0.583 |
| Medical history | |||
| Hypertension, n (%) | 459 (68.6) | 232 (64.8) | 0.161 |
| Dyslipidemia, n (%) | 352 (52.6) | 185 (51.6) | 0.472 |
| Diabetes mellitus, n (%) | 192 (28.7) | 114 (31.8) | 0.121 |
| Smoking, n (%) | 328 (49.0) | 198 (55.3) | 0.032 |
| Previous MI, n (%) | 59 (8.8) | 28 (7.8) | 0.297 |
| Previous PCI, n (%) | 69 (10.3) | 37 (10.3) | 0.777 |
| Previous CABG, n (%) | 7 (1.0) | 3 (0.8) | 0.777 |
| Laboratory data | |||
| WBC, ×102/μL | 106.7 ± 38.0 | 104.4 ± 37.3 | 0.472 |
| Hemoglobin, g/dl | 13.9 ± 2.2 | 13.9 ± 2.1 | 0.885 |
| Platelet, ×104/μL | 21.9 ± 6.6 | 22.0 ± 5.8 | 0.459 |
| HbA1c, % | 5.9 (5.6–6.5) | 6.0 (5.6–6.5) | 0.235 |
| BS, mg/dL | 156 (130–200) | 156 (130–200) | 0.275 |
| eGFR, mL/min/1.73m2 | 67.4 ± 24.1 | 65.2 ± 21.3 | 0.334 |
| LDL-CHO, mg/dL | 121.1 ± 35.7 | 122.6 ± 34.8 | 0.685 |
| HDL-CHO, mg/dL | 46.1 ± 11.6 | 47.4 ± 12.8 | 0.577 |
| Albumin, mg/dL | 4.0 ± 0.5 | 4.0 ± 0.5 | 0.877 |
| Uric acid, mg/dL | 6.0 ± 1.6 | 5.8 ± 1.5 | 0.236 |
| CRP, mg/dL | 0.13 (0.06–0.42) | 0.13 (0.06–0.39) | 0.303 |
| CK, U/L | 154 (96–411) | 162 (97–413) | 0.581 |
| High-sensitivity troponin I, ng/mL (upper limit of normal: 0.032) | 0.27 (0.04–2.64) | 0.26 (0.05–2.58) | 0.529 |
| BNP, pg/mL (upper limit of normal: 18.4) | 47.0 (18.2–151.7) | 45.1 (17.2–152.5) | 0.401 |
| Killip classification | |||
| I, II, III, IV, n | 535/74/25/35 | 292/28/15/22 | 0.319 |
| Onset-to-admission time, min | 200 (110–400) | 200 (115–392) | 0.381 |
| LVEF (on admission), % | 50.6 ± 11.5 | 48.9 ± 10.0 | 0.321 |
| Culprit lesion | |||
| LMT, n (%) | 20 (2.9) | 5 (1.4) | 0.084 |
| LAD, n (%) | 348 (52.0)) | 199 (55.5) | 0.110 |
| RCA, n (%) | 243 (36.3) | 117 (32.7) | 0.114 |
| LCX, n (%) | 57 (8.5) | 37 (10.3) | 0.252 |
| Multi-vessel disease, n (%) | 269 (38.4) | 125 (34.9) | 0.278 |
| Pre TIMI grade 0.1, n (%) | 440 (65.7) | 239 (66.9) | 0.401 |
| Post TIMI grade 3, n (%) | 625 (93.4) | 323 (90.5) | 0.090 |
| Peak CK level, mg/dL | 2062 (932–3899) | 2096 (973–3934) | 0.350 |
| Mechanical support on admission | |||
| Respirator, n (%) | 36 (5.4) | 18 (5.0) | 0.436 |
| Temporary pacing, n (%) | 51 (7.6) | 33 (9.2) | 0.238 |
| IABP, n (%) | 99 (14.7) | 44 (12.3) | 0.169 |
| PCPS, n (%) | 21 (3.1) | 11 (3.0) | 0.523 |
| In-hospital death, n (%) | 34 (5.1) | 23 (6.4) | 0.318 |
| Variables | Survivor | Death | p-Value |
|---|---|---|---|
| WBC, ×102 /μL | 105.9 ± 1.5 | 122.6 ± 6.5 | 0.013 |
| Hemoglobin, g/dL | 14.0 ± 2.1 | 12.6 ± 2.1 | <0.001 |
| Platelet, ×104/μL | 22.1 ± 0.6 | 17.3 ± 1.1 | <0.001 |
| HbA1c, % | 5.9 (5.7–6.5) | 6.1 (5.6–6.9) | 0.541 |
| BS, mg/dL | 155 (129–194) | 200 (159–291) | <0.001 |
| eGFR, mL/min/1.73m2 | 68.5 ± 0.9 | 46.1 ± 4.0 | <0.001 |
| LDL-CHO, mg/dL | 125.5 ± 1.4 | 117.3 ± 6.1 | 0.191 |
| HDL-CHO, mg/dL | 44.6 ± 0.5 | 43.5 ± 2.0 | 0.182 |
| Albumin, mg/dL | 4.1 ± 0.1 | 3.4 ± 0.1 | <0.001 |
| Uric acid, mg/dL | 5.9 ± 0.1 | 7.4 ± 0.3 | <0.001 |
| CRP, mg/dL | 0.13 (0.06–0.39) | 0.35 (0.08–1.06) | 0.012 |
| CK, U/L | 152 (96–393) | 312 (133–2148) | 0.005 |
| High-sensitivity troponin I, ng/mL (99th percentile for whole healthy adult population: 0.026) | 0.26 (0.04–2.18) | 2.32 (0.29–40.4) | <0.001 |
| BNP, pg/mL | 44.9 (16.8–138.0) | 240 (55.4–805) | <0.001 |
| Variables | Odds Ratio | 95% Confidence Interval | p-Value | Given Score |
|---|---|---|---|---|
| Platelet <15 × 104/μL | 3.45 | 1.50–7.97 | 0.003 | 2 points |
| BS ≥200 mg/dL | 2.63 | 1.20–5.80 | 0.020 | 1 point |
| eGFR <45 mL/min/1.73m2 | 3.65 | 1.60–8.11 | 0.001 | 2 points |
| Albumin ≤3.5mg/dL | 3.37 | 1.52–7.47 | 0.003 | 2 points |
| High-sensitivity troponin I >1.6 ng/dL (normal upper limit × 50) | 2.76 | 1.27–6.01 | 0.010 | 1 point |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goriki, Y.; Tanaka, A.; Nishihira, K.; Kawaguchi, A.; Natsuaki, M.; Watanabe, N.; Ashikaga, K.; Kuriyama, N.; Shibata, Y.; Node, K. A Novel Predictive Model for In-Hospital Mortality Based on a Combination of Multiple Blood Variables in Patients with ST-Segment-Elevation Myocardial Infarction. J. Clin. Med. 2020, 9, 852. https://doi.org/10.3390/jcm9030852
Goriki Y, Tanaka A, Nishihira K, Kawaguchi A, Natsuaki M, Watanabe N, Ashikaga K, Kuriyama N, Shibata Y, Node K. A Novel Predictive Model for In-Hospital Mortality Based on a Combination of Multiple Blood Variables in Patients with ST-Segment-Elevation Myocardial Infarction. Journal of Clinical Medicine. 2020; 9(3):852. https://doi.org/10.3390/jcm9030852
Chicago/Turabian StyleGoriki, Yuhei, Atsushi Tanaka, Kensaku Nishihira, Atsushi Kawaguchi, Masahiro Natsuaki, Nozomi Watanabe, Keiichi Ashikaga, Nehiro Kuriyama, Yoshisato Shibata, and Koichi Node. 2020. "A Novel Predictive Model for In-Hospital Mortality Based on a Combination of Multiple Blood Variables in Patients with ST-Segment-Elevation Myocardial Infarction" Journal of Clinical Medicine 9, no. 3: 852. https://doi.org/10.3390/jcm9030852
APA StyleGoriki, Y., Tanaka, A., Nishihira, K., Kawaguchi, A., Natsuaki, M., Watanabe, N., Ashikaga, K., Kuriyama, N., Shibata, Y., & Node, K. (2020). A Novel Predictive Model for In-Hospital Mortality Based on a Combination of Multiple Blood Variables in Patients with ST-Segment-Elevation Myocardial Infarction. Journal of Clinical Medicine, 9(3), 852. https://doi.org/10.3390/jcm9030852





