Cognition in Children with Arachnoid Cysts
Abstract
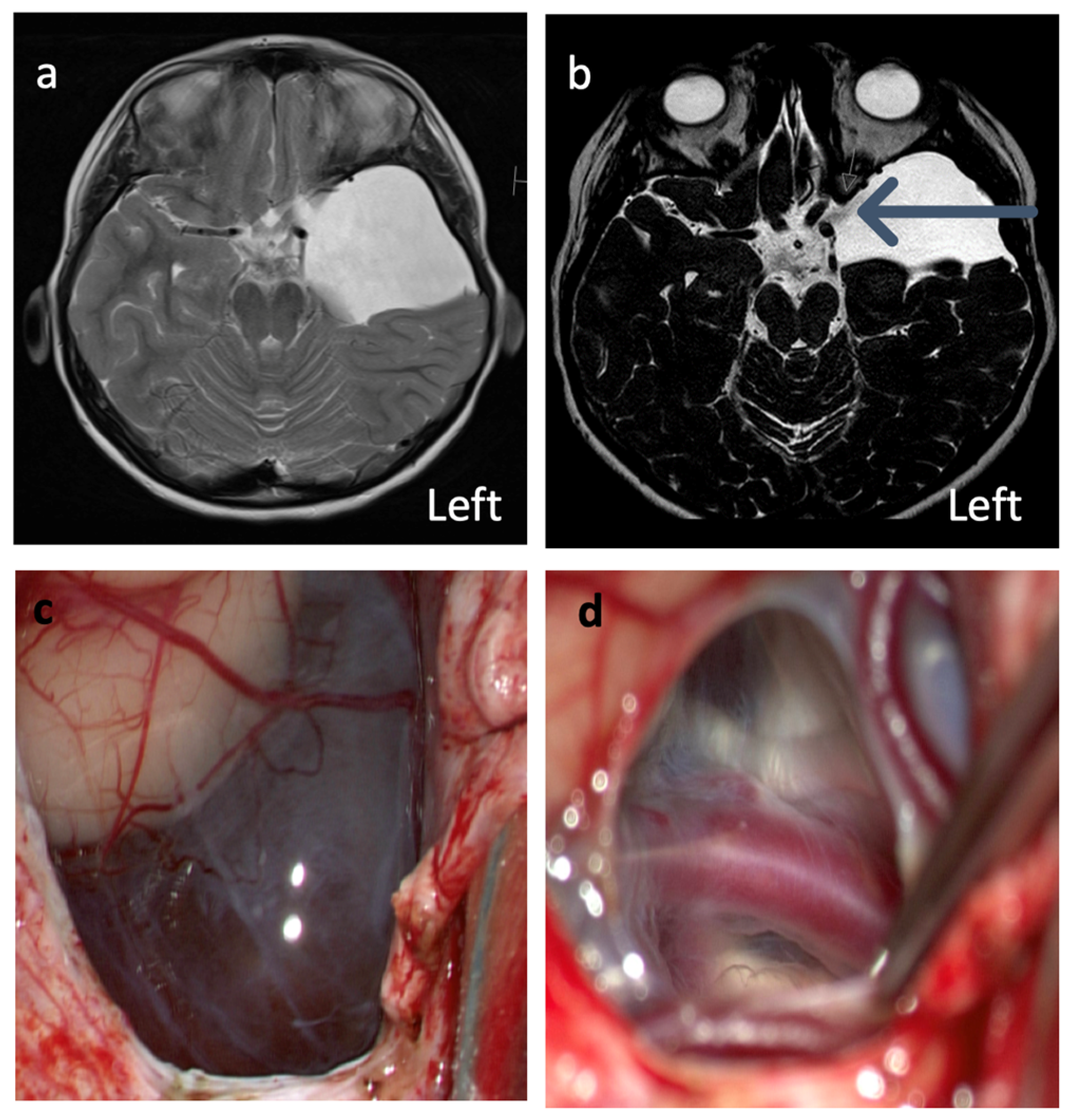
1. Introduction
2. Materials and Methods
2.1. Weschler Intelligence Scale for Children
2.2. FAS
2.3. Boston Naming Test
2.4. NEPSY-II
2.5. Rey Complex Figure Test
2.6. Nordic Five-to-Fifteen
2.7. Additional Evaluations
2.8. Clinical Decision Process
2.9. Statistical Analysis
3. Results
3.1. WISC-IV
3.2. NEPSY-II
3.3. FAS
3.4. BNT
3.5. RCFT
3.6. Opthalmology and EEG
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fulkerson, D.H.; Vogel, T.D.; Baker, A.A.; Patel, N.B.; Ackerman, L.L.; Smith, J.L.; Boaz, J.C. Cyst-ventricle stent as primary or salvage treatment for posterior fossa arachnoid cysts. J. Neurosurg. Pediatr. 2011, 7, 549–556. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Li, Y.; Zhai, X.; Zhou, Y.; Liang, P. The clinical classification and treatment of middle cranial fossa arachnoid cysts in children. Clin. Neurol. Neurosurg. 2013, 115, 411–418. [Google Scholar] [CrossRef]
- Al-Holou, W.N.; Yew, A.Y.; Boomsaad, Z.E.; Garton, H.J.; Muraszko, K.M.; Maher, C.O. Prevalence and natural history of arachnoid cysts in children. J. Neurosurg. Pediatr. 2010, 5, 578–585. [Google Scholar] [CrossRef]
- Gjerde, P.B.; Schmid, M.; Hammar, Å.; Wester, K. Intracranial arachnoid cysts: Impairment of higher cognitive functions and postoperative improvement. J. Neurodev. Disord. 2013, 5, 21. [Google Scholar] [CrossRef]
- Tunes, C.; Flones, I.; Helland, C.; Wilhelmsen, K.; Goplen, F.; Wester, K.G. Pre- and post-operative dizziness and postural instability in temporal arachnoid cyst patients. Acta Neurol. Scand. 2014, 129, 335–342. [Google Scholar] [CrossRef]
- Torgersen, J.; Helland, C.; Flaatten, H.; Wester, K. Reversible dyscognition in patients with a unilateral, middle fossa arachnoid cyst revealed by using a laptop based neuropsychological test battery (CANTAB). J. Neurol. 2010, 257, 1909–1916. [Google Scholar] [CrossRef]
- Isaksen, E.; Leet, T.H.; Helland, C.A.; Wester, K. Maze learning in patients with intracranial arachnoid cysts. Acta Neurochir. 2013, 155, 841–848. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; PsychCorp.: San Antonia, TX, USA, 2003. [Google Scholar]
- Bishop, D. Handbook of Normative Data for Neuropsychological Assessment; Mitrushina, M.N., Boone, K.B., D’Elia, L.F., Eds.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Kaplan, E.; Goodglass, H.; Weintraub, S. Boston Naming Test-2 (BNT-2), 2nd ed.; Pro-Ed: Austin, TX, USA, 2001. [Google Scholar]
- Goldstein, S. NEPSY. In Encyclopedia of Child Behavior and Development; Springer: Boston, MA, USA, 2011. [Google Scholar]
- Goldstein, S.A.M. Rey Complex Figure Test. In Encyclopedia of Child Behavior and Development; Springer: Boston, MA, USA, 2011. [Google Scholar]
- Janols, L.-O.; Korkman, M.; Mickelsson, K.; Strand, G.; Trillingsgaard, A.; Gillberg, C.; Kadesjö, B. The FTF (five to fifteen): The development of a parent questionnaire for the assessment of ADHD and comorbid conditions. Eur. Child Adolesc. Psychiatry 2004, 13 (Suppl. 3), 3–13. [Google Scholar]
- Galassi, E.; Tognetti, F.; Gaist, G.; Fagioli, L.; Frank, F.; Frank, G. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: Classification and pathophysiological aspects. Surg. Neurol. 1982, 17, 363–369. [Google Scholar] [CrossRef]
- Amelot, A.; Beccaria, K.; Blauwblomme, T.; Bourgeois, M.; Paternoster, G.; Cuny, M.L.; Zerah, M.; Sainte-Rose, C.; Puget, S. Microsurgical, endoscopic, and shunt management of pediatric temporosylvian arachnoid cysts: A comparative study. J. Neurosurg. Pediatr. 2019, 23, 749–757. [Google Scholar] [CrossRef]
- Laird, C.; Whitaker, S. The Use of IQ and Descroptions of People with Intellectual Disabilities in the Scientific Literature. Br. J. Dev. Disabil. 2011, 46, 175–183. [Google Scholar] [CrossRef]
- Whitaker, S. The Stability of IQ in People with Low Intellectual Ability: An Analysis of the Literature. Intellect. Dev. Disabil. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Gundersen, H.; Helland, C.A.; Raeder, M.B.; Hugdahl, K.; Wester, K. Visual attention in patients with intracranial arachnoid cysts. J. Neurol. 2007, 254, 60–66. [Google Scholar] [CrossRef]
- Park, Y.S.; Eom, S.; Shim, K.W.; Kim, D.S. Neurocognitive and psychological profiles in pediatric arachnoid cyst. Child’s Nerv. Syst. 2009, 25, 1071–1076. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, J.Y.; Phi, J.H.; Cho, B.K.; Shin, M.S.; Kim, S.K. Neurocognitive profile in children with arachnoid cysts before and after surgical intervention. Child’s Nerv. Syst. 2019, 35, 517–522. [Google Scholar] [CrossRef]
- Ryan, J.J.; Glass, L.A.; Bartels, J.M. Stability of the WIISC-IV in a sample of elementary and middle school children. Appl. Neuropsychol. 2010, 17, 68–72. [Google Scholar] [CrossRef]
- Kaufman, A.; Flanagan, D.P. Essential of WISC-IV Assesment; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2004. [Google Scholar]
- Thorsen, A.L.; Meza, J.; Hinshaw, S.; Lundervold, A.J. Processing Speed Mediates the Longitudinal Association between ADHD Symptoms and Preadolescent Peer Problems. Front. Psychol. 2017, 8, 2154. [Google Scholar] [CrossRef]
- Gordon, R.; Smith-Spark, J.H.; Newton, E.J.; Henry, L.A. Executive Function and Academic Achievement in Primary School Children: The Use of Task-Related Processing Speed. Front. Psychol. 2018, 9, 582. [Google Scholar] [CrossRef]
- Helland, C.A.; Wester, K. Intracystic pressure in patients with temporal arachnoid cysts: A prospective study of preoperative complaints and postoperative outcome. J. Neurol. Neurosurg. Psychiatry 2007, 78, 620–623. [Google Scholar] [CrossRef]
- Rabiei, K.; Hogfeldt, M.J.; Doria-Medina, R.; Tisell, M. Surgery for intracranial arachnoid cysts in children-a prospective long-term study. Child’s Nerv. Syst. 2016, 32, 1257–1263. [Google Scholar] [CrossRef]
- Lee, E.J.; Ra, Y.S. Clinical and neuroimaging outcomes of surgically treated intracranial cysts in 110 children. J. Korean Neurosurg. Soc. 2012, 52, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Mørkve, S.H.; Helland, C.A.; Amus, J.; Lund-Johansen, M.; Wester, K.G. Surgical Decompression of Arachnoid Cysts Leads to Improved Quality of Life: A Prospective Study. Neurosurgery 2016, 78, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, J.Y.; Phi, J.H.; Kim, S.K.; Wang, K.C. Stricter indications are recommended for fenestration surgery in intracranial arachnoid cysts of children. Child’s Nerv. Syst. 2015, 31, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Helland, C.A.; Wester, K. A population-based study of intracranial arachnoid cysts: Clinical and neuroimaging outcomes following surgical cyst decompression in children. J. Neurosurg. 2006, 105 (Suppl. 5), 385–390. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, S.; Santamaria, J. What is the relationship between arachnoid cysts and seizure foci? Epilepsia 1997, 38, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Yu, M.; Wang, W. Clinical and radiological outcomes of surgical treatment for symptomatic arachnoid cysts in adults. J. Clin. Neurosci. 2015, 22, 1456–1461. [Google Scholar] [CrossRef]
- Mazurkiewicz-Bełdzińska, M.; Dilling-Ostrowska, E. Presentation of intracranial arachnoid cysts in children: Correlation between localization and clinical symptoms. Med. Sci. Monit. 2002, 8, CR462–CR465. [Google Scholar]
- Wang, C.; Han, G.; You, C.; Liu, C.; Wang, J.; Xiong, Y. Individual surgical treatment of intracranial arachnoid cyst in pediatric patients. Neurol. India 2013, 61, 400–405. [Google Scholar]
- Agopian-Dahlenmark, L.; Mathiesen, T.; Bergendal, A. Cognitive dysfunction and subjective symptoms in patients with arachnoid cyst before and after surgery. Acta Neurochir. 2020. [Google Scholar] [CrossRef]
- Gjerde, P.B.; Litleskare, S.; Lura, N.G.; Tangen, T.; Helland, C.A.; Wester, K. Anxiety and Depression in Patients with Intracranial Arachnoid Cysts-A Prospective Study. World Neurosurg. 2019, 132, e645–e653. [Google Scholar] [CrossRef]
- Wester, K. Auditory based neuropsychology in neurosurgery. Hear. Res. 2008, 238, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.B.; Yu, S.Y.; Cao, L.; Bai, J.W.; Wang, X.S.; Li, C.Z.; Zhang, Y.Z. Endoscopic treatment of suprasellar cysts without hydrocephalus. J. Neurosurg. Pediatr. 2016, 18, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Mankotia, D.S.; Sardana, H.; Sinha, S.; Sharma, B.S.; Suri, A.; Borkar, S.A.; Satyarthee, G.D.; Chandra, P.S. Pediatric interhemispheric arachnoid cyst: An institutional experience. J. Pediatr. Neurosci. 2016, 11, 29–34. [Google Scholar] [PubMed]
- Aljohani, H.; Romano, A.; Iaccarino, C.; Ganau, M.; Diemidio, P.; Chibbaro, S. Pure Endoscopic Management of Fourth Ventricle Arachnoid Cyst: Case Report and Literature Review. Asian J. Neurosurg. 2018, 13, 184–187. [Google Scholar] [CrossRef]
- Knie, B.; Morota, N.; Ihara, S.; Tamura, G.; Ogiwara, H. Pediatric intraventricular arachnoid cysts in the body of lateral ventricle: Surgical outcome and its embryologic background. Child’s Nerv. Syst. 2016, 32, 2197–2204. [Google Scholar] [CrossRef]
| Patient ID | Sex | Presenting Symptoms | Cyst Laterality | Age at Surgery (years) | Follow-up (years) | Preop. Cyst Volume (mL) | Postop. Cyst Volume (mL) | Galassi Type | Postoperative Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | Delayed motor development, language difficulties | Left | 13 | 3 | 50 | 22 | 1 | Improved |
| 2 | Male | Paresthesias, headache, dyslexia, difficulties concentrating | Left | 12.75 | 2.5 | 72 | 40 | 1 | Headache resolved |
| 3 | Male | Epilepsy | Right | 11.5 | 0.8 | 18 | 12 | 1 | Seizure free |
| 4 | Female | Nausea and vomiting | Left | 7.75 | 1.5 | 78 | 39 | 2 | Nausea and vomiting resolved |
| 5 | Female | Language and concentration difficulties | Left | 11 | 2.5 | 21 | 11 | 1 | Improved |
| 6 | Female | Language difficulties, headache | Right | 6 | 2 | 341 | 207 | 3 | Unchanged |
| 7 | Male | Tics and paroxysmal perceptual difficulties, headache and retroorbital pain | Left | 7.75 | 1.5 | 18 | 7 | 1 | Improved regarding pain. Tics unchanged |
| 8 | Male | Attention Deficit Hyperactivity Disorder and headache | Left | 10.75 | 0.9 | 75 | 4 | 1 | Headache resolved |
| 9 | Female | Precocious puberty and cognitive difficulties | Left | 9 | 1 | 41 | 41 | 1 | Unchanged |
| 10 | Male | Headache and nausea | Right | 7 | 2 | 22 | 24 | 1 | Headache and nausea resolved |
| 11 | Male | Epilepsy | Right | 9.3 | 2.9 | 13 | 3 | 1 | Seizure free |
| 12 | Male | Precocious puberty and cognitive difficulties | Right | 13 | 2 | 55 | 17 | 1 | Improved cognition. |
| 13 | Male | Non-verbal learning difficulties | Right | Diagnosis at 7.5 years | - | 98 | - | 2 | Declined surgery |
| 14 | Female | Attention Deficit Hyperactivity Disorder | Left | Diagnosis at 7.7 years | . | 14 | - | 1 | Normal cognitive test results No surgery |
| 15 | Female | Headache secondary to hygroma, concentration difficutlies | Left | Diagnosis at 12.7 years | 4 | 37 | 1 | Normal cognitive testing Acute hygroma evacuation but no elective surgery for cyst |
| Presurgical mean (SD) | Postsurgical mean (SD) | Difference (95% CI) | p | |
|---|---|---|---|---|
| WISC-IV | ||||
| FSIQ | 84.8 (8.7) | 93.0 (11.9) | 8.2 (3.2–13.2) | 0.005 |
| VCI | 86.7 (13.1) | 94.7 (13.1) | 8.0 (4.2–11.8) | 0.001 |
| PRI | 99.1 (11.4) | 103.8 (9.6) | 4.7 (−2.6–12.0) | 0.180 |
| WMI | 82.5 (9.7) | 84.5 (15.7) | 2.0 (−5.5–9.5) | 0.566 |
| PSI | 81.5 (10.4) | 92.5 (12.6) | 11.0 (4.4–17.6) | 0.004 |
| NEPSY-II | ||||
| MF, IR | 9.5 (3.6) | 10.8 (3.7) | 1.3 (0.1–2.5) | 0.033 |
| MF, DR | 9.5 (3.3) | 11.8 (3.2) | 2.3 (0.6–4.1) | 0.018 |
| MS | 9.5 (5.2) | 10.4 (3.5) | 0.9 (-4.1–5.9) | 0.696 |
| MW | 10.7 (2.8) | 12.7 (2.9) | 2 (0.3–3.7) | 0.027 |
| Presurgical | Postsurgical | p | ||
| FAS | ||||
| Raw scores, median (range) Age-standardized, mean (SD) | 17 (1–28) −0.7 (0.8) | 17 (5–35) −0.3 (2.1) | 0.168 0.524 | |
| BNT | ||||
| Raw scores, median (range) Age-standardized, mean (SD) | 35 (22–44) −2.1 (2.6) | 33 (31–45) −1.7 (1.4) | 0.023 0.473 | |
| RCFT | ||||
| 3 min | 33 (< 20–52) | 32 (< 20–58) | 0.646 | |
| 30 min | 34 (< 20–51) | 30 (< 20–55) | 0.414 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandvik, U.; Adolfsson, T.; Jacobson, D.N.; Tedroff, K. Cognition in Children with Arachnoid Cysts. J. Clin. Med. 2020, 9, 850. https://doi.org/10.3390/jcm9030850
Sandvik U, Adolfsson T, Jacobson DN, Tedroff K. Cognition in Children with Arachnoid Cysts. Journal of Clinical Medicine. 2020; 9(3):850. https://doi.org/10.3390/jcm9030850
Chicago/Turabian StyleSandvik, Ulrika, Tomas Adolfsson, Dan N. Jacobson, and Kristina Tedroff. 2020. "Cognition in Children with Arachnoid Cysts" Journal of Clinical Medicine 9, no. 3: 850. https://doi.org/10.3390/jcm9030850
APA StyleSandvik, U., Adolfsson, T., Jacobson, D. N., & Tedroff, K. (2020). Cognition in Children with Arachnoid Cysts. Journal of Clinical Medicine, 9(3), 850. https://doi.org/10.3390/jcm9030850





