Environmental Factors as Modulators of the Relationship between Obstructive Sleep Apnea and Lesions in the Circulatory System
Abstract
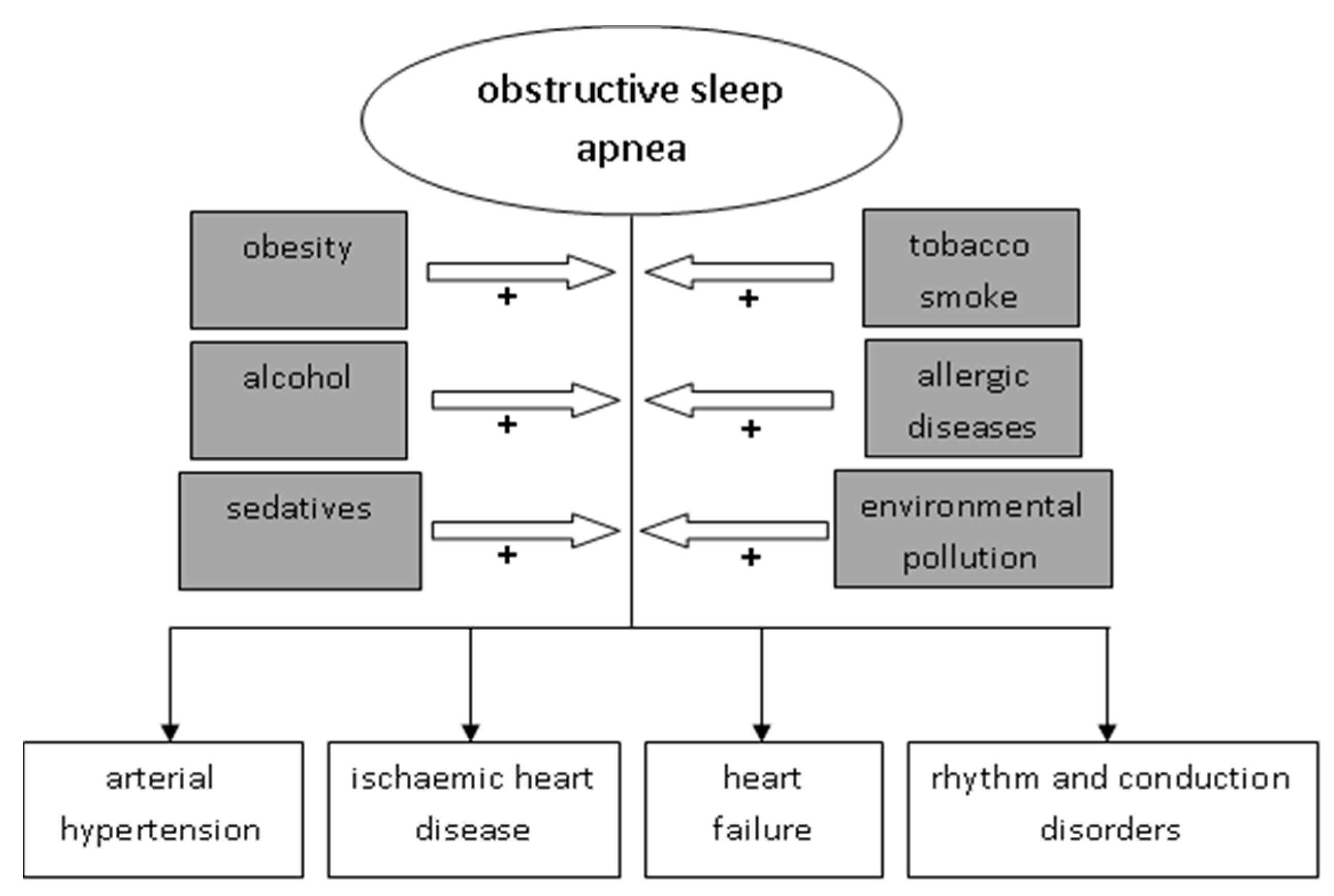
1. Introduction
2. OSA and Lesions in the Circulatory System
2.1. OSA and Arterial Hypertension
2.2. OSA and Ischemic Heart Disease
2.3. OSA and Heart Failure
2.4. OSA and Rhythm and Conduction Disorders
3. Environmental Factors and OSA
3.1. Obesity and OSA
3.2. Alcohol, Sedatives and OSA
3.3. Tobacco Smoking
3.4. Allergic Diseases, Environmental Pollution and OSA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Ravesloot, M.J.L.; Van Maanen, J.P.; Hilgevoord, A.A.J.; Van Wagensveld, B.A.; De Vries, N. Obstructive sleep apnea is underrecognized and underdiagnosed in patients undergoing bariatric surgery. Eur. Arch. Oto. Rhino. Laryngol. 2012, 269, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.G.; Johnson, D.C. Frequency of sleep apnea in stroke and TIA patients: A meta-analysis. J. Clin. Sleep Med. 2010, 6, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Postrzech-Adamczyk, K.; Nahorecki, A.; Zatońska, K.; Lawson, J.; Wołyniec, M.; Skomro, R.; Szuba, A. Prevalence and risk of obstructive sleep apnea and arterial hypertension in the adult population in Poland: An observational subset of the international Prospective Urban Rural Epidemiology (PURE) Study. Adv. Exp. Med. Biol. 2019. [Google Scholar] [CrossRef]
- Leuenberger, U.; Jacob, E.; Sweer, L.; Waravdekar, N.; Zwillich, C.; Sinoway, L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J. Appl. Physiol. 1995, 79, 581–588. [Google Scholar] [CrossRef]
- Pływaczewski, R.; Brzecka, A.; Bielicki, P.; Czajkowska-Malinowska, M.; Cofta, S.; Jonczak, L.; Radlinski, J.; Tażbirek, M.; Wasilewska, J. Zalecenia Polskiego Towarzystwa Chorób Płuc dotyczące rozpoznawania i leczenia zaburzeń oddychania w czasie snu u dorosłych. Pneumonol. Alergol. Pol. 2013, 81, 221–258. [Google Scholar]
- Whyte, K.F.; Allen, M.B.; Jeffrey, A.A.; Gould, G.A.; Douglas, N.J. Clinical features of the sleep apnoea/hypopnoea syndrome. Q. J. Med. 1989, 72, 659–666. [Google Scholar]
- Hoffstein, V.; Mateika, S.; Erson, D. Snoring: Is it in the ear of the beholder? Sleep 1994, 17, 522–526. [Google Scholar] [CrossRef]
- Ye, L.; Pien, G.W.; Ratcliffe, S.J.; Björnsdottir, E.; Arnardottir, E.S.; Pack, A.I.; Benediktsdottir, B.; Gislason, T. The different clinical faces of obstructive sleep apnoea: A cluster analysis. Eur. Respir. J. 2014, 44, 1600–1607. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.; Duran-Cantolla, J.; Martı’nez-Vila, E.; Gallego, J.; Rubio, R.; Aizpuru, F.; De La Torre, G. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke 2006, 37, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Vasheghani-Farahani, A.; Kazemnejad, F.; Sadeghniiat-Haghighi, K.; Saadat, S.; Tavakolipoor, P.; Yazdani, T.; Alidoosti, M.; Ghasem-Amooeian, V.; Ashraf, H. Obstructive sleep apnea and severity of coronary artery disease. Casp. J. Intern. Med. 2018, 9, 276–282. [Google Scholar] [CrossRef]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.; Sahadevan, J.; Redline, S. Sleep Heart Health Study Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Peppard, P.E. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar] [PubMed]
- Pratt-Ubunama, M.N.; Nishizaka, M.K.; Boedefeld, R.L.; Cofield, S.S.; Harding, S.M.; Calhoun, D.A. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007, 131, 453–459. [Google Scholar] [CrossRef]
- Fitzgerald, M.P.; Mulligan, M.; Parthasarathy, S. Nocturic frequency is related to severity of obstructive sleep apnea, improves with continuous positive airways treatment. Am. J. Obstet. Gynecol. 2006, 194, 1399–1403. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Marin, J.M.; Agusti, A.; Villar, I.; Forner, M.; Nieto, D.; Carrizo, S.J.; Barbé, F.; Vicente, E.; Wei, Y.; Nieto, F.J.; et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. J. Am. Med. Assoc. 2012, 307, 2169–2176. [Google Scholar] [CrossRef]
- Fava, C.; Dorigoni, S.; Dalle Vedove, F.; Danese, E.; Montagnana, M.; Guidi, G.C.; Narkiewicz, K.; Minuz, P. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 2014, 145, 762–771. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Q.; Guo, Z.; Dai, Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: A meta-analysis of randomized controlled trials. J. Clin. Hypertens. 2016, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Sapiña-Beltrán, E.; Torres, G.; Benítez, I.; Santamaría-Martos, F.; Durán-Cantolla, J.; Egea, C.; Sánchez-de-la-Torre, M.; Barbé, F.; Dalmases, M. Differential blood pressure response to continuous positive airway pressure treatment according to the circadian pattern in hypertensive patients with obstructive sleep apnoea. Eur. Respir. J. 2019, 54, 1900098. [Google Scholar] [CrossRef] [PubMed]
- Glantz, H.; Thunstrom, E.; Herlitz, J.; Cederin, B.; Nasic, S.; Ejdeback, J.; Peker, Y. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann. Am. Thorac. Soc. 2013, 10, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Sethi, R.; Li, R.; Ho, H.H.; Hein, T.; Jim, M.H.; Loo, G.; Koo, C.Y.; Gao, X.F.; Chandra, S.; et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef]
- Khan, S.U.; Duran, C.A.; Rahman, H.; Lekkala, M.; Saleem, M.A.; Kaluski, E. A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur. Heart J. 2018, 39, 2291–2297. [Google Scholar] [CrossRef]
- Furlow, B. SAVE trial: No cardiovascular benefits for CPAP in OSA. Lancet Respir. Med. 2016, 4, 860. [Google Scholar] [CrossRef]
- Arzt, M.; Woehrle, H.; Oldenburg, O.; Graml, A.; Suling, A.; Erdmann, E.; Teschler, H.; Wegscheider, K. Prevalence and predictors of sleep-disordered breathing in patients with stable chronic heart failure: The SchlaHF Registry. JACC Heart Fail. 2016, 4, 116–125. [Google Scholar] [CrossRef]
- Oldenburg, O.; Wellmann, B.; Buchholz, A.; Bitter, T.; Fox, H.; Thiem, U.; Horstkotte, D.; Wegscheider, K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur. Heart J. 2016, 37, 1695–1703. [Google Scholar] [CrossRef]
- Selim, B.J.; Koo, B.B.; Qin, L.; Jeon, S.; Won, C.; Redeker, N.S.; Lampert, R.J.; Concato, J.P.; Bravata, D.M.; Ferguson, J.; et al. The association between nocturnal cardiac arrhythmias and sleep-disordered breathing: The DREAM study. J. Clin. Sleep Med. 2016, 12, 829–837. [Google Scholar] [CrossRef]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef]
- Ng, C.Y.; Liu, T.; Shehata, M.; Stevens, S.; Chugh, S.S.; Wang, X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 2011, 108, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, W.T.; Nasir, U.B.; Alqalyoobi, S.; O’Neal, W.T.; Mawri, S.; Sabbagh, S.; Soliman, E.Z.; Al-Mallah, M.H. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am. J. Cardiol. 2015, 116, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Fein, A.S.; Shvilkin, A.; Shah, D.; Haffajee, C.I.; Das, S.; Kumar, K.; Kramer, D.B.; Zimetbaum, P.J.; Buxton, A.E.; Josephson, M.E.; et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2013, 62, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Stone, K.L.; Varosy, P.D. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: Outcomes of sleep disorders in older men (MrOS sleep) study. Arch. Intern. Med. 2009, 169, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Roche, F.; Xuong, A.N.; Court-Fortune, I.; Costes, F.; Pichot, V.; Duverney, D.; Vergnon, J.M.; Gaspoz, J.M.; Barthélémy, J.C. Relationship among the severity of sleep apnea syndrome, cardiac arrhythmias, and autonomic imbalance. Pacing Clin. Electrophysiol. 2003, 26, 669–677. [Google Scholar] [CrossRef]
- Gami, A.S.; Howard, D.E.; Olson, E.J.; Somers, V.K. Day-night pattern of sudden death in obstructive sleep apnea. N. Engl. J. Med. 2005, 352, 1206–1214. [Google Scholar] [CrossRef]
- Gami, A.S.; Olson, E.J.; Shen, W.K.; Wright, R.S.; Ballman, K.V.; Hodge, D.O.; Herges, R.M.; Howard, D.E.; Somers, V.K. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 2013, 62, 610–616. [Google Scholar] [CrossRef]
- Voigt, L.; Haq, S.A.; Mitre, C.A.; Lombardo, G.; Kassotis, J. Effect of obstructive sleep apnea on QT dispersion: A potential mechanism of sudden cardiac death. Cardiology 2011, 118, 68–73. [Google Scholar] [CrossRef]
- Collis, T.; Devereux, R.B.; Roman, M.J.; De Simone, G.; Yeh, J.L.; Howard, B.V.; Fabsitz, R.R.; Welty, T.K. Relations of stroke volume and cardiac output to body composition: The Strong Heart Study. Circulation 2001, 103, 820–855. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Otto, M.E.; Belohlavek, M.; Romero-Corral, A.; Gami, A.S.; Gilman, G.; Svatikova, A.; Amin, R.S.; Lopez-Jimenez, F.; Khandheria, B.K.; Somers, V.K. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am. J. Cardiol. 2007, 99, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Borradaile, K.E.; Sanders, M.H.; Millman, R.; Zammit, G.; Newman, A.B.; Wadden, T.A.; Kelley, D.; Wing, R.R.; Pi-Sunyer, F.X.; et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: The Sleep AHEAD study. Arch. Intern. Med. 2009, 169, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Sam, K.; Mok, W.Y.W.; Cheung, M.T.; Fong, D.Y.T.; Lam, J.C.; Lam, D.C.; Yam, L.Y.; Ip, M.S. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax 2007, 62, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. J. Am. Med. Assoc. 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Issa, F.G.; Sullivan, C.E. Alcohol, snoring and sleep apnoea. J. Neurol. Neurosurg. Psychiatry 1982, 45, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Scrima, L.; Broudy, M.; Nay, K.N.; Cohn, M.A. Increased severity of obstructive sleep apnea after bedtime alcohol ingestion: Diagnostic potential and proposed mechanism of action. Sleep 1982, 5, 318–328. [Google Scholar] [CrossRef]
- Shorten, G.D.; Opie, N.J.; Graziotti, P.; Morris, I.; Khangure, M. Assessment of upper airway anatomy in awake, sedated and anaesthetised patients using magnetic resonance imaging. Anaesth. Intensive Care 1994, 22, 165–169. [Google Scholar] [CrossRef]
- Hudgel, D.W.; Hendricks, C. Palate and hypopharynx-sites of inspiratory narrowing of the upper airway during sleep. Am. Rev. Respir. Dis. 1988, 138, 1542–1547. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A.; Wellman, A.; White, D.P. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep 2014, 37, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol and the risk of sleep apnoea: A systematic review and meta-analysis. Sleep Med. 2018, 42, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, W.S.; Tang, S.E.; Lin, H.C.; Peng, C.K.; Chu, H.T.; Kao, C.H. Benzodiazepines associated with acute respiratory failure in patients with obstructive sleep apnea. Front. Pharmacol. 2019, 9, 1513. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Hock, L.M.; Bowman, T.J. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001, 5, 167–172. [Google Scholar] [CrossRef]
- Wetter, D.W.; Young, T.B.; Bidwell, T.R.; Badr, M.S.; Palta, M. Smoking as a risk factor for sleep-disordered breathing. Arch. Intern. Med. 1994, 154, 2219–2224. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.H.; Park, S.Y.; Won, H.R.; Lee, H.J.; Yang, H.S.; Kim, H.J. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J. Clin. Sleep Med. 2012, 8, 367–374. [Google Scholar] [CrossRef]
- Deleanu, O.C.; Pocora, D.; Mihaicuta, S.; Ulmeanu, R.; Zaharie, A.M.; Mihaltan, F.D. Influence of smoking on sleep and obstructive sleep apnea syndrome. Pneumologia 2016, 65, 28–35. [Google Scholar]
- Bielicki, P.; Trojnar, A.; Sobieraj, P.; Wąsik, M. Smoking status in relation to obstructive sleep apnea severity (OSA) and cardiovascular comorbidity in patients with newly diagnosed OSA. Adv. Respir. Med. 2019, 87, 103–109. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Chiu, N.Y.; Chang, C.C.; Chang, T.G.; Lane, H.Y. The association between cigarette smoking and obstructive sleep apnea. Tob. Induc. Dis. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. Smoking interacts with sleep apnea to increase cardiovascular risk. Sleep Med. 2008, 9, 247–253. [Google Scholar] [CrossRef]
- Greenberg, N.; Carel, R.S.; Dubnov, J.; Derazne, E.; Portnov, B.A. Prevalence of asthma among young men residing in urban areas with different sources of air pollution. Isr. Med. Assoc. J. 2019, 12, 785–789. [Google Scholar] [PubMed]
- Bédard, A.; Sofiev, M.; Arnavielhe, S.; Antó, J.M.; Garcia-Aymerich, J.; Thibaudon, M.; Bergmann, K.C.; Dubakiene, R.; Bedbrook, A.; Onorato, G.L.; et al. Interactions between air pollution and pollen season for rhinitis using mobile technology: A mask-pollar study. J. Allergy Clin. Immunol. Pract. 2019, 8, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Ma, S.; Wang, K.; Lou, H.; Wang, Y.; Zhang, L.; Wang, C.; Akdis, C.A. Particulate matter 2.5 causes deficiency in barrier integrity in human nasal epithelial cells. Allergy Asthma Immunol. Res. 2020, 12, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Kim, H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J. Allergy Clin. Immunol. 1997, 99, 757–762. [Google Scholar] [CrossRef]
- Chan, C.C.K.; Au, C.T.; Lam, H.S.; Lee, D.L.Y.; Wing, Y.K.; Li, A.M. Intranasal corticosteroids for mild childhood obstructive sleep apnea - a randomized, placebo-controlled study. Sleep Med. 2015, 16, 358–363. [Google Scholar] [CrossRef]
- Goldbart, A.D.; Greenberg-Dotan, S.; Tal, A. Montelukast for children with obstructive sleep apnea: A double-blind, placebo-controlled study. Pediatrics 2012, 130, 575–580. [Google Scholar] [CrossRef]
- Park, H.-K.; Cheng, K.-C.; Tetteh, A.O.; Hildemann, L.M.; Nadeau, K.C. Effectiveness of air purifier on health outcomes and indoor particles in homes of children with allergic diseases in Fresno, California: A pilot study. J. Asthma 2017, 54, 341–346. [Google Scholar] [CrossRef]
- Weinreich, G.; Wessendorf, T.E.; Pundt, N.; Weinmayr, G.; Hennig, F.; Moebus, S.; Möhlenkamp, S.; Erbel, R.; Jöckel, K.H.; Teschler, H.; et al. Association of short-term ozone and temperature with sleep disordered breathing. Eur. Respir. J. 2015, 46, 1361–1369. [Google Scholar] [CrossRef]
- Zanobetti, A.; Redline, S.; Schwartz, J.; Rosen, D.; Patel, S.; O’Connor, G.T.; Lebowitz, M.; Coull, B.A.; Gold, D.R. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am. J. Respir. Crit. Care Med. 2010, 182, 819–825. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanik, D.; Martynowicz, H.; Mazur, G.; Poręba, R.; Gać, P. Environmental Factors as Modulators of the Relationship between Obstructive Sleep Apnea and Lesions in the Circulatory System. J. Clin. Med. 2020, 9, 836. https://doi.org/10.3390/jcm9030836
Urbanik D, Martynowicz H, Mazur G, Poręba R, Gać P. Environmental Factors as Modulators of the Relationship between Obstructive Sleep Apnea and Lesions in the Circulatory System. Journal of Clinical Medicine. 2020; 9(3):836. https://doi.org/10.3390/jcm9030836
Chicago/Turabian StyleUrbanik, Dominika, Helena Martynowicz, Grzegorz Mazur, Rafał Poręba, and Paweł Gać. 2020. "Environmental Factors as Modulators of the Relationship between Obstructive Sleep Apnea and Lesions in the Circulatory System" Journal of Clinical Medicine 9, no. 3: 836. https://doi.org/10.3390/jcm9030836
APA StyleUrbanik, D., Martynowicz, H., Mazur, G., Poręba, R., & Gać, P. (2020). Environmental Factors as Modulators of the Relationship between Obstructive Sleep Apnea and Lesions in the Circulatory System. Journal of Clinical Medicine, 9(3), 836. https://doi.org/10.3390/jcm9030836






