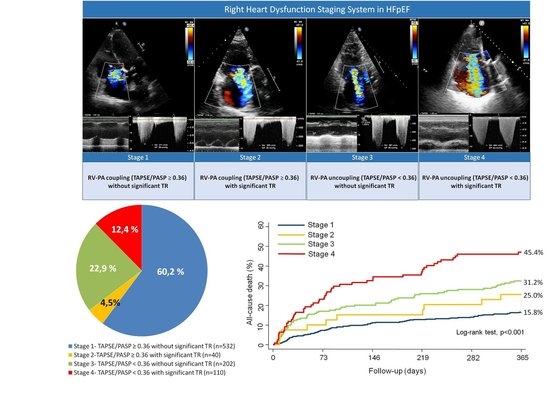
Right Ventricular Dysfunction Staging System for Mortality Risk Stratification in Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Experimental Section
2.1. Study Group and Protocol
2.2. Echocardiography
2.3. Outcomes
2.4. Ethical Concerns
2.5. Statistical Analysis
3. Results
3.1. Study Population Characteristics
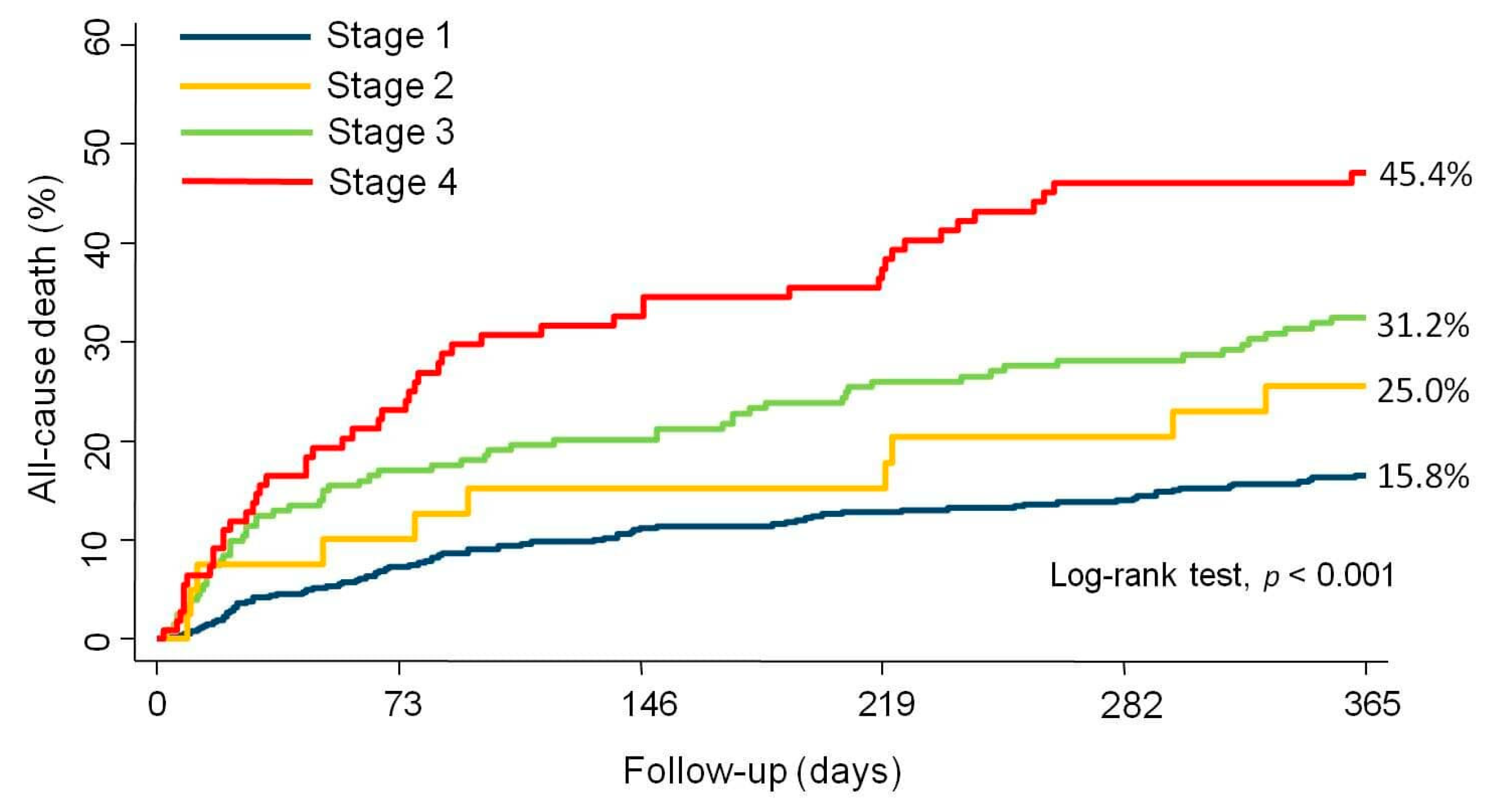
3.2. All-cause Mortality Across RVD Stages
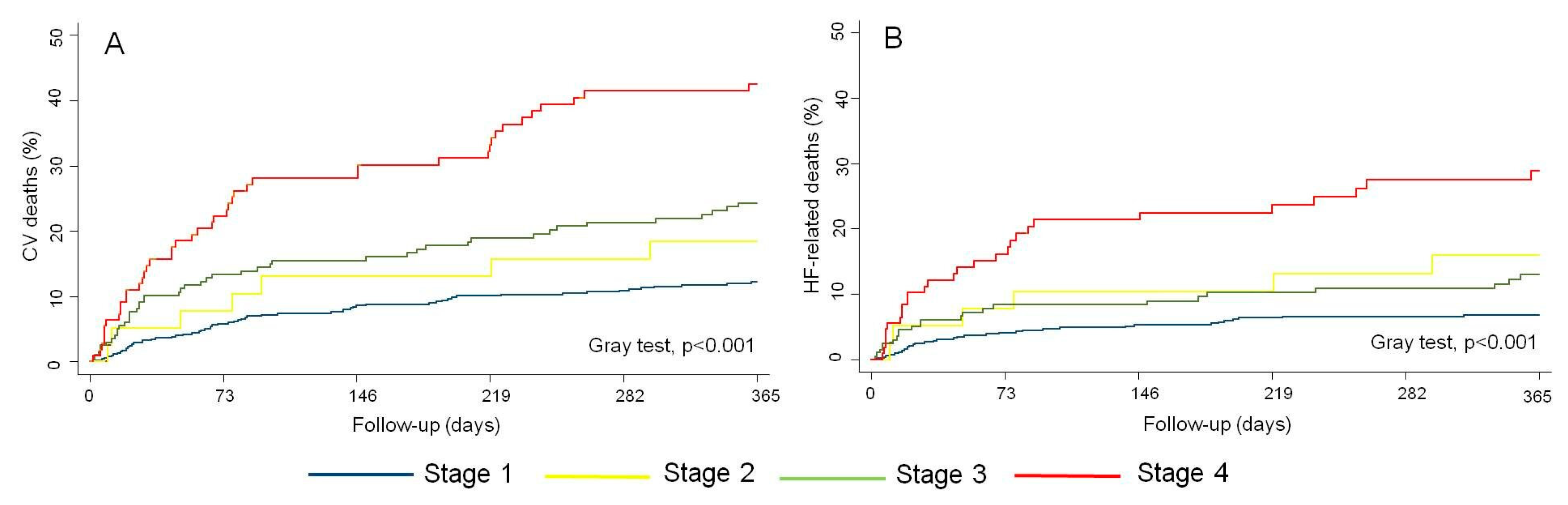
3.3. CV and HF-Related Mortality Across RVD Stages
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur. Heart J. 2018, 39, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.V.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Chaterjee, N.A.; Steiner, J.; Lewis, G.D. It is time to look at heart failure with preserved ejection fraction from the right side. Circulation 2014, 130, 2272–2277. [Google Scholar] [CrossRef][Green Version]
- Gorter, T.M.; van Veldhuisen, D.J.; Bauersachs, J.; Borlaug, B.A.; Celutkiene, J.; Coats, A.J.S.; Crespo-Leiro, M.G.; Guazzi, M.; Harjola, V.P.; Heymans, S.; et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: Mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 2018, 20, 16–37. [Google Scholar] [CrossRef]
- Parikh, K.S.; Sharma, K.; Fuizat, M.; Surks, H.K.; George, J.T.; Honarpour, N.; Depre, C.; Desvigne-Vickens, P.; Nkulikinyinka, R.; Lewis, G.D.; et al. Heart failure with preserved ejection fraction expert panel report. Current controversies and implications for clinical trials. J. Am. Coll. Cardiol. HF 2018, 6, 619–632. [Google Scholar]
- Borlaug, B.; Kane, G.C.; Melenovsky, V.; Olson, T.P. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur. Heart J. 2016, 37, 3294–3302. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Naeije, M. Pulmonary hypertension in heart failure. J. Am. Coll. Cardiol. 2017, 69, 1718–1734. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Marwick, T.H. Impaired right heart and pulmonary vascular function in HFpEF. Time for more risk markers? JACC Cardiovasc. Imaging 2017, 10, 1222–1224. [Google Scholar] [CrossRef]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction. Stratification of clinical phenotypes and outcomes. J. Am. Coll. Cardiol. Img. 2017, 10, 1211–1221. [Google Scholar] [CrossRef]
- Gerges, M.; Gerges, C.; Pristitto, A.M.; Lang, M.B.; Trip, T.; Jakowistch, J.; Binder, T.; Lang, I.M. Pulmonary hypertension in heart failure, epidemiology, right ventricular function and survival. Am. J. Respir. Crit. Care Med. 2015, 192, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.; Waxman, A.B.; Denti, P.; Delhaas, T. Anatomic relationship of the complex tricuspid valve, right ventricle and pulmonary vasculature. A review. JAMA Cardiol. 2019, 4, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Santas, E.; Chorro, F.J.; Miñana, G.; Méndez, J.; Muñoz, J.; Escribano, D.; García-Blas, S.; Valero, E.; Bodí, V.; Núñez, E.; et al. Tricuspid regurgitation and mortality risk across left ventricular systolic function in acute heart failure. Circ. J. 2015, 79, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; Hoendermis, E.S.; van Valdhuisen, D.J.; Voors, A.A.; Lam, C.S.P.; Geelhoed, B.; Willems, T.P.; van Melle, J.P. Right ventricular dysfunction in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Eur. J. Heart Fail. 2016, 18, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Roger, V.L.; Rodeheffer, R.J.; Borlaug, B.A.; Enders, F.T.; Redfield, M.M. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J. Am. Coll. Cardiol. 2009, 53, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A report from the American Society of Echocardiography; endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- Zohgbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for Evaluation of the Severity of Native Valvular Regurgitation with Two-dimensional and Doppler Echocardiography. A report from the American Society of Echocardiography’s Nomenclature and Standards Committee and The Task Force on Valvular Regurgitation, developed in conjunction with the American College of Cardiology Echocardiography Committee, The Cardiac Imaging Committee Council on Clinical Cardiology, the American Heart Association, and the European Society of Cardiology Working Group on Echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar]
- Fine, J.P.; Gray, R.J. A proportional hazard model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Taramaso, M.; Gavazzoni, M.; Pozzoli, A.; Dreyfus, G.D.; Bolling, S.F.; George, I.; Kapos, I.; Tanner, F.C.; Zuber, M.; Maisano, F.; et al. Tricuspid regurgitation. Predicting the need for intervention, procedural success, and recurrence of disease. J. Am. Coll. Cardiol. Imaging 2019, 12, 605–621. [Google Scholar]
- Gorter, T.M.; van Veldhuisen, D.J.; Voors, A.A.; Hummel, Y.M.; Lam, C.S.P.; Berger, R.M.F.; van Melle, J.P.; Hoendermis, E.S. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur. J. Cardiovasc. Imaging 2018, 19, 425–432. [Google Scholar] [CrossRef]
- Santas, E.; Palau, P.; Guazzi, M.; de la Espriella, R.; Miñana, G.; Sanchis, J.; Bayés-Genís, A.; Lupón, J.; Chorro, F.J.; Núñez, J. Uselfulness of right ventricular to pulmonary circulation coupling as an indicator of risk for recurrent admissions in heart failure with preserved ejection fraction. Am. J. Card. 2019, 124, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Cardounel, A.; Abdelkarim, I.; Kilic, A.; Althouse, A.D.; Sharbaugh, M.S.; Gupta, A.; Xu, J.; Fukui, M.; Simon, M.A.; et al. Right ventricular to pulmonary artery coupling in patients undergoing transcatheter aortic valve implantation. Heart 2019, 105, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Taramaso, M.; Gavazzoni, M.; Maisano, F. Is tricuspid regurgitation a prognostic interventional target or is it just an indicator of worst prognosis in heart failure patients? Eur. Heart J. 2019, 40, 485–487. [Google Scholar] [CrossRef]
- De la Espriella, R.; Santas, E.; Chorro, F.J.; Miñana, G.; Soler, M.; Bodí, V.; Valero, E.; Núñez, E.; Bayés-Genís, A.; Lupón, J.; et al. Functional tricuspid regurgitation and recurrent admissions in patients with acute heart failure. Int. J. Cardiol. 2019, 291, 83–88. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur. Heart J. 2019, 40, 689–698. [Google Scholar] [CrossRef]
- Wang, N.; Fulcher, J.; Abeysuriya, N.; McGrady, M.; Wilcox, I.; Celermajer, D.; Lal, S. Tricuspid regurgitation is associated with increased mortality independent of pulmonary pressures and right heart failure: A systematic review and meta-analysis. Eur. Heart J. 2019, 40, 476–484. [Google Scholar] [CrossRef]
- Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; Goedemans, L.; Mertens, B.J.A.; Gursoy, E.; van Genderen, O.S.; AjmonAjmone Marsane, N.; Delgado, V.; Bax, J.J. Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation 2019, 140, 836–845. [Google Scholar] [CrossRef]
- Bax, J.J.; Di Carli, M.; Narula, J.; Delgado, V. Multimodality imaging in ischemic heart failure. Lancet 2019, 393, 1056–1070. [Google Scholar] [CrossRef]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Vollema, E.M.; Amanullah, M.R.; Ng, A.C.T.; van der Bijl, P.; Prevedello, F.; Sin, Y.G.; Prihadi, E.A.; Marsan, N.A.; Ding, Z.P.; Généreux, P.; et al. Staging cardiac damage with symptomatic aortic valve stenosis. J. Am. Coll. Cardiol. 2019, 74, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Bisbal, F.; Baranchuk, A.; Braundwald, E.; Bayés de Luna, A.; Bayés-Genís, A. Atrial failure as a clinical entity. J. Am. Coll. Cardiol. 2020, 75, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Howard, L.S.; Gomberg-Maitland, M.; Hoeper, M.M. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation 2020, 141, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Bosch, L.; Lam, C.S.P.; Gong, L.; Chang, S.P.; Sim, D.; Yeo, D.; Jaufeerally, F.; Long, K.T.G.; Ong, H.Y.; Ng, T.P.; et al. Right ventricular dysfunction in left-sided heart failure with preserved vs reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
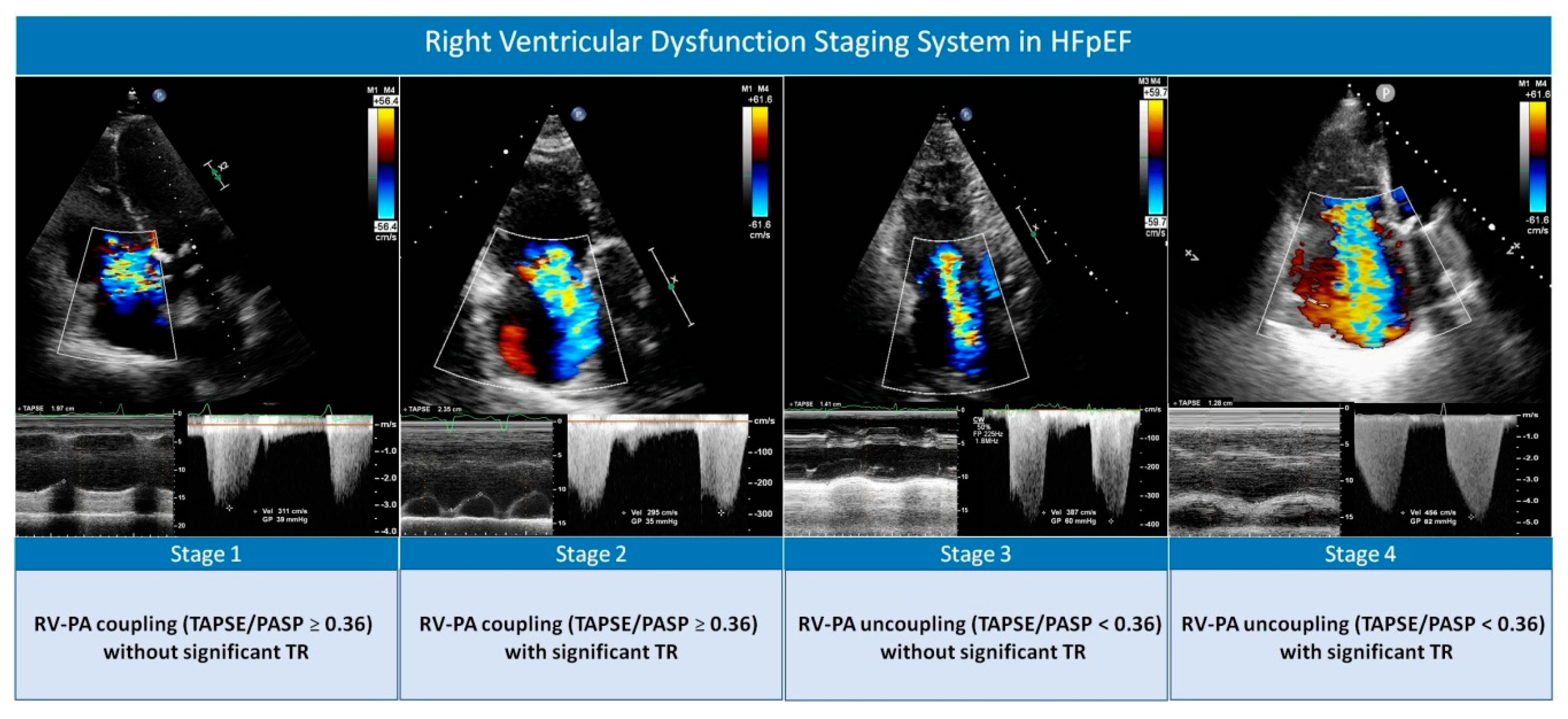
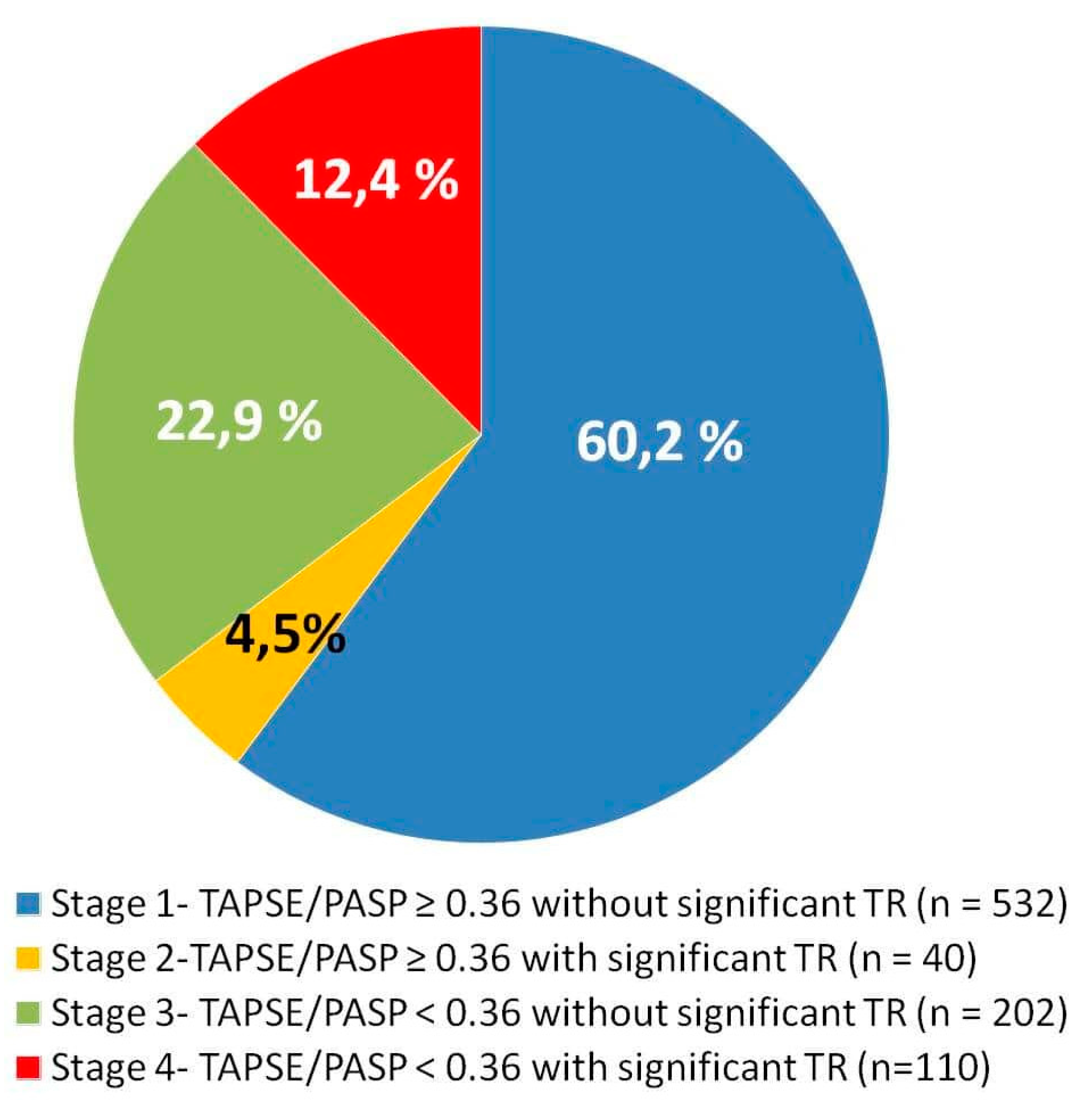

| Total Population (n = 884) | TAPSE/PASP ≥ 0.36 without Significant TR (n = 532) | TAPSE/PASP ≥ 0.36 with Significant TR (n = 40) | TAPSE/PASP < 0.36 without Significant TR (n = 202) | TAPSE/PASP < 0.36 with Significant TR (n = 110) | p Value | |
|---|---|---|---|---|---|---|
| Age, years | 76.1 ± 9.7 | 75.9 ± 9.9 | 74.0 ± 11.1 | 77.7 ± 8.9 | 77.2 ± 8.3 | 0.036 |
| Women | 569 (64.4) | 339 (63.7) | 30 (75.0) | 144 (71.3) | 82 (74.5) | 0.041 |
| First HF admission | 494 (55.7) | 332 (62.4) | 23 (57.5) | 96 (47.5) | 48 (43.6) | <0.001 |
| Prior NYHA class III/IV | 159 (17.9) | 63 (11.2) | 9 (22.5) | 55 (27.2) | 32 (29.1) | <0.001 |
| Hypertension | 700 (79.2) | 429 (80.6) | 27 (67.5) | 163 (80.7) | 81 (73.6) | 0.097 |
| Diabetes Mellitus | 347 (39.2) | 208 (39.1) | 13 (32.5) | 87 (43.1) | 39 (35.4) | 0.445 |
| Dyslipidemia | 452 (51.1) | 284 (53.4) | 18 (45.0) | 100 (49.5) | 50 (45.4) | 0.346 |
| Current smoker | 59 (6.7) | 48 (9.0) | 4 (10.0) | 5 (2.5) | 2 (1.8) | 0.002 |
| Ischemic heart disease | 185 (20.9) | 112 (21.0) | 7 (17.5) | 51 (25.2) | 15 (13.6) | 0.107 |
| Charlson index >2 | 274 (31) | 160 (30.1) | 9 (22.5) | 71 (35.1) | 34 (30.9) | 0.363 |
| Heart rate (beats/min) | 96.4 ± 30.7 | 98.8 ± 31.7 | 94.7 ± 29.1 | 94.5 ± 29.2 | 89.4 ± 27.4 | 0.005 |
| SBP (mmHg) | 144.5 ± 29.6 | 146.8 ± 29.8 | 133.0 ± 17.1 | 145.4 ± 32.3 | 135.6 ± 24.4 | <0.001 |
| DBP (mmHg) | 77.7 ± 18.9 | 79.2 ± 19.5 | 74.8 ± 15.9 | 76.7 ± 18.1 | 73.5 ± 16.8 | 0.017 |
| QRS >120 ms | 202 (22.8) | 122 (22.9) | 7 (17.5) | 43 (21.3) | 30 (27.3) | 0.541 |
| Atrial fibrillation | 547 (61.9) | 297 (55.8) | 31 (77.5) | 139 (68.8) | 80 (72.7) | <0.001 |
| Hemoglobin (g/dL) | 11.9 ± 1.8 | 11.9 ± 1.9 | 11.9 ± 1.8 | 11.8 ± 1.6 | 12.0 ± 1.9 | 0.604 |
| Sodium (mEq/l) | 138.2 ± 5.0 | 138.5 ± 4.7 | 138.2 ± 4.2 | 138.0 ± 5.5 | 137.3 ± 5.7 | 0.173 |
| NT-proBNP (pg/mL) * | 3653 (5628) | 3088 (4351) | 2026 (3280) | 5282 (7017) | 5635 (6364) | <0.001 |
| Serum Creatinine (mg/dL) | 1.22 ± 0.59 | 1.19 ± 0.59 | 1.10 ± 0.53 | 1.26 ± 0.58 | 1.31 ± 0.61 | 0.016 |
| BUN (mg/dL) | 60.9 ± 33.1 | 56.8 ± 29.1 | 56.5 ± 30.8 | 65.7 ± 35.7 | 73.7 ± 41.0 | <0.001 |
| GFR ( mL/min/1.73 m2) | 61.9 ± 29.8 | 63.2 ± 25.9 | 65.8 ± 25.3 | 59.9 ± 30.5 | 58.3 ± 43.9 | <0.001 |
| CA125 (U/mL) * | 56 (103) | 48 (83) | 79 (126) | 60 (98) | 99 (146) | 0.001 |
| LVEF (%) | 61.7 ± 7.5 | 61.8 ± 7.2 | 62.1 ± 7.6 | 61.8 ± 8.2 | 61.2 ± 7.7 | 0.864 |
| LAD (mm) | 45.7 ± 8.2 | 43.9 ± 7.4 | 48.3 ± 11.2 | 46.5 ± 7.3 | 51.5 ± 9.1 | <0.001 |
| LAVI (mL/m2) | 44.6 ± 12.1 | 41.3 ± 11.5 | 43.9 ± 12.9 | 46.4 ± 15.7 | 50.0 ± 16.9 | <0.001 |
| DT (ms) | 222.5 ± 62.4 | 225.3 ± 59.2 | 241.4 ± 77.2 | 214.4 ± 60.1 | 216 ± 72.0 | 0.026 |
| E/e’ ratio | 19.3 ± 11.3 | 18.7 ± 11.8 | 19.3 ± 10.5 | 21.3 ± 10.6 | 19.1 ± 9.4 | 0.359 |
| TAPSE (mm) | 19.2 ± 3.3 | 19.9 ± 3.2 | 20.6 ± 4.0 | 16.8 ± 3.0 | 16.8 ± 2.8 | <0.001 |
| S’ tricuspid wave (cm/s) | 11.3 ± 3.1 | 11.8 ± 3.2 | 12.0 ± 3.0 | 9.9 ± 2.5 | 9.9 ± 2.6 | <0.001 |
| PASP (mm Hg) | 47.5 ± 16.0 | 38.8 ± 38.4 | 45.7 ± 9.2 | 63.7 ± 26.7 | 70.4 ± 16.4 | <0.001 |
| Functional MR III/IV | 188 (21.3) | 79 (14.8) | 14 (35.0) | 48 (23.8) | 47 (42.7) | <0.001 |
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
|---|---|---|---|---|
| All-cause Mortality | ||||
| Unadjusted | Adjusted * | |||
| Stage 1 (reference) | ||||
| Stage 2 | 1.634 (0.848–3.149) | 0.142 | 1.482 (0.755–2.912) | 0.253 |
| Stage 3 | 2.209 (1.593–3.062) | <0.001 | 1.822 (1.308–2.538) | <0.001 |
| Stage 4 | 3.568 (2.513–5.066) | <0.001 | 2.263 (1.540–3.325) | <0.001 |
| CV mortality | ||||
| Unadjusted | Adjusted * | |||
| Stage 1 (reference) | ||||
| Stage 2 | 1.552 (0.711–3.388) | 0.270 | 1.456 (0.640–3.309) | 0.640 |
| Stage 3 | 2.091 (1.423–3.074) | <0.001 | 1.730 (1.157–2.585) | 0.007 |
| Stage 4 | 4.207 (2.859–6.192) | 0.001 | 2.677 (1.701–4.212) | <0.001 |
| HF mortality | ||||
| Unadjusted | Adjusted† | |||
| Stage 1 (reference) | ||||
| Stage 2 | 2.403 (1.017–5.676) | 0.046 | 2.565 (1.062–6.193) | 0.036 |
| Stage 3 | 1.843 (1.085–3.130) | 0.024 | 1.546 (0.904–2.644) | 0.112 |
| Stage 4 | 4.438 (2.696–7.305) | <0.001 | 2.845 (1.562–5.183) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santas, E.; De la Espriella, R.; Chorro, F.J.; Palau, P.; Miñana, G.; Heredia, R.; Amiguet, M.; Merenciano, H.; Sanchis, J.; Lupón, J.; et al. Right Ventricular Dysfunction Staging System for Mortality Risk Stratification in Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2020, 9, 831. https://doi.org/10.3390/jcm9030831
Santas E, De la Espriella R, Chorro FJ, Palau P, Miñana G, Heredia R, Amiguet M, Merenciano H, Sanchis J, Lupón J, et al. Right Ventricular Dysfunction Staging System for Mortality Risk Stratification in Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine. 2020; 9(3):831. https://doi.org/10.3390/jcm9030831
Chicago/Turabian StyleSantas, Enrique, Rafael De la Espriella, Francisco Javier Chorro, Patricia Palau, Gema Miñana, Raquel Heredia, Martina Amiguet, Héctor Merenciano, Juan Sanchis, Josep Lupón, and et al. 2020. "Right Ventricular Dysfunction Staging System for Mortality Risk Stratification in Heart Failure with Preserved Ejection Fraction" Journal of Clinical Medicine 9, no. 3: 831. https://doi.org/10.3390/jcm9030831
APA StyleSantas, E., De la Espriella, R., Chorro, F. J., Palau, P., Miñana, G., Heredia, R., Amiguet, M., Merenciano, H., Sanchis, J., Lupón, J., Bayés-Genís, A., & Núñez, J. (2020). Right Ventricular Dysfunction Staging System for Mortality Risk Stratification in Heart Failure with Preserved Ejection Fraction. Journal of Clinical Medicine, 9(3), 831. https://doi.org/10.3390/jcm9030831