Comparative In Vitro Study of Various α2-Adrenoreceptor Agonist Drugs for Ticagrelor Reversal
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Preparation of Washed Platelet Suspension
2.2. Agonists and Inhibitors
2.3. Platelet Aggregation
2.4. Measurement of Intracellular cAMP
2.5. Statistical Analysis
3. Results
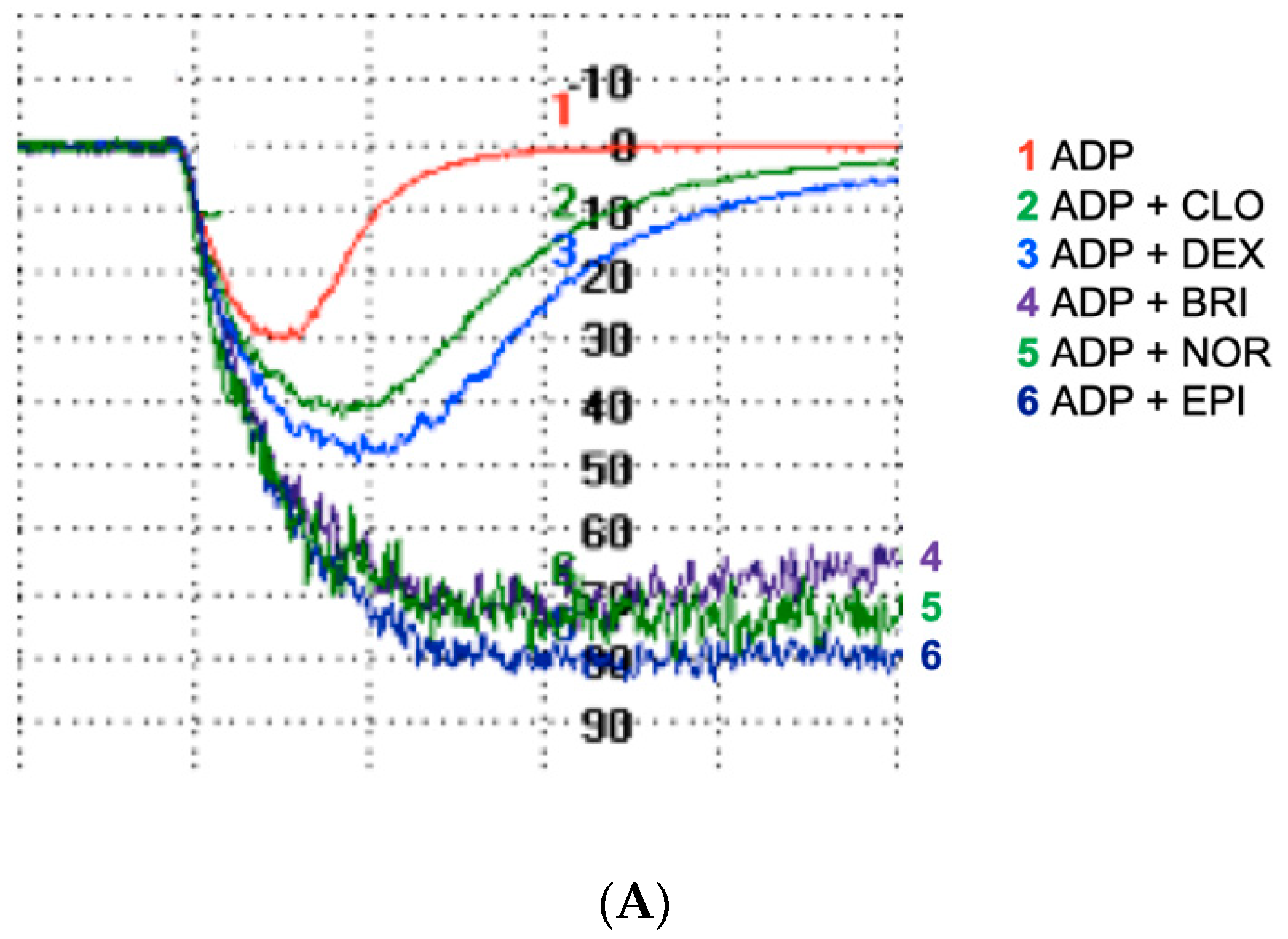
3.1. All α2-Adrenoreceptor Agonists Potentiate ADP-Induced Platelet Aggregation in the Absence of Ticagrelor
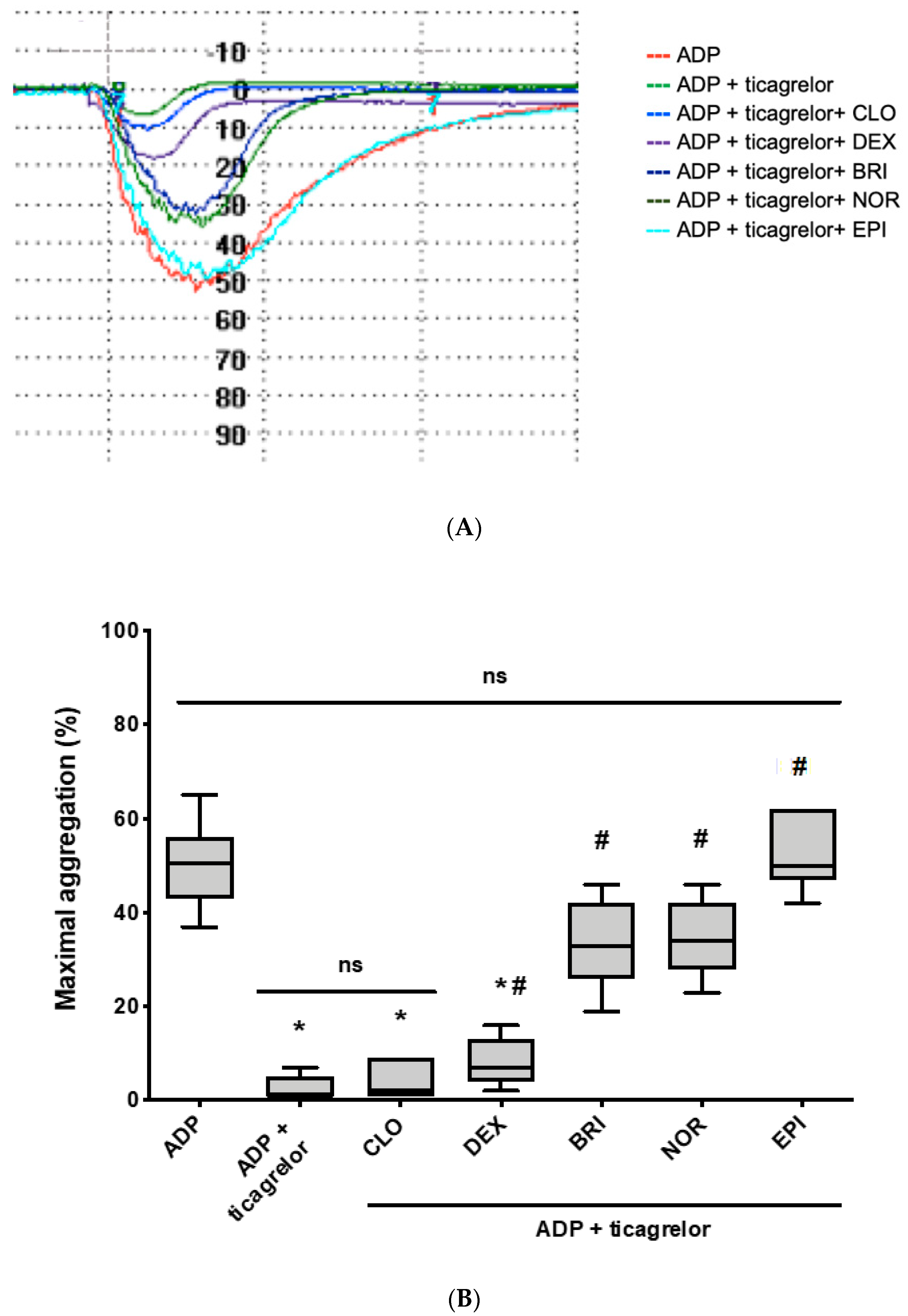
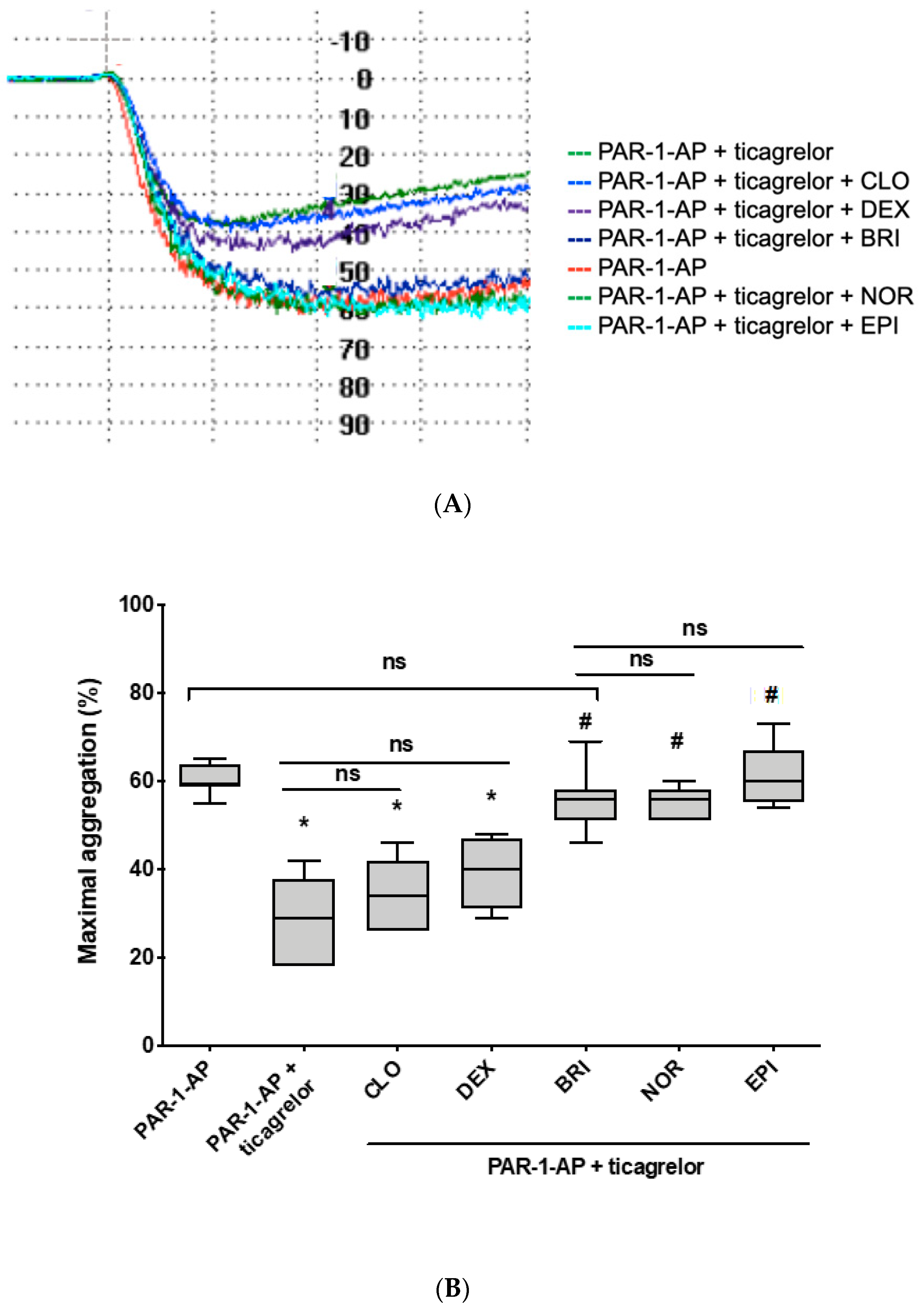
3.2. Norepinephrine and Brimonidine Partially Restore ADP- and PAR-1-AP-Induced Platelet Aggregation Inhibited by Ticagrelor, While Clonidine and Dexmedetomidine Are Ineffective
3.3. Norepinephrine and Brimonidine Fully Inhibit PGE1-Stimulated cAMP Production Whereas Clonidine and Dexmedetomidine Induce Only Weak Inhibition
4. Discussion
5. Study Limitations and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies, 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar]
- Martin, A.; Berndt, C.; Calmette, L.; Philip, I.; Decouture, B.; Gaussem, P.; Gouin-Thibault, I.; Samama, C.; Bachelot-Loza, C.; Godier, A. The effectiveness of platelet supplementation for the reversal of ticagrelor-induced inhibition of platelet aggregation: An in-vitro study. Eur. J. Anaesthesiol. 2016, 33, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Godier, A.; Taylor, G.; Gaussem, P. Inefficacy of Platelet Transfusion to Reverse Ticagrelor. N. Engl. J. Med. 2015, 372, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernandez-Mondejar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fourth edition. Crit. Care 2016, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Calmette, L.; Martin, A.C.; Le Bonniec, B.; Zlotnik, D.; Gouin-Thibault, L.; Bachelot-Loza, C.; Gaussem, P.; Godier, A. Ticagrelor reversal: In vitro assessment of four haemostatic agents. J. Clin. Pathol. 2017, 70, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Pehrsson, S.; Hansson, K.; Nelander, K.; Nylander, S. Boosting the coagulation restores haemostasis in ticagrelor-treated mice. Blood Coagul. Fibrinolysis 2016, 27, 1–919. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Mitchell, P.D.; Butler, K. The effect of desmopressin on bleeding time and platelet aggregation in healthy volunteers administered ticagrelor. J. Clin. Pharm. Ther. 2014, 39, 186–191. [Google Scholar] [CrossRef]
- Buchanan, A.; Newton, P.; Pehrsson, S.; Inghardt, T.; Antonsson, T.; Svensson, P.; Sjögren, T.; Öster, L.; Janefeldt, A.; Sandinge, A.-S.; et al. Structural and functional characterization of a specific antidote for ticagrelor. Blood 2015, 125, 3484–3490. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Pollack, C.V.; Weitz, J.I.; Jennings, L.K.; Xu, S.; Arnold, S.E.; Umstead, B.R.; Mays, M.C.; Lee, J.S. Antibody-Based Ticagrelor Reversal Agent in Healthy Volunteers. N. Engl. J. Med. 2019, 380, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arter. Thromb. Vasc. Boil. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Martin, A.-C.; Zlotnik, D.; Bonete, G.P.; Baron, E.; Decouture, B.; Belleville-Rolland, T.; Le Bonniec, B.; Poirault-Chassac, S.; Alessi, M.-C.; Gaussem, P.; et al. Epinephrine restores platelet functions inhibited by ticagrelor: A mechanistic approach. Eur. J. Pharmacol. 2020, 866, 172798. [Google Scholar] [CrossRef] [PubMed]
- Algeo, S.; Appleton, C.P.; Martin, G.V.; Lee, R.W.; Mulkey, R.; Olajos, M.; Goldman, S. Effects of alpha 2-adrenergic stimulation with UK 14,304-18 on the heart and peripheral circulation of intact dogs. J. Cardiovasc. Pharmacol. 1985, 7, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Ardlie, N.G.; Perry, D.W.; Packham, M.A.; Mustard, J.F. Influence of Apyrase on Stability of Suspensions of Washed Rabbit Platelets. Exp. Boil. Med. 1971, 136, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Kafka, M.S.; Tallman, J.F.; Smith, C.C.; Costa, J.L. Alpha-adrenergic receptors on human platelets. Life Sci. 1977, 21, 1429–1437. [Google Scholar] [CrossRef]
- Piletz, J.E.; Andorn, A.C.; Unnerstall, J.R.; Halaris, A. Binding of [3H]-p-aminoclonidine to alpha 2-adrenoceptor states plus a non-adrenergic site on human platelet plasma membranes. Biochem. Pharmacol. 1991, 42, 569–584. [Google Scholar] [CrossRef]
- Piletz, J.E.; Sletten, K. Nonadrenergic imidazoline binding sites on human platelets. J. Pharmacol. Exp. Ther. 1993, 267, 1493–1502. [Google Scholar]
- Smolenski, A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J. Thromb. Haemost. 2012, 10, 167–176. [Google Scholar] [CrossRef]
- Kawamoto, S.; Hirakata, H.; Sugita, N.; Fukuda, K. Bidirectional effects of dexmedetomidine on human platelet functions in vitro. Eur. J. Pharmacol. 2015, 766, 122–128. [Google Scholar] [CrossRef]
- Adam, E.H.; Baro, D.; Schmidt, P.; Mutlak, H.; Zacharowski, K.; Hanke, A.A.; Weber, C.F. Aggregometric assessment of clonidine’s impact on the efficacy of dual platelet inhibition. Clin. Lab. 2014, 60, 1533–1539. [Google Scholar] [CrossRef]
- Janatmakan, F.; Nesioonpour, S.; Zadeh, F.J.; Teimouri, A.; Vaziri, M. Comparing the Effect of Clonidine and Dexmedetomidine on Intraoperative Bleeding in Spine Surgery. Anesthesiol. Pain Med. 2019, 9, e83967. [Google Scholar] [CrossRef]
- Mizrak, A.; Karatas, E.; Saruhan, R.; Kara, F.; Oner, U.; Saricicek, V.; Baysal, E. Does dexmedetomidine affect intraoperative blood loss and clotting tests in pediatric adenotonsillectomy patients? J. Surg. Res. 2013, 179, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.K.; Ober, J.C.; McNatt, J.; Benedict, C.R.; Rosolowsky, M.; Anderson, H.V.; Cui, K.; Maffrand, J.P.; Campbell, W.B.; Buja, L.M. ADP plays an important role in mediating platelet aggregation and cyclic flow variations in vivo in stenosed and endothelium-injured canine coronary arteries. Circ. Res. 1992, 70, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Samama, C.M.; Bonnin, P.; Bonneau, M.; Pignaud, G.; Mazoyer, E.; Bailliart, O.; Maffrand, J.P.; Viars, P.; Caen, J.P.; O Drouet, L. Comparative arterial antithrombotic activity of clopidogrel and acetyl salicylic acid in the pig. Thromb. Haemost. 1992, 68, 500–505. [Google Scholar] [PubMed]
- Singh, S.; Damén, T.; Nygren, A.; Hakimi, C.S.; Ramström, S.; Dellborg, M.; Lindahl, T.L.; Hesse, C.; Jeppsson, A. Adrenaline Improves Platelet Reactivity in Ticagrelor-Treated Healthy Volunteers. Thromb. Haemost. 2019, 119, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Gedeborg, R.; Silander, H.C.; Ronne-Engström, E.; Rubertsson, S.; Wiklund, L. Adverse effects of high-dose epinephrine on cerebral blood flow during experimental cardiopulmonary resuscitation. Crit. Care Med. 2000, 28, 1423–1430. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porta Bonete, G.; Godier, A.; Gaussem, P.; Belleville-Rolland, T.; Leuci, A.; Poirault-Chassac, S.; Bachelot-Loza, C.; Martin, A.-C. Comparative In Vitro Study of Various α2-Adrenoreceptor Agonist Drugs for Ticagrelor Reversal. J. Clin. Med. 2020, 9, 809. https://doi.org/10.3390/jcm9030809
Porta Bonete G, Godier A, Gaussem P, Belleville-Rolland T, Leuci A, Poirault-Chassac S, Bachelot-Loza C, Martin A-C. Comparative In Vitro Study of Various α2-Adrenoreceptor Agonist Drugs for Ticagrelor Reversal. Journal of Clinical Medicine. 2020; 9(3):809. https://doi.org/10.3390/jcm9030809
Chicago/Turabian StylePorta Bonete, Guillaume, Anne Godier, Pascale Gaussem, Tiphaine Belleville-Rolland, Alexandre Leuci, Sonia Poirault-Chassac, Christilla Bachelot-Loza, and Anne-Céline Martin. 2020. "Comparative In Vitro Study of Various α2-Adrenoreceptor Agonist Drugs for Ticagrelor Reversal" Journal of Clinical Medicine 9, no. 3: 809. https://doi.org/10.3390/jcm9030809
APA StylePorta Bonete, G., Godier, A., Gaussem, P., Belleville-Rolland, T., Leuci, A., Poirault-Chassac, S., Bachelot-Loza, C., & Martin, A.-C. (2020). Comparative In Vitro Study of Various α2-Adrenoreceptor Agonist Drugs for Ticagrelor Reversal. Journal of Clinical Medicine, 9(3), 809. https://doi.org/10.3390/jcm9030809




