Helicobacter pylori Gastritis in Children—The Link between Endoscopy and Histology
Abstract
1. Introduction
2. Materials and Methods
2.1. Endoscopy
2.2. Histopathological Examination
2.3. Statistical Analysis
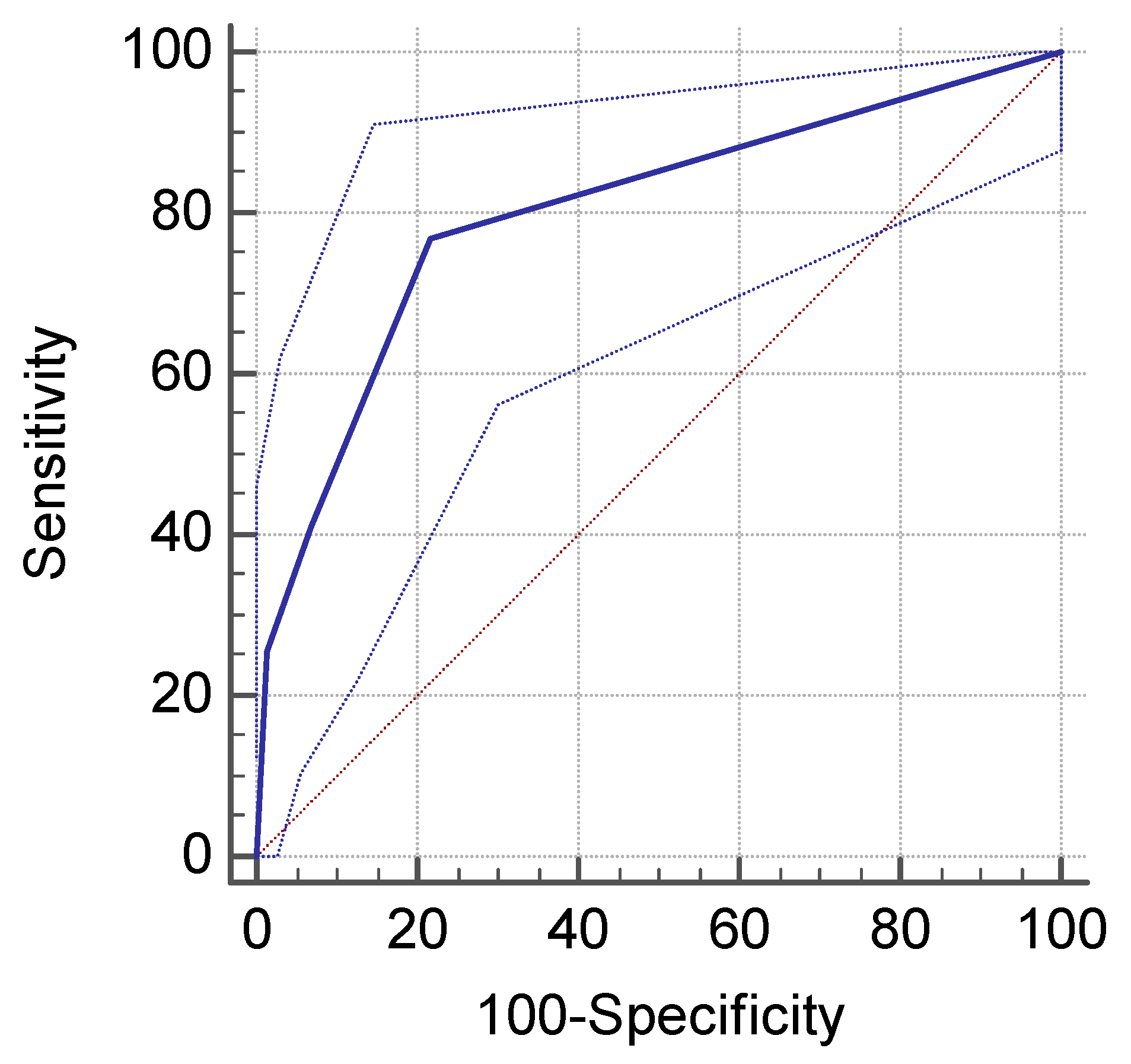
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Axon, A. Helicobacter pylori and public health. Helicobacter 2014, 19, 68–73. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Marshall, B.; Warren, J. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Schistosomes, Liver Flukes and Helicobacter Pylori; Iarc Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 1994; Volume 61. [Google Scholar]
- Mărginean, C.; Cotoi, O.; Pitea, A.; Mocanu, S.; Mărginean, C. Assessment of the relationship between helicobacter pylori infection, endoscopic appearance and histological changes of the gastric mucosa in children with gastritis (a single center experience). Rom. J. Morphol. Embriol. 2013, 54, 709–715. [Google Scholar]
- Spulber, G. Aspecte Clinice, Endoscopice, Bacteriologice şi Histopatologice în Gastrite la Copil. Ph.D. Thesis, “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania, 2011. [Google Scholar]
- Olar, L.; Mitrut, P.; Florou, C.; Mălăescu, G.D.; Predescu, O.I.; Rogozea, L.M.; Mogoantă, L.; Ionovici, N.; Pirici, I. Evaluation of helicobacter pylori infection in patients with eso-gastro-duodenal pathology. Rom. J. Morphol. Embriol. 2017, 58, 809–815. [Google Scholar]
- Piazuelo, M.B.; Correa, P. Gastric cancer: Overview. Colomb. Med. 2013, 44, 192–201. [Google Scholar]
- Correa, P. Helicobacter pylori and gastric carcinogenesis. Am. J. Surg. Pathol. 1995, 19, 37–43. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. European helicobacter and microbiota study group and consensus panel. Management of helicobacter pylori infection-the maastricht v/florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Rokkas, T.; Rokka, A.; Portincasa, P. A systematic review and meta-analysis of the role of helicobacter pylori eradication in preventing gastric cancer. Ann. Gastroenterol. 2017, 30, 414–423. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Sharaf, R.R.; Aziz, R.K. Helicobactier pylori: A poor man’s gut pathogen? Gut Pathog. 2010, 2, 2–12. [Google Scholar] [CrossRef]
- Rothenbacher, D.; Inceoglu, J.; Bode, G.; Brenner, H. Acquisition of helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. J. Pediatr. 2000, 136, 744–748. [Google Scholar] [CrossRef]
- Torres, J.; Perez-Perez, G.; Goodman, K.J.; Atherton, J.C.; Gold, B.D.; Harris, P.R.; laGarza, A.M.; Guarner, J.; Munoz, O. A comprehensive review of the natural history of helicobacter pylori infection in children. Arch. Med. Res. 2000, 31, 431–469. [Google Scholar] [CrossRef]
- Iwanczak, B.; Francavailla, R. Helicobacter pylori infection in pediatrics. Helicobacter 2014, 19, 46–51. [Google Scholar] [CrossRef]
- Jones, N.L.; Koletzko, S.; Goodman, K.; Bontems, P.; Cadranel, S.; Casswall, T.; Czinn, S.; Gold, B.D.; Guarner, J.; Elitsur, Y.; et al. Joint espghan/naspghan guidelines for the management of helicobacter pylori in children and adolescents (update 2016). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 991–1003. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of gastritis, houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Burgard, M.; Kotilea, K.; Mekhael, J.; Miendje-Deyi, V.Y.; De Prez, C.; Vanderpas, J.; Cadranel, S.; Bontems, P. Evolution of helicobacter pylori associated with gastroduodenal ulcers or erosions in children over the past 23 years: Decline or steady state? Helicobacter 2019, 24, e12629. [Google Scholar] [CrossRef]
- Carvalho, M.A.; Machado, N.C.; Ortolan, E.V.; Rodrigues, M.A. Upper gastrointestinal histopathological findings in non-ulcer dyspeptic children and adolescents with helicobacter pylori infection. J. Pediatric Gastroenterol. Nutr. 2012, 55, 523–529. [Google Scholar] [CrossRef]
- Sheiko, M.A.; Feinstein, J.A.; Capocelli, K.E.; Kramer, R.E. The concordance of endoscopic and histologic findings of 1000 pediatric egds. Gastrointest. Endosc. 2015, 81, 1385–1391. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Tsay, A.; McAllister, B.P.; Karamchandani, D.M. Hit or a miss: Concordance between histopathologic-endoscopic findings in gastric mucosal biopsies. Ann. Diagn. Pathol. 2019, 38, 106–114. [Google Scholar] [CrossRef]
- Carr, N.J.; Leadbetter, H.; Marriott, A. Correlation between the endoscopic and histologic diagnosis of gastritis. Ann. Diagn. Pathol. 2012, 16, 13–15. [Google Scholar] [CrossRef]
- Okamura, T.; Iwaya, Y.; Kitahara, K.; Suga, T.; Tanaka, E. Accuracy of endoscopic diagnosis for mild atrophic gastritis infected with helicobacter pylori. Clin. Endosc. 2018, 51, 362–367. [Google Scholar] [CrossRef]
- Yang, H.R.; Choi, H.S.; Paik, J.H.; Lee, H.S. Endoscopic and histologic analysis of gastric mucosa-associated lymphoid tissue in children with helicobacter pylori infection. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 298–304. [Google Scholar] [CrossRef]
- Hidaka, N.; Nakayama, Y.; Horiuchi, A.; Kato, S.; Sano, K. Endoscopic identification of helicobacter pylori gastritis in children. Dig. Endosc. 2010, 22, 90–94. [Google Scholar] [CrossRef]
- Serban, R.; Grigorescu-Sido, P.; Gheban, D.; Kiss, E. Helicobacter pylori gastritis in children: Endoscopical and histological aspects. Rom. J. Gastroenterol. 2002, 11, 297–301. [Google Scholar]
- Jaramillo-Rodríguez, Y.; Nares-Cisneros, J.; Martínez-Ordaz, V.A.; Velasco-Rodríguez, V.M.; Márquez, F.C.; Manríquez-Covarrubias, L.E. Chronic gastritis associated with helicobacter pylori in mexican children: Histopathological patterns. Pediatr. Dev. Pathol. 2011, 14, 93–98. [Google Scholar] [CrossRef]
- Whitney, A.E.; Guarner, J.; Hutwagner, L.; Gold, B.D. Helicobacter pylori gastritis in children and adults: Comparative histopathologic study. Ann. Diagn. Pathol. 2000, 4, 279–285. [Google Scholar] [CrossRef]
- Mitchell, H.M.; Bohane, T.D.; Tobias, V.; Bullpitt, P.; Daskalopoulos, G.; Carrick, J.; Mitchell, J.D.; Lee, A. Helicobacter pylori infection in children: Potential clues to pathogenesis. J. Pediatr. Gastroenterol. Nutr. 1993, 16, 120–125. [Google Scholar] [CrossRef]
- Gallo, N.; Zambon, C.F.; Navaglia, F.; Basso, D.; Guariso, G.; Grazia Piva, M.; Greco, E.; Mazza, S.; Fogar, P.; Rugge, M.; et al. Helicobacter pylori infection in children and adults: A single pathogen but a different pathology. Helicobacter 2003, 8, 21–28. [Google Scholar] [CrossRef]
- Yang, H.R. Updates on the diagnosis of helicobacter pylori infection in children: What are the differences between adults and children? Pediatr. Gastroenterol. Hepatol. Nutr 2016, 19, 96–103. [Google Scholar] [CrossRef]
- Serrano, C.; Wright, S.W.; Bimczok, D.; Shaffer, C.L.; Cover, T.L.; Venegas, A.; Salazar, M.G.; Smythies, L.E.; Harris, P.R.; Smith, P.D. Down-regulated th17 responses are associated with reduced gastritis in helicobacter pylori-infected children. Mucosal Immunol. 2013, 6, 950–959. [Google Scholar] [CrossRef]
- Friesen, C.A.; Colombo, J.M.; Schurman, J.V. The evolving role of mucosal histology in the evaluation of pediatric functional dyspepsia: A review. Gastrointest. Disord. 2019, 1, 13. [Google Scholar] [CrossRef]
- Mejia, C.R.; Vera, C.A.; Huiza-Espinoza, L. Association between follicular gastritis and helicobacter pylori in children seen at a public hospital in peru. Rev. Gastroenterol. Mex. 2016, 81, 80–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bedoya, A.; Garay, J.; Sanzon, F.; Bravo, L.E.; Bravo, J.C.; Correa, H.; Craver, R.; Fontham, E.; Du, J.X.; Correa, P. Histopathology of gastritis in helicobacter pylori-infected children from populations at high and low gastric cancer risk. Hum. Pathol. 2003, 34, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.R.; Smythies, L.E.; Smith, P.D.; Perez-Perez, G.I. Role of childhood infection in the sequelae of h. Pylori disease. Gut Microbes 2013, 4, 426–438. [Google Scholar] [CrossRef]
- Hernandez, C.; Serrano, C.; Einisman, H.; Villagran, A.; Pena, A.; Duarte, I.; Torres, J.; Riera, F.; Harris, P.R. Peptic ulcer disease in helicobacter pylori-infected children: Clinical findings and mucosal immune response. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 773–778. [Google Scholar] [CrossRef]
- Bontems, P.; Kalach, N.; Vanderpas, J.; Iwanczak, B.; Casswall, T.; Koletzko, S.; Oderda, G.; Martinez-Gomez, M.J.; Urruzuno, P.; Kindermann, A.; et al. Helicobacter pylori infection in european children with gastro-duodenal ulcers and erosions. Pediatr. Infect. Dis. J. 2013, 32, 1324–1329. [Google Scholar] [CrossRef]
- Dimitrov, G.; Gottrand, F. Does gastric atrophy exist in children? World J. Gastroenterol. 2006, 12, 6274–6279. [Google Scholar] [CrossRef]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The staging of gastritis with the olga system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef]
- Sierra, M.S.; Hastings, E.V.; Goodman, K.J. What do we know about benefits of h. Pylori treatment in childhood? Gut Microbes 2013, 4, 549–567. [Google Scholar] [CrossRef]
- Spee, L.A.A.; Madderom, M.B.; Pijpers, M.; van Leeuwen, Y.; Berger, M.Y. Association between helicobacter pylori and gastrointestinal symptoms in children. Pediatrics 2010, 125, e651–e669. [Google Scholar] [CrossRef]
- Roberts, S.E.; Morrison-Rees, S.; Samuel, D.G.; Thorne, K.; Akbari, A.; Williams, J.G. Review article: The prevalence of helicobacter pylori and the incidence of gastric cancer across europe. Aliment. Pharmacol. Ther. 2016, 43, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Popescu, A.; Van Blankenstein, M.; Şirli, R.; Focşa, M.; Dǎnilǎ, M. The prevalence of helicobacter pylori infection in western romania. Rom. J. Gastroenterol. 2003, 12, 15–18. [Google Scholar] [PubMed]
| Variable | Median (Range), n (%) |
|---|---|
| Gender | |
| Male | 84 (33.8%) |
| Female | 164 (66.2%) |
| Age (years) | 14 (0-18) |
| 0–6 | 4 (1.6%) |
| 7–12 | 94 (37.9%) |
| 13–18 | 150 (60.5%) |
| Age Group | Helicobacter Pylori Positive (H. pylori+) | Helicobacter Pylori Negative (H. pylori-) | p-value |
|---|---|---|---|
| 0–6 | |||
| Male | 0 | 3 (75%) | 0.14 |
| Female | 0 | 1 (25%) | 0.99 |
| 7–12 | |||
| Female | 14 (14.8%) | 40 (42.5%) | 0.0001 |
| Male | 12 (12.7%) | 28 (30%) | 0.006 |
| 13–18 | |||
| Female | 44 (29.5%) | 65 (43.3%) | 0.01 |
| Male | 12 (8%) | 29 (19.2%) | 0.007 |
| Symptoms | H. pylori- n = 166 | H. pylori+ n = 82 | p-value |
|---|---|---|---|
| Dyspeptic syndrome | 102 (62%) | 52 (63.4%) | 0.90 |
| Abdominal pain | 28 (17.4%) | 22 (26.8%) | 0.1 |
| Emesis | 9 (5.4%) | 4 (4.8%) | 0.91 |
| Anemia | 3 (1.8%) | 5 (6%) | 0.16 |
| Suspected celiac disease | 5 (3%) | 3 (3.6%) | 0.89 |
| Catabolic syndrome | 9 (5.4%) | 2 (2.4%) | 0.45 |
| Upper digestive hemorrhage | 0 | 2 (2.4%) | 0.21 |
| Gastro-esophageal reflux | 3 (1.8%) | 0 | 0.54 |
| Suspected Crohn disease | 1 (0.6%) | 1 (1.2%) | 0.79 |
| Anorexia | 1 (0.6%) | 1 (1.2%) | 0.79 |
| Endoscopic Pattern | H. pylori- n = 166 | H. pylori+ n = 82 | p-value |
|---|---|---|---|
| Minimal changes | 148 (89.1%) | 41 (50%) | <0.0001 |
| Nodularity | 9 (5.4%) | 30 (36.5%) | <0.0001 |
| Paving stone | 9 (5.4%) | 11 (13.4%) | 0.04 |
| Hyperemia | 163 (98.1%) | 81 (98.7%) | 0.85 |
| Edema | 76 (45.7%) | 50 (60.9%) | 0.03 |
| Erosions | 11 (6.6%) | 4 (4.8%) | 0.78 |
| Histopathological Findings | H. pylori- n = 166 | H. pylori+ n = 82 | p-value |
|---|---|---|---|
| Activity | 6 (3.7%) | 82 (100%) | <0.001 |
| Chronic inflammation | 50 (31.4%) | 50 (60.9%) | <0.0001 |
| Atrophy | 27 (75%) | 9 (25%) | <0.0001 |
| Intestinal metaplasia | 1 (0.6%) | 2 (2.4%) | 0.54 |
| Endoscopic Findings | Histopathologic Findings | No. Biopsies | H. Pylori+ |
|---|---|---|---|
| Minimal, non-characteristic changes | Normal | 46 (24.3%) | 0 |
| Abnormal | 143 (75.7%) | 40 | |
| Chronic inactive gastritis | 87 | 16 | |
| Chronic active gastritis | 29 | 24 | |
| Other | 27 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domșa, A.-M.T.; Lupușoru, R.; Gheban, D.; Șerban, R.; Borzan, C.M. Helicobacter pylori Gastritis in Children—The Link between Endoscopy and Histology. J. Clin. Med. 2020, 9, 784. https://doi.org/10.3390/jcm9030784
Domșa A-MT, Lupușoru R, Gheban D, Șerban R, Borzan CM. Helicobacter pylori Gastritis in Children—The Link between Endoscopy and Histology. Journal of Clinical Medicine. 2020; 9(3):784. https://doi.org/10.3390/jcm9030784
Chicago/Turabian StyleDomșa, Ana-Maria Teodora, Raluca Lupușoru, Dan Gheban, Radu Șerban, and Cristina Maria Borzan. 2020. "Helicobacter pylori Gastritis in Children—The Link between Endoscopy and Histology" Journal of Clinical Medicine 9, no. 3: 784. https://doi.org/10.3390/jcm9030784
APA StyleDomșa, A.-M. T., Lupușoru, R., Gheban, D., Șerban, R., & Borzan, C. M. (2020). Helicobacter pylori Gastritis in Children—The Link between Endoscopy and Histology. Journal of Clinical Medicine, 9(3), 784. https://doi.org/10.3390/jcm9030784





