Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review
Abstract
1. Introduction
2. Experimental Section
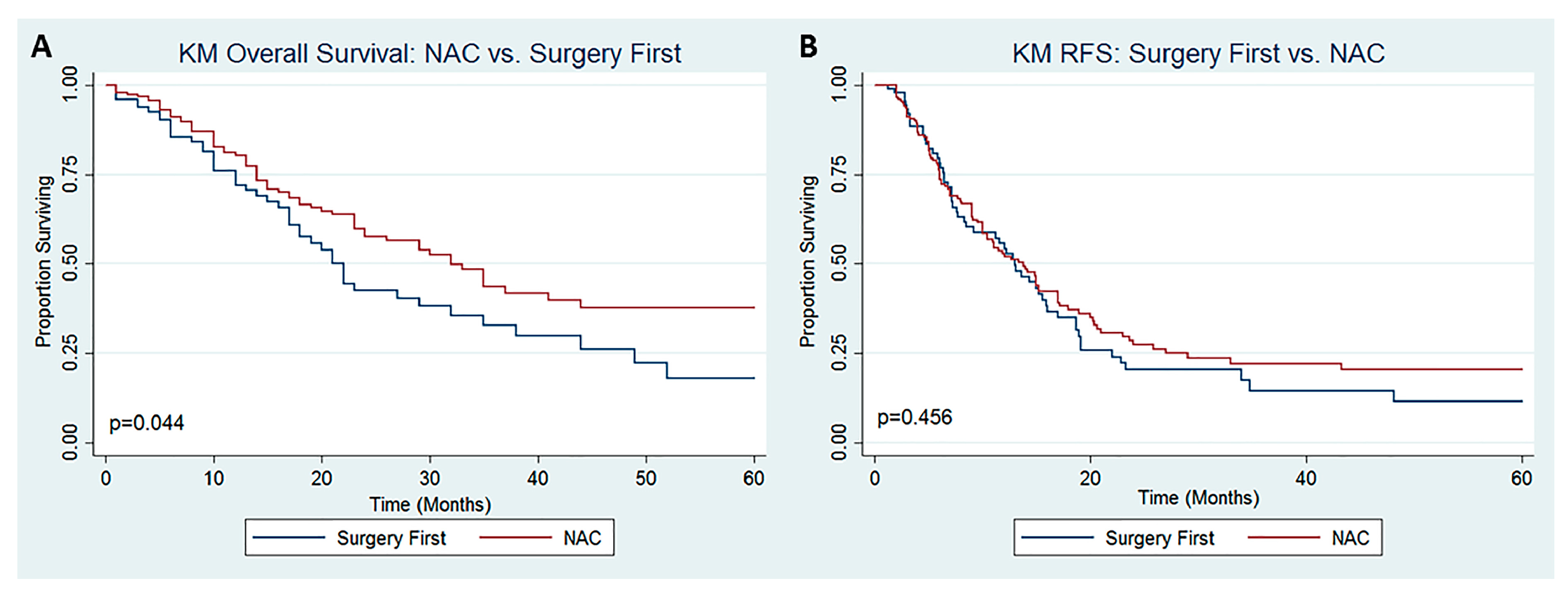
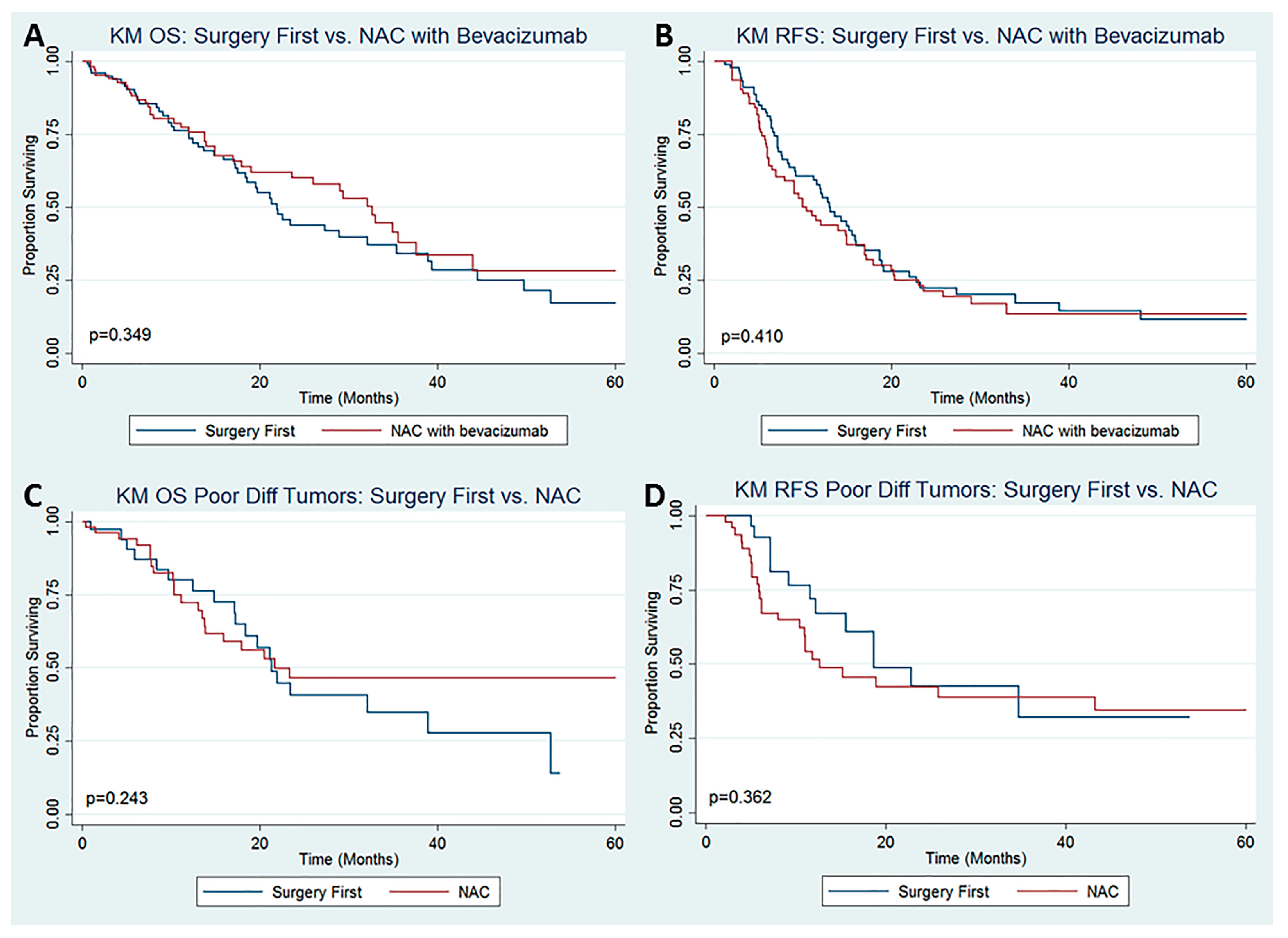
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegal, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Colon Cancer. Version 2.2019. [Internet]. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 1 November 2019).
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.; Seymour, M.T.; Saltz, L.; Punt, C.J.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of Colorectal Peritoneal Carcinomatosis with Systemic Chemotherapy: A Pooled Analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. J. Clin. Oncol. 2011, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.B.; Simkens, L.H.J.; Lemmens, V.E.P.P.; Koopman, M.; Teerenstra, S.; Bleichrodt, R.; De Hingh, I.H.; Punt, C. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur. J. Surg. Oncol. 2012, 38, 617–623. [Google Scholar] [CrossRef]
- Lieu, C.H.; Renfro, L.A.; De Gramont, A.; Meyers, J.P.; Maughan, T.S.; Seymour, M.T.; Saltz, L.B.; Goldberg, R.M.; Sargent, D.J.; Eckhardt, S.G.; et al. Association of Age with Survival in Patients with Metastatic Colorectal Cancer: Analysis from the ARCAD Clinical Trials Program. J. Clin. Oncol. 2014, 32, 2975–2982. [Google Scholar] [CrossRef] [PubMed]
- Glehan, O.; Kwiatkowski, F.; Sugarbaker, P.; Elias, D.; Levine, E.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for the Management of Peritoneal Carcinomatosis from Colorectal Cancer: A Multi-Institutional Study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.-M.; Ferron, G.; Guilloit, J.-M.; Meeus, P.; Goéré, D.; et al. Complete Cytoreductive Surgery Plus Intraperitoneal Chemohyperthermia with Oxaliplatin for Peritoneal Carcinomatosis of Colorectal Origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef]
- Elias, D.; Delperro, J.; Sideris, L.; Benhamou, E.; Pocard, M.; Baton, O.; Giovannini, M.; Lasser, P. Treatment of Peritoneal Carcinomatosis from Colorectal Cancer: Impact of Complete Cytoreductive Surgery and Difficulties in Conducting Randomized Trials. J. Surg. Oncol. 2004, 11, 518–521. [Google Scholar] [CrossRef]
- Shen, P.; Hawksworth, J.; Lovato, J.; Loggie, B.W.; Geisinger, K.R.; Fleming, R.A.; Levine, E.A. Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy with Mitomycin C for Peritoneal Carcinomatosis from Nonappendiceal Colorectal Carcinoma. Ann. Surg. Oncol. 2004, 11, 178–186. [Google Scholar] [CrossRef]
- Yan, T.D.; Black, D.; Savady, R.; Sugarbaker, P.H. Systematic Review on the Efficacy of Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Colorectal Carcinoma. J. Clin. Oncol. 2019, 24, 4011–4019. [Google Scholar] [CrossRef]
- Franko, J.; Ibrahim, Z.; Gusani, N.J.; Holtzman, M.P.; Bartlett, D.L.; Zeh, H.J. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemoperfusion Versus Systemic Chemotherapy Alone for Colorectal Peritoneal Carcinomatosis. Cancer 2010, 116, 3756–3762. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; Van Slooten, G.W.; Van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy and Palliative Surgery in Patients with Peritoneal Carcinomatosis of Colorectal Cancer. J. Clin. Oncol. 2003, 15, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, R.; Mehta, A.M.; Chandrakumaran, K.; Cecil, T.; Moran, B.J.; Carr, N.; Verwaal, V.J.; Mohamed, F.; Mirnezami, A.H. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br. J. Cancer 2014, 111, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.; Youssef, H.; Gensurg, F. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann. Surg. Oncol. 2017, 24, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Passot, G.; Vaudoyer, D.; Cotte, E.; You, B.; Isaac, S.; Gilly, F. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to curative approach for colorectal carcinomatosis. Ann. Surg. 2012, 256, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Gilly, F.N.; Hospitalo, C.; Lyon, U.; Be, P. Phase II studies: International registry of colorectal carcinomatosis. EJSO 2006, 32, 648–654. [Google Scholar] [CrossRef]
- Ceelen, W.; Van Nieuwenhove, Y.; Putte, D.V.; Pattyn, P. Neoadjuvant Chemotherapy with Bevacizumab May Improve Outcome after Cytoreduction and Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) for Colorectal Carcinomatosis. Ann. Surg. Oncol. 2014, 21, 3023–3028. [Google Scholar] [CrossRef]
- Eveno, C.; Passot, G.; Goere, D.; Soyer, P.; Gayat, E.; Glehen, O.; Elias, M.; Pocard, M. Bevacizumab Doubles the Early Postoperative Complication Rate after Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Carcinomatosis of Colorectal Origin. Ann. Surg. Oncol. 2014, 21, 1792–1800. [Google Scholar] [CrossRef]
- Adam, R.; Wicherts, D.A.; De Haas, R.J.; Ciacio, O.; Lévi, F.; Paule, B.; Ducreux, M.; Azoulay, D.; Bismuth, H.; Castaing, D. Patients with Initially Unresectable Colorectal Liver Metastases: Is There a Possibility of Cure? J. Clin. Oncol. 2019, 27, 1829–1835. [Google Scholar] [CrossRef]
- Parikh, A.; Gentner, B.; Tsung-Teh, W.; Curley, S.A.; Ellis, L.M.; Vauthey, J. Perioperative Complications in Patients Undergoing Major Liver Resection with or without Neoadjuvant Chemotherapy. J. Gastrointest. Surg. 2003, 7, 1082–1088. [Google Scholar] [CrossRef]
- Harmon, R.; Sugarbaker, P. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int. Semin. Surg. Oncol. 2005, 2. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.; Sanabria, J.; Strasberg, S. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery (U.S.) 1992, 111, 518–526. [Google Scholar]
- Dedrick, R.; Flessner, M. Pharmacokinetic Problems in Peritoneal Drug Administration: Tissue Penetration and Surface Exposure. J. Natl. Cancer Inst. 1997, 89, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. A UNICANCER phase III trial of hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J. Clin. Oncol. 2018, 36, LBA3503. [Google Scholar] [CrossRef]
- Devilee, R.A.; Simkens, G.A.; Van Oudheusden, T.R.; Rutten, H.J.; Creemers, G.; Tije, A.J.T.; De Hingh, I.H. Increased Survival of Patients with Synchronous Colorectal Peritoneal Metastases Receiving Preoperative Chemotherapy before Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2016, 23, 2841–2848. [Google Scholar] [CrossRef]
- Glehen, O.; Cotte, E.; Schreiber, V.; Vignal, J.; Gilly, F.N. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br. J. Surg. 2004, 91, 747–754. [Google Scholar] [CrossRef]
- Rovers, K.; Bakkers, C.; Simkens, G.; A Burger, J.W.; Nienhuijs, S.W.; Creemers, G.-J.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; Ayez, N.; et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: Protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer 2019, 19, 390. [Google Scholar]
- Quenet, F.; Goéré, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, M. Results of two Bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef]
- Hompes, D.; Aalbers, A.; Boot, H.; Van Velthuysen, M.-L.; Vogel, W.; Prevoo, W.; Van Tinteren, H.; Verwaal, V. A prospective pilot study to assess neoadjuvant chemotherapy for unresectable peritoneal carcinomatosis from colorectal cancer. Color. Dis. 2014, 16, O264–O272. [Google Scholar] [CrossRef]
- Vergote, I.; Trope, C.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; Van Der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Hentzen, J.E.K.R.; Rovers, K.P.; Kuipers, H.; Van Der Plas, W.Y.; Been, L.B.; Hoogwater, F.J.H.; Van Ginkel, R.J.; Hemmer, P.H.J.; Van Dam, G.M.; De Hingh, I.H.J.T.; et al. Impact of Synchronous Versus Metachronous Onset of Colorectal Peritoneal Metastases on Survival Outcomes After Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Multicenter, Retrospective, Observational Study. Ann. Surg. Oncol. 2019, 26, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
| All Patients | NAC | SF | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | (%) | n | (%) | ||
| N | 298 | 100% | 196 | 66% | 102 | 34% | |
| Follow-up (months), mean ± SD (range) | 18.6 ± 18.7 (0.2–124) | 19.2 ± 20.1 (0.3–124) | 17.3 ± 16.1 (0.2–70.8) | 0.372 | |||
| Age (years), mean ± SD (range) | 54.1 ± 12.9 (20–95) | 53.2 ± 12.5 (22–78) | 55.8 ± 13.7 (20–95) | 0.121 | |||
| BMI | 27.7 ± 6.0 (17.3–60.7) | 27.3 ± 6.2 (17.3–60.7) | 28.6 ± 5.6 (18.9–43.8) | 0.084 | |||
| Female | 161 | 54% | 104 | 53% | 57 | 56% | 0.643 |
| ASA Class | 0.065 | ||||||
| I | 0 | 0% | 0 | 0% | 0 | 0% | |
| II | 31 | 11% | 17 | 10% | 14 | 14% | |
| III | 223 | 81% | 150 | 85% | 73 | 74% | |
| IV | 22 | 8% | 10 | 6% | 12 | 2% | |
| ECOG Performance Status | 0.432 | ||||||
| 0 | 140 | 59% | 83 | 57% | 57 | 63% | |
| 1 | 84 | 36% | 54 | 37% | 30 | 33% | |
| 2 | 11 | 5% | 8 | 3% | 3 | 3% | |
| 3 | 1 | 0% | 8 | 3% | 3 | 3% | |
| Comorbidities | |||||||
| Hypertension | 100 | 36% | 59 | 59% | 41 | 41% | 0.178 |
| Diabetes | 23 | 8% | 17 | 8% | 9 | 9% | 0.729 |
| CHF | 8 | 3% | 7 | 4% | 1 | 1% | 0.163 |
| Prior cardiac event | 16 | 6% | 9 | 5% | 7 | 7% | 0.496 |
| Prior CVA | 5 | 2% | 4 | 2% | 1 | 1% | 0.499 |
| COPD | 3 | 1% | 0 | 0% | 3 | 3% | 0.020 |
| CKD | 5 | 2% | 4 | 2% | 1 | 1% | 0.683 |
| PVD | 13 | 5% | 13 | 7% | 0 | 0% | 0.007 |
| Ascities | 20 | 7% | 11 | 6% | 9 | 9% | 0.374 |
| Systemic Anticoagulation | 21 | 7% | 17 | 9% | 4 | 4% | 0.128 |
| Chronic Steroid Use | 5 | 2% | 5 | 3% | 0 | 0% | 0.092 |
| Rheumatic disease | 6 | 2% | 4 | 2% | 2 | 2% | 0.963 |
| Tobacco use | 0.550 | ||||||
| Current | 23 | 8% | 17 | 10% | 6 | 6% | |
| Former | 68 | 24% | 44 | 25% | 24 | 24% | |
| Symptomatic | |||||||
| GI Bleed | 15 | 5% | 10 | 5% | 5 | 5% | 0.933 |
| Obstruction | 15 | 5% | 11 | 6% | 4 | 4% | 0.515 |
| Diarrhea | 6 | 2% | 3 | 2% | 3 | 3% | 0.464 |
| Constipation | 14 | 5% | 11 | 6% | 3 | 3% | 0.281 |
| Pain | 73 | 26% | 48 | 27% | 25 | 25% | 0.706 |
| Nausea/Vomiting | 11 | 4% | 8 | 3% | 3 | 1% | 0.541 |
| Anorexia | 8 | 3% | 7 | 4% | 1 | 1% | 0.161 |
| Fatigue | 29 | 10% | 18 | 10% | 11 | 11% | 0.792 |
| Anemia | 37 | 14% | 21 | 12% | 16 | 17% | 0.246 |
| GERD/Dyspepsia | 40 | 15% | 35 | 20% | 5 | 5% | 0.001 |
| Synchronous peritoneal disease | 107 | 36% | 73 | 37% | 34 | 33% | 0.196 |
| Previous cytoreduction | 49 | 16% | 29 | 15% | 30 | 20% | 0.288 |
| Previous HIPEC | 12 | 4% | 3 | 1% | 9 | 3% | 0.002 |
| Tumor Differentiation—Poor and/or Signet Ring Cell | 77 | 26% | 60 | 31% | 17 | 18% | 0.087 |
| n | % | |
|---|---|---|
| FOLFIRI + Bevacizumab | 45 | 23.0% |
| FOLFOX + Bevacizumab | 42 | 21.4% |
| FOLFOX | 37 | 18.9% |
| FOLFIRI | 20 | 10.2% |
| Xeloda + Bevacizumab | 9 | 4.6% |
| FOLFOXIRI + Bevacizumab | 5 | 2.6% |
| 5FU ± Leucovorin | 3 | 1.5% |
| Xeloda | 3 | 1.5% |
| XELOX | 3 | 1.5% |
| XELOX + Bevacizumab | 2 | 1.0% |
| Other | 24 | 12.3% |
| Other + Bevacizumab | 3 | 1.5% |
| All Patients | NAC | SF | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| PCI, mean ± SD (range) | 12.9 ± 8.2 (1–39) | 12.1 ± 7.9 (1–38) | 14.3 ± 8.5 (2–39) | 0.034 | |||
| CCR | 0.218 | ||||||
| 0 | 212 | 71.10% | 145 | 74.00% | 67 | 65.70% | |
| 1 | 49 | 16.40% | 31 | 15.80% | 18 | 17.60% | |
| ≥ 2 | 37 | 12.40% | 20 | 10.20% | 17 | 16.70% | |
| HIPEC | 281 | 95.90% | 190 | 96.90% | 91 | 93.80% | 0.204 |
| Chemotherapy Mitomycin C | 273 | 97.00% | 184 | 97.40% | 89 | 97.80% | 0.822 |
| Oxaliplatin | 7 | 2.50% | 5 | 2.60% | 2 | 2.20% | |
| HIPEC Infusion Time (minutes), mean ± SD (range) | 88.0 ± 9.7 (30–120) | 88.4 ± 9.1 (30–120) | 87.2 ± 10.7 (30–100) | 0.364 | |||
| Operative Time (hours), mean ± SD (range) | 7.8 ± 2.9 (0–20.8) | 7.7 ± 2.9 (0–20.8) | 8.7 ± 2.7 (2.5–14.5) | 0.013 | |||
| EBL (mL), mean ± SD (range) | 437.4 ± 641.5 (0–6000) | 447.0 ± 740.9 (0–6000) | 419.1 ± 388.9 (0–2000) | 0.672 | |||
| Any postoperative complication | 180 | 60.40% | 119 | 60.70% | 61 | 59.80% | 0.879 |
| Highest Clavien–Dindo grade | 0.650 | ||||||
| I | 20 | 10.90% | 11 | 9.00% | 9 | 14.50% | |
| II | 98 | 53.30% | 64 | 52.50% | 34 | 54.80% | |
| III | 46 | 25.00% | 34 | 27.90% | 12 | 19.40% | |
| IV | 15 | 8.20% | 10 | 8.20% | 5 | 8.10% | |
| V | 5 | 2.70% | 3 | 2.50% | 2 | 3.20% | |
| Hospital LOS (days), mean ± SD (range) | 12.1 ± 8.0 (0–68) | 12.2 ± 8.2 (3–68) | 12.1 ± 7.7 (0–49) | 0.974 | |||
| Adjuvant chemotherapy | 78 | 38.60% | 62 | 40.30% | 16 | 33.30% | 0.389 |
| Neoadjuvant Radiation | 8 | 3.30% | 8 | 4.30% | 0 | 0% | 0.110 |
| Adjuvant Radiation | 7 | 3.80% | 5 | 3.50% | 2 | 4.90% | 0.696 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender | ||||
| Female | Ref | Ref | ||
| Male | 1.19 (0.83, 1.70) | 0.344 | ||
| Age (years) | 1.00 (0.98, 1.01) | 0.748 | ||
| BMI | 0.97 (0.94, 1.01) | 0.104 | ||
| ASA Class | ||||
| II | Ref | Ref | ||
| III | 1.52 (0.79, 2.94) | 0.209 | ||
| IV | 1.36 (0.58, 3.22) | 0.478 | ||
| Previous HIPEC | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.95 (0.91, 4.19) | 0.088 | 1.46 (0.62, 3.43) | 0.384 |
| Symptomatic | ||||
| No | Ref | Ref | ||
| Yes | 1.30 (0.91, 1.87) | 0.143 | ||
| Synchronous Peritoneal Disease | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.65 (0.45, 0.961) | 0.031 | 0.63 (0.42, 0.94) | 0.024 |
| PCI Score | 1.07 (1.05, 1.10) | < 0.001 | 1.05 (1.03, 1.08) | < 0.001 |
| CCR | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 3.56 (2.29, 5.52) | < 0.001 | 2.18 (1.31, 3.63) | 0.003 |
| ≥ 2 | 4.59 (2.78, 7.57) | < 0.001 | 2.37 (1.21, 4.67) | 0.012 |
| HIPEC | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.33 (0.16, 0.68) | 0.003 | 0.57 (0.24, 1.36) | 0.204 |
| Tumor Differentiation | ||||
| Well Differentiated | Ref | Ref | ||
| Moderately Differentiated | 1.35 (0.63, 2.87) | 0.442 | ||
| Poorly Differentiated | 1.40 (0.64, 3.06) | 0.402 | ||
| Not Reported | 1.35 (0.63, 2.89) | 0.435 | ||
| Any postoperative complication | ||||
| No | Ref | Ref | ||
| Yes | 1.24 (0.86, 1.79) | 0.251 | ||
| Neoadjuvant Chemotherapy | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.69 (0.48, 0.99) | 0.045 | 0.80 (0.54, 1.17) | 0.247 |
| Adjuvant Chemotherapy | ||||
| No | Ref | Ref | ||
| Yes | 1.00 (0.63, 1.58) | 0.998 | ||
| Neoadjuvant Radiation | ||||
| No | Ref | Ref | ||
| Yes | 1.65 (0.60, 4.53) | 0.331 | ||
| Adjuvant Radiation | ||||
| No | Ref | |||
| Yes | 0.77 (0.27, 2.16) | 0.613 | ||
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Gender | ||||
| Female | Ref | Ref | ||
| Male | 0.99 (0.73, 1.36) | 0.963 | ||
| Age (years) | 1.00 (0.99, 1.01) | 0.943 | ||
| BMI | 0.99 (0.96, 1.02) | 0.990 | ||
| ASA Class | ||||
| II | Ref | Ref | Ref | Ref |
| III | 1.56 (0.90, 2.73) | 0.116 | 1.60 (0.90, 2.83) | 0.107 |
| IV | 2.03 (0.99, 4.17) | 0.054 | 1.90 (0.91, 3.98) | 0.089 |
| Previous HIPEC | ||||
| No | Ref | Ref | ||
| Yes | 1.52 (0.75, 3.11) | 0.249 | ||
| Symptomatic | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.72 (0.52, 1.00) | 0.041 | 0.80 (0.56, 1.14) | 0.213 |
| Synchronus Peritoneal Disease | ||||
| No | Ref | Ref | ||
| Yes | 0.90 (0.65, 1.24) | 0.507 | ||
| PCI Score | 1.04 (1.03, 1.07) | < 0.001 | 1.05 (1.02, 1.07) | < 0.001 |
| CCR | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 1.52 (1.02, 2.29) | 0.041 | 0.74 (0.45, 1.22) | 0.242 |
| ≥ 2 | 0.82 (0.41, 1.61) | 0.557 | 0.57 (0.26, 1.27) | 0.172 |
| HIPEC | ||||
| No | Ref | Ref | ||
| Yes | 0.59 (0.22, 1.61) | 0.306 | ||
| Tumor Differentiation | ||||
| Well Differentiated | Ref | Ref | ||
| Moderately Differentiated | 1.13 (0.62, 2.06) | 0.701 | ||
| Poorly Differentiated | 1.26 (0.68, 2.34) | 0.457 | ||
| Not Reported | 0.64 (0.34, 1.21) | 0.173 | ||
| Any postoperative complication | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.51 (1.09, 2.10) | 0.014 | 1.37 (0.74, 1.47) | 0.091 |
| Neoadjuvant Chemotherapy | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.89 (0.64, 1.22) | 0.458 | 1.04 (0.74, 1.47) | 0.834 |
| Adjuvant Chemotherapy | ||||
| No | Ref | Ref | ||
| Yes | 1.01 (0.70, 1.46) | 0.955 | ||
| Neoadjuvant Radiation | ||||
| No | Ref | Ref | ||
| Yes | 2.13 (0.86, 5.27) | 0.101 | ||
| Adjuvant Radiation | ||||
| No | Ref | Ref | ||
| Yes | 0.67 (0.27, 1.66) | 0.388 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beal, E.W.; Suarez-Kelly, L.P.; Kimbrough, C.W.; Johnston, F.M.; Greer, J.; Abbott, D.E.; Pokrzywa, C.; Raoof, M.; Lee, B.; Grotz, T.E.; et al. Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review. J. Clin. Med. 2020, 9, 748. https://doi.org/10.3390/jcm9030748
Beal EW, Suarez-Kelly LP, Kimbrough CW, Johnston FM, Greer J, Abbott DE, Pokrzywa C, Raoof M, Lee B, Grotz TE, et al. Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review. Journal of Clinical Medicine. 2020; 9(3):748. https://doi.org/10.3390/jcm9030748
Chicago/Turabian StyleBeal, Eliza W., Lorena P. Suarez-Kelly, Charles W. Kimbrough, Fabian M. Johnston, Jonathan Greer, Daniel E. Abbott, Courtney Pokrzywa, Mustafa Raoof, Byrne Lee, Travis E. Grotz, and et al. 2020. "Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review" Journal of Clinical Medicine 9, no. 3: 748. https://doi.org/10.3390/jcm9030748
APA StyleBeal, E. W., Suarez-Kelly, L. P., Kimbrough, C. W., Johnston, F. M., Greer, J., Abbott, D. E., Pokrzywa, C., Raoof, M., Lee, B., Grotz, T. E., Leiting, J. L., Fournier, K., Lee, A. J., Dineen, S. P., Powers, B., Veerapong, J., Baumgartner, J. M., Clarke, C., Mogal, H., ... Cloyd, J. M. (2020). Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review. Journal of Clinical Medicine, 9(3), 748. https://doi.org/10.3390/jcm9030748







