Hip Morphology in Mucolipidosis Type II
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging
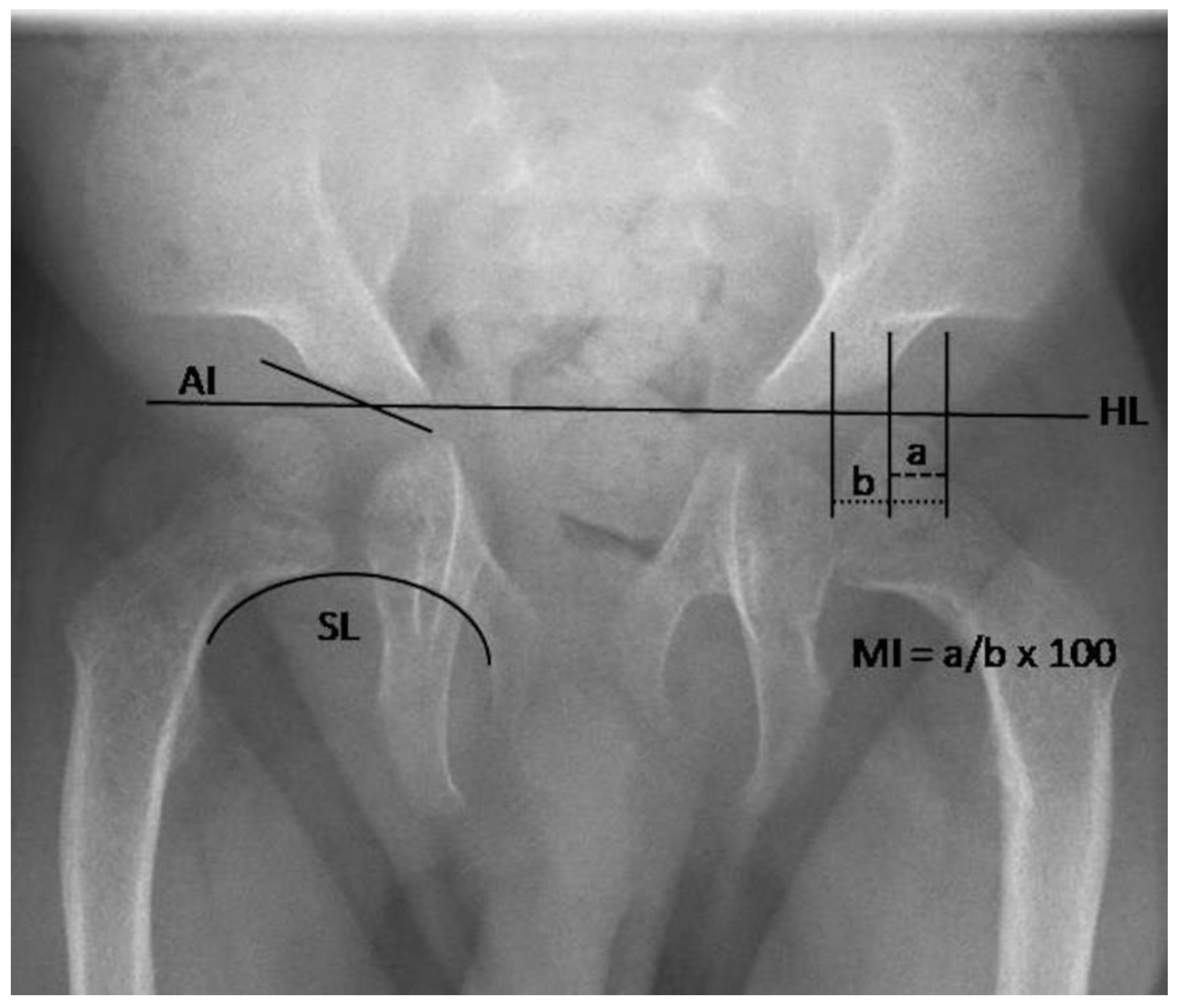
2.2.1. Radiography
2.2.2. Ultrasound
2.2.3. MRI
2.3. Statistics
2.4. Ethics
3. Results
3.1. Patients
3.2. Imaging
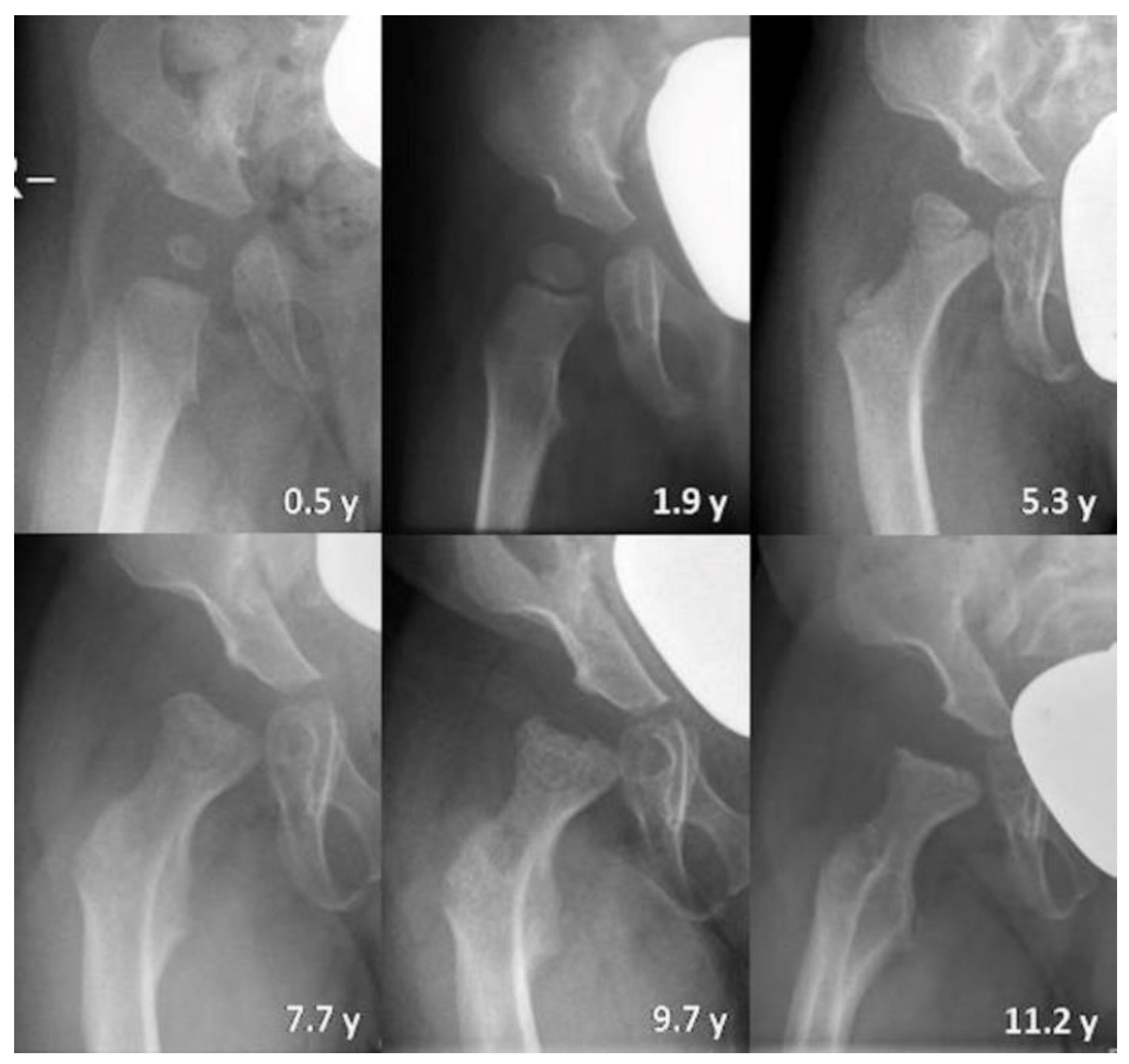
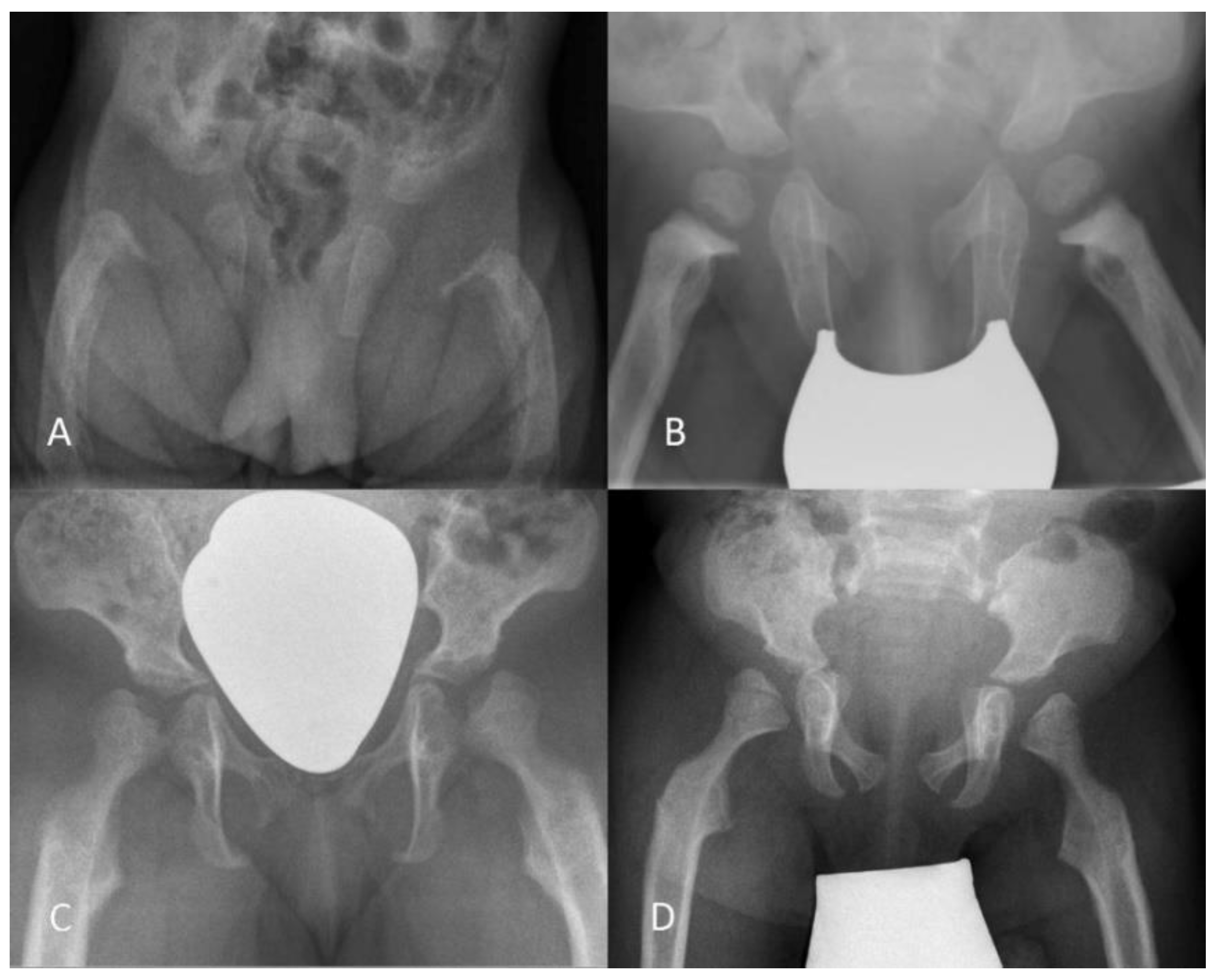
3.2.1. Radiography
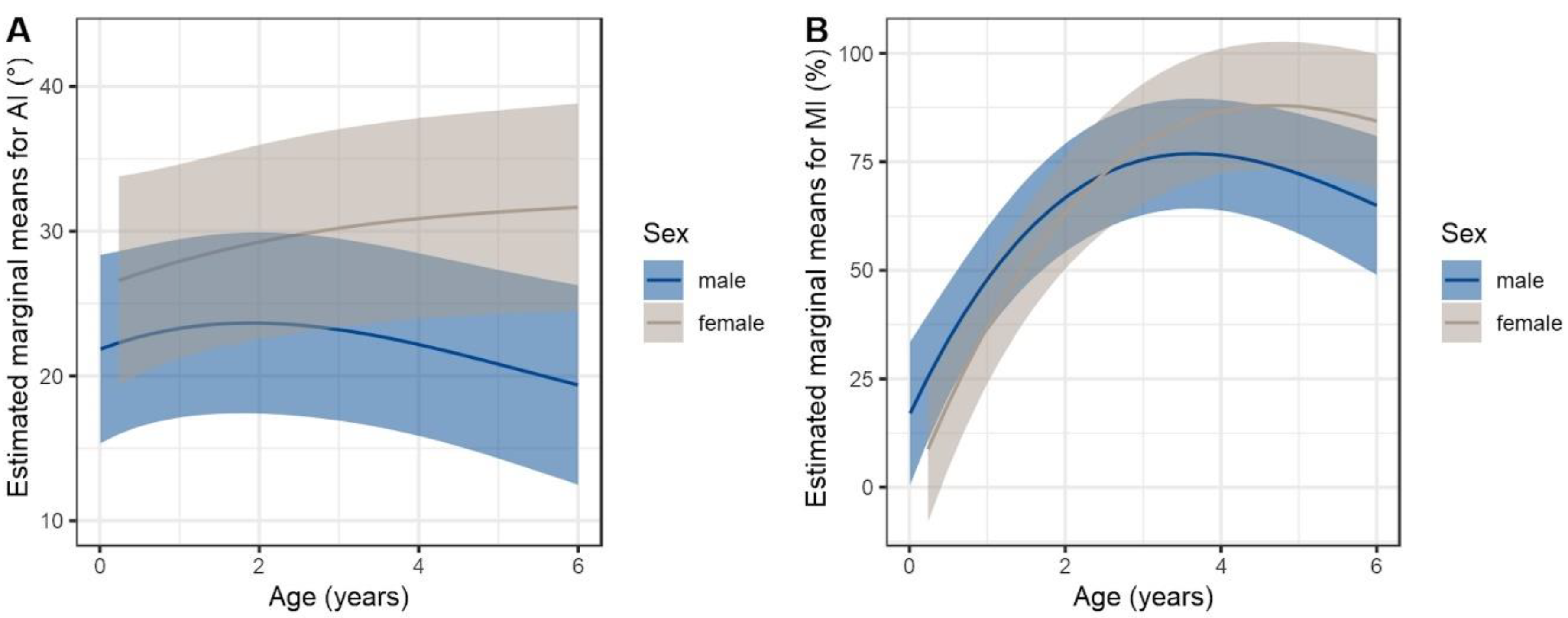
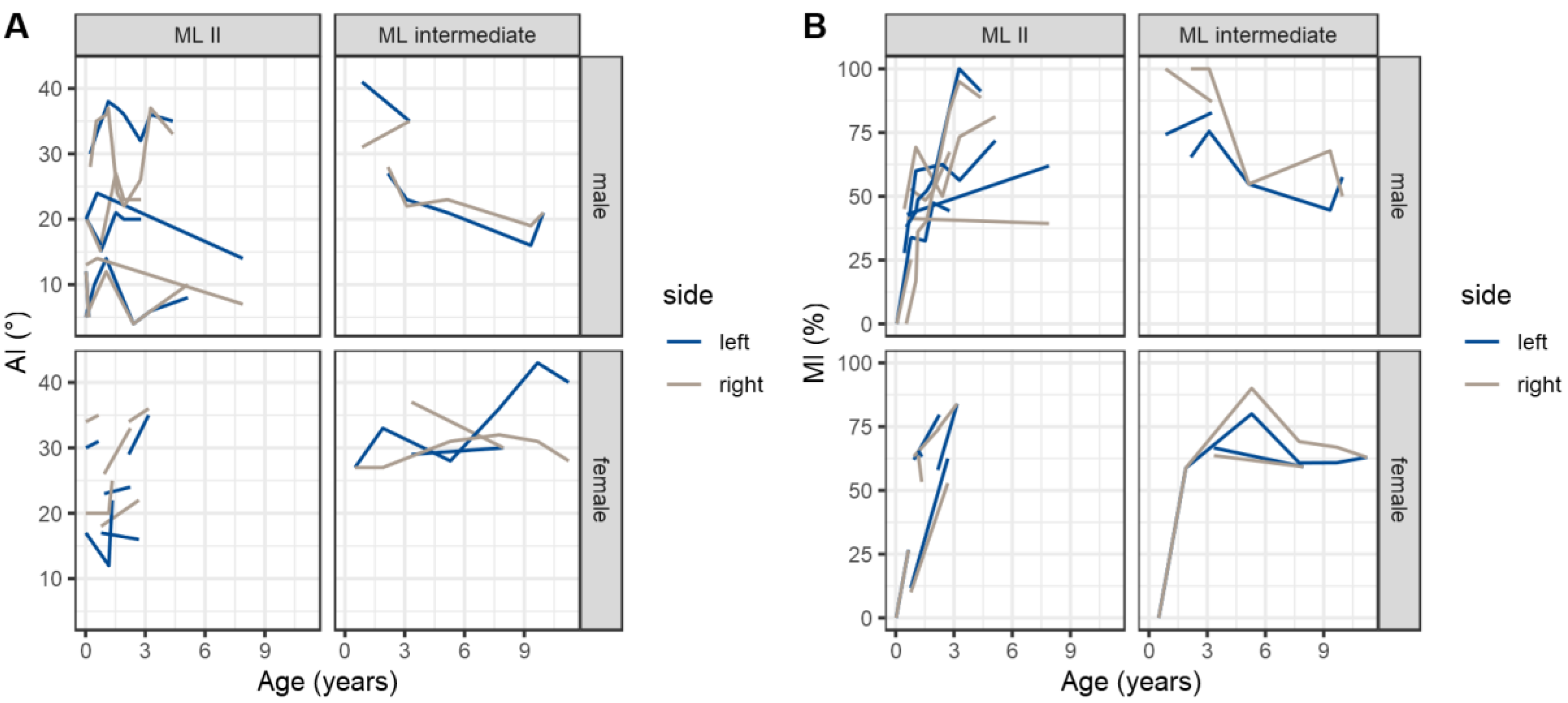
3.2.2. Quantitative Assessment of Radiographs
3.2.3. Qualitative Assessment of Radiographs
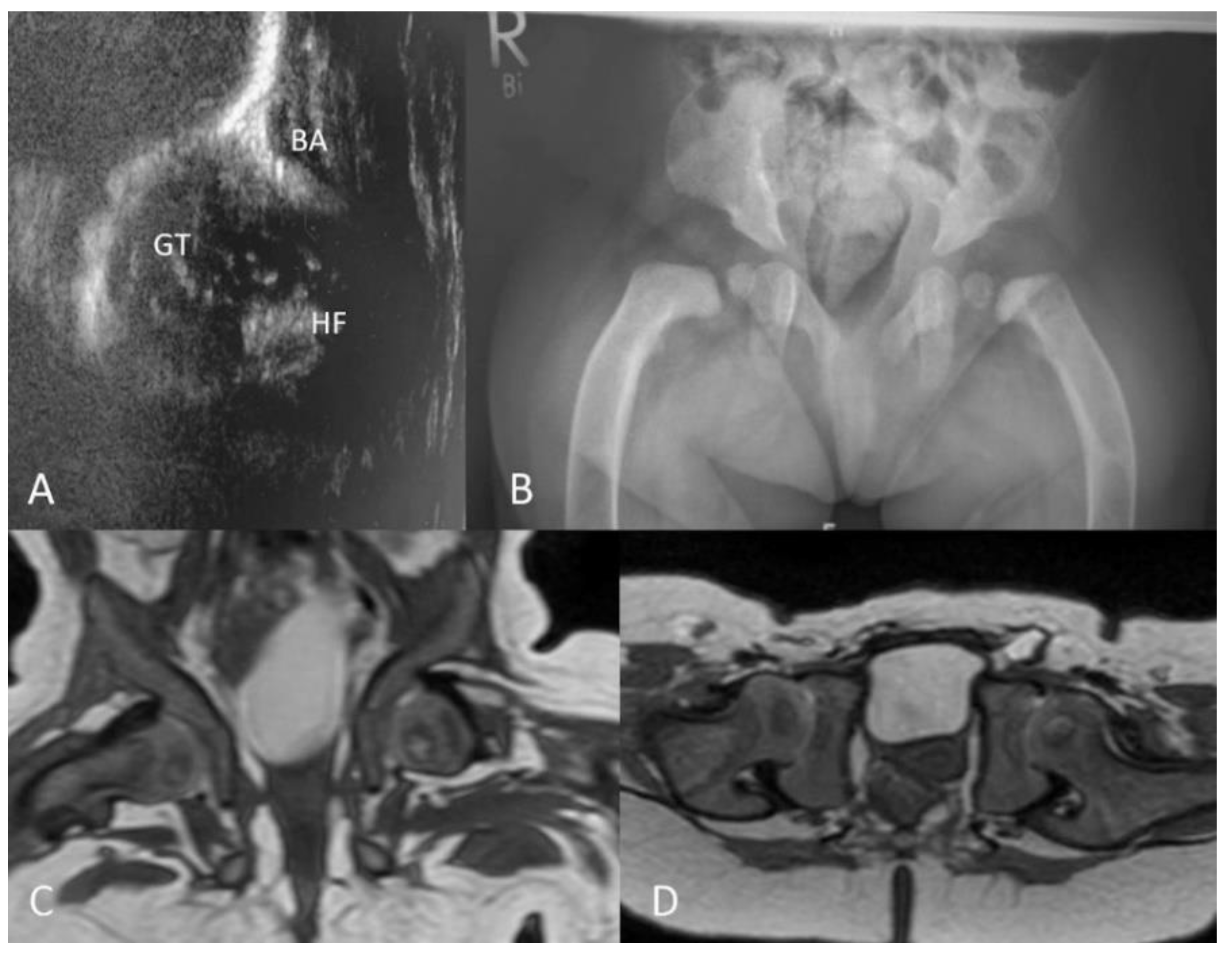
3.2.4. Ultrasound
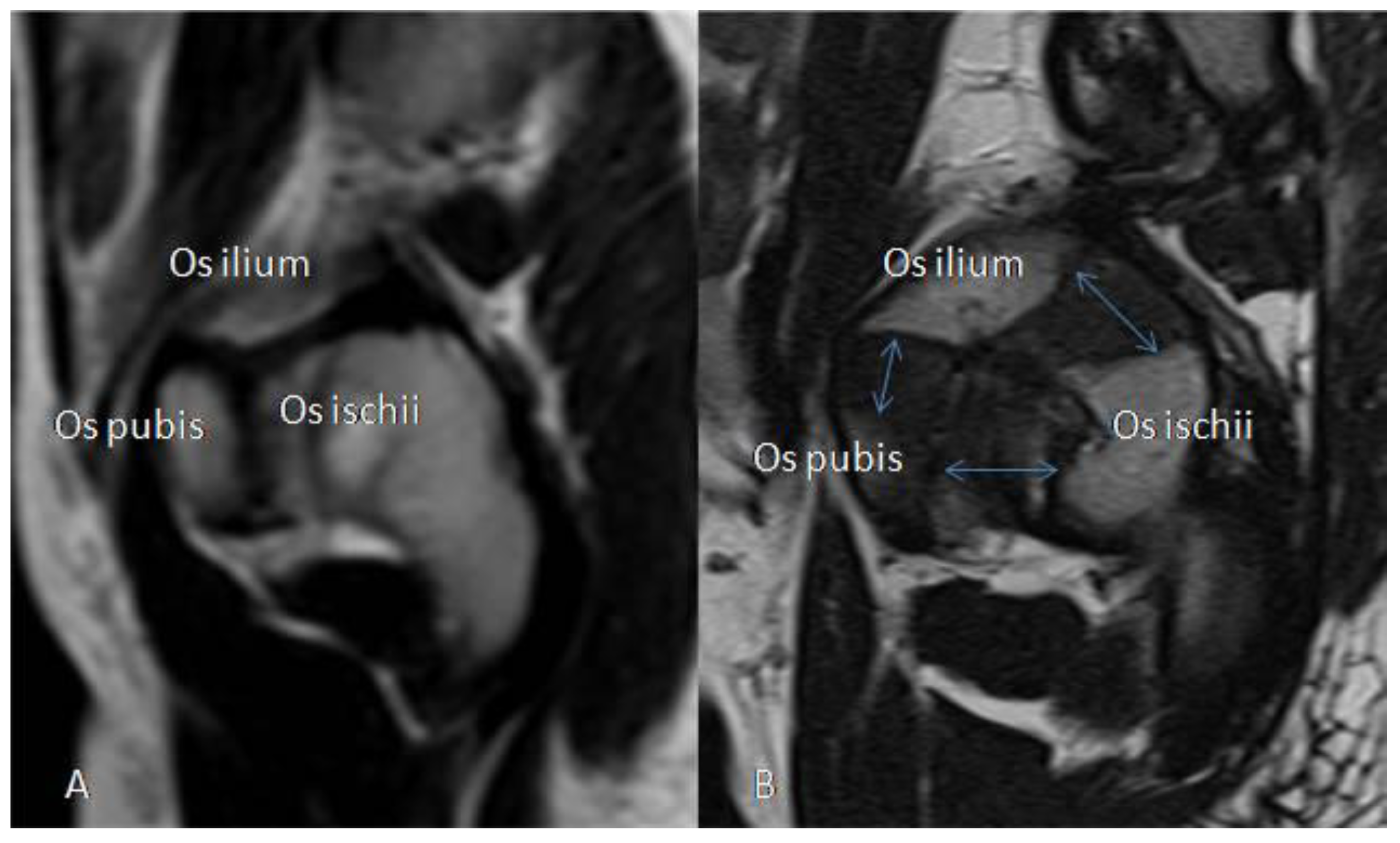
3.2.5. MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kollmann, K.; Pohl, S.; Marschner, K.; Encarnacao, M.; Sakwa, I.; Tiede, S.; Poorthuis, B.J.; Lubke, T.; Muller-Loennies, S.; Storch, S.; et al. Mannose phosphorylation in health and disease. Eur. J. Cell Biol. 2010, 89, 117–123. [Google Scholar] [CrossRef]
- Velho, R.V.; Harms, F.L.; Danyukova, T.; Ludwig, N.F.; Friez, M.J.; Cathey, S.S.; Filocamo, M.; Tappino, B.; Gunes, N.; Tuysuz, B.; et al. The lysosomal storage disorders mucolipidosis type II, type III alpha/beta, and type III gamma: Update on GNPTAB and GNPTG mutations. Hum. Mutat. 2019, 40, 842–864. [Google Scholar] [CrossRef]
- Bargal, R.; Zeigler, M.; Abu-Libdeh, B.; Zuri, V.; Mandel, H.; Ben Neriah, Z.; Stewart, F.; Elcioglu, N.; Hindi, T.; Le Merrer, M.; et al. When Mucolipidosis III meets Mucolipidosis II: GNPTA gene mutations in 24 patients. Mol. Genet. Metab. 2006, 88, 359–363. [Google Scholar] [CrossRef]
- Cathey, S.S.; Kudo, M.; Tiede, S.; Raas-Rothschild, A.; Braulke, T.; Beck, M.; Taylor, H.A.; Canfield, W.M.; Leroy, J.G.; Neufeld, E.F.; et al. Molecular order in mucolipidosis II and III nomenclature. Am. J. Med. Genet. A 2008, 146A, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Tiede, S.; Storch, S.; Lubke, T.; Henrissat, B.; Bargal, R.; Raas-Rothschild, A.; Braulke, T. Mucolipidosis II is caused by mutations in GNPTA encoding the alpha/beta GlcNAc-1-phosphotransferase. Nat. Med. 2005, 11, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- David-Vizcarra, G.; Briody, J.; Ault, J.; Fietz, M.; Fletcher, J.; Savarirayan, R.; Wilson, M.; McGill, J.; Edwards, M.; Munns, C.; et al. The natural history and osteodystrophy of mucolipidosis types II and III. J. Paediatr. Child Health 2010, 46, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cathey, S.S.; Leroy, J.G.; Wood, T.; Eaves, K.; Simensen, R.J.; Kudo, M.; Stevenson, R.E.; Friez, M.J. Phenotype and genotype in mucolipidoses II and III alpha/beta: A study of 61 probands. J. Med. Genet. 2010, 47, 38–48. [Google Scholar] [CrossRef]
- Leroy, J.G.; Sillence, D.; Wood, T.; Barnes, J.; Lebel, R.R.; Friez, M.J.; Stevenson, R.E.; Steet, R.; Cathey, S.S. A novel intermediate mucolipidosis II/IIIalphabeta caused by GNPTAB mutation in the cytosolic N-terminal domain. Eur. J. Hum. Genet. 2014, 22, 594–601. [Google Scholar] [CrossRef][Green Version]
- Leroy, J.G.; Cathey, S.S.; Friez, M.J. GNPTAB-Related Disorders. In GeneReviews®; Adam, M.P., Pagon, A.H., Eds.; University of Washington: Seattle, DC, USA, 2008; (Updated 2019). [Google Scholar]
- Leroy, J.G.; Demars, R.I. Mutant enzymatic and cytological phenotypes in cultured human fibroblasts. Science 1967, 157, 804–806. [Google Scholar] [CrossRef]
- Spranger, J.W.; Wiedemann, H.R. The genetic mucolipidoses. Diagnosis and differential diagnosis. Humangenetik 1970, 9, 113–139. [Google Scholar]
- Patriquin, H.B.; Kaplan, P.; Kind, H.P.; Giedion, A. Neonatal mucolipidosis II (I-cell disease): Clinical and radiologic features in three cases. AJR Am. J. Roentgenol. 1977, 129, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Taber, P.; Gyepes, M.T.; Philippart, M.; Ling, S. Roentgenographic manifestations of Leroy’s I-cell disease. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1973, 118, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.M.; Lachman, R.S. Early characteristic radiographic changes in mucolipidosis II. Pediatric Radiol. 2016, 46, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Hefti, F. Kinderorthopädie in der Praxis, 1st ed.; Springer: Berlin, Germany, 1997; p. 173. [Google Scholar]
- Reimers, J. The stability of the hip in children. A radiological study of the results of muscle surgery in cerebral palsy. Acta Orthop. Scand Suppl. 1980, 184, 1–100. [Google Scholar] [CrossRef]
- Tonnis, D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clin. Orthop. Relat. Res. 1976, 119, 39–47. [Google Scholar]
- Novais, E.N.; Pan, Z.; Autruong, P.T.; Meyers, M.L.; Chang, F.M. Normal Percentile Reference Curves and Correlation of Acetabular Index and Acetabular Depth Ratio in Children. J. Pediatric Orthop. 2018, 38, 163–169. [Google Scholar] [CrossRef]
- Tannast, M.; Hanke, M.S.; Zheng, G.; Steppacher, S.D.; Siebenrock, K.A. What are the radiographic reference values for acetabular under- and overcoverage? Clin. Orthop. Relat. Res. 2015, 473, 1234–1246. [Google Scholar] [CrossRef]
- Jones, D.H. Shenton’s line. J. Bone Jt. Surg. Br. 2010, 92, 1312–1315. [Google Scholar] [CrossRef]
- Graf, R. Classification of hip joint dysplasia by means of sonography. Arch. Orthop. Trauma Surg. 1984, 102, 248–255. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 26. [Google Scholar] [CrossRef]
- Alegra, T.; Sperb-Ludwig, F.; Guarany, N.R.; Ribeiro, E.M.; Lourenco, C.M.; Kim, C.A.; Valadares, E.R.; Galera, M.F.; Acosta, A.X.; Horovitz, D.D.G.; et al. Clinical Characterization of Mucolipidoses II and III: A Multicenter Study. J. Pediatric Genet. 2019, 8, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Blank, E.; Linder, D. I-cell disease (mucolipidosis II): A lysosomopathy. Pediatrics 1974, 54, 797–805. [Google Scholar] [PubMed]
- Okada, S.; Owada, M.; Sakiyama, T.; Yutaka, T.; Ogawa, M. I-cell disease: Clinical studies of 21 Japanese cases. Clin. Genet. 1985, 28, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Terashima, Y.; Tsuda, K.; Isomura, S.; Sugiura, Y.; Nogami, H. I-cell disease. Report of three cases. Am. J. Dis. Child 1975, 129, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, T.; Ishida, K. Recent advances in the prevention, early diagnosis, and treatment of congenital dislocation of the hip in Japan. Clin. Orthop. Relat. Res. 1984, 184, 34–40. [Google Scholar] [CrossRef]
- Siffert, R.S. Patterns of deformity of the developing hip. Clin. Orthop. Relat. Res. 1981, 14–29. [Google Scholar] [CrossRef]
- Leroy, J.G.; Martin, J.J. Mucolipidosis II (I-cell disease): Present status of knowledge. Birth Defects Orig. Artic. Ser. 1975, 11, 283–293. [Google Scholar]
- Martin, J.J.; Leroy, J.G.; Farriaux, J.P.; Fontaine, G.; Desnick, R.J.; Cabello, A. I-cell disease (mucolipidosis II): A report on its pathology. Acta Neuropathol. 1975, 33, 285–305. [Google Scholar] [CrossRef]
- Pazzaglia, U.E.; Beluffi, G.; Castello, A.; Coci, A.; Zatti, G. Bone changes of mucolipidosis II at different ages. Postmortem study of three cases. Clin. Orthop. Relat. Res. 1992, 276, 283–290. [Google Scholar]
- Spritz, R.A.; Doughty, R.A.; Spackman, T.J.; Murane, M.J.; Coates, P.M.; Koldovsky, O.; Zackai, E.H. Neonatal presentation of I-cell disease. J. Pediatric 1978, 93, 954–958. [Google Scholar] [CrossRef]
- Kollmann, K.; Pestka, J.M.; Kuhn, S.C.; Schone, E.; Schweizer, M.; Karkmann, K.; Otomo, T.; Catala-Lehnen, P.; Failla, A.V.; Marshall, R.P.; et al. Decreased bone formation and increased osteoclastogenesis cause bone loss in mucolipidosis II. EMBO Mol. Med. 2013, 5, 1871–1886. [Google Scholar] [CrossRef] [PubMed]
- Marschner, K.; Kollmann, K.; Schweizer, M.; Braulke, T.; Pohl, S. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science 2011, 333, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N. I-cell disease--mucolipidosis II. Postgrad Med. J. 1973, 49, 359–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, M.C.; Eberson, C.P. Growth and development of the child’s hip. Orthop. Clin. N. Am. 2006, 37, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, U.E.; Beluffi, G.; Campbell, J.B.; Bianchi, E.; Colavita, N.; Diard, F.; Gugliantini, P.; Hirche, U.; Kozlowski, K.; Marchi, A.; et al. Mucolipidosis II: Correlation between radiological features and histopathology of the bones. Pediatric Radiol. 1989, 19, 406–413. [Google Scholar] [CrossRef]
| n (Sex) | Genotype GNPTAB Allele 1/allele 2 | Phenotype | Age at Death (Years) | Age at First Radiograph (Years) | Age at Last Radiograph (Years) | Age at First Ultrasound (Month) | Age at First MRI (Years) |
|---|---|---|---|---|---|---|---|
| 1 (F) | c.1001G>A/c.1001G>A | MLII | N/A | 0.9 | 2.3 | 11.2 | N/A |
| 2 (F) | c.1337G>A/c.3098T>C | MLII | 8.7 | 2.2 | 3.2 | 0.1 | N/A |
| 3 (F) | c.3335+1G>A/c.3335+1G>A | MLII | N/A | 0.0 | 0.7 | 0.3 | 0.6 |
| 4 (M) | c.3503_3504del/c.1052dup | MLII | N/A | 0.8 | 2.8 | 9.1 | N/A |
| 5 (M) | Unknown | MLII | 9.4 | 0.0 | 5.1 | 1.6 | 0.2 |
| 6 (F) | Unknown | MLII | 3.4 | 0.0 | 2.7 | 0.2 | N/A |
| 7 (M) | c.3503_3504del/c.3503_3504del | MLII | N/A | 0.1 | 0.8 | 0.6 | N/A |
| 8 (M) | c.516dup/c.516dup | MLII | 8.0 | 0.0 | 7.9 | N/A | N/A |
| 9 (M) | c.3503_3504del/c.3503_3504del | MLII | N/A | 1.2 | N/A | N/A | N/A |
| 10 (M) | c.1090C>T/c.3091C>T | MLII | 4.7 | 0.2 | 4.4 | 2.8 | N/A |
| 11 # (M) | c.3503_3504del/c.3503_3504del | MLII | 0.3 | 0.0 | 0.2 | N/A | N/A |
| 12 # (F) | c.3503_3504del/c.3503_3504del | MLII | 1.3 | 0.0 | 1.3 | N/A | N/A |
| 13 (F) | c.10A>C/c.10A>C | ML intermediate | N/A | 3.3 | 8.0 | N/A | N/A |
| 14 (M) | Unknown | ML intermediate | N/A | 0.8 | 3.2 | 0.0 | N/A |
| 15 (M) | c.10A>C/c.2502del | ML intermediate | 11.8 | 2.2 | 10.0 | N/A | 6.7 |
| 16 (M) | c.344_345del/c.1022del | ML intermediate | N/A | 0.5 | 11.2 | 0.9 | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammer, L.S.; Oussoren, E.; Muschol, N.M.; Pohl, S.; Rubio-Gozalbo, M.E.; Santer, R.; Stuecker, R.; Vettorazzi, E.; Breyer, S.R. Hip Morphology in Mucolipidosis Type II. J. Clin. Med. 2020, 9, 728. https://doi.org/10.3390/jcm9030728
Ammer LS, Oussoren E, Muschol NM, Pohl S, Rubio-Gozalbo ME, Santer R, Stuecker R, Vettorazzi E, Breyer SR. Hip Morphology in Mucolipidosis Type II. Journal of Clinical Medicine. 2020; 9(3):728. https://doi.org/10.3390/jcm9030728
Chicago/Turabian StyleAmmer, Luise Sophie, Esmeralda Oussoren, Nicole Maria Muschol, Sandra Pohl, Maria Estela Rubio-Gozalbo, René Santer, Ralf Stuecker, Eik Vettorazzi, and Sandra Rafaela Breyer. 2020. "Hip Morphology in Mucolipidosis Type II" Journal of Clinical Medicine 9, no. 3: 728. https://doi.org/10.3390/jcm9030728
APA StyleAmmer, L. S., Oussoren, E., Muschol, N. M., Pohl, S., Rubio-Gozalbo, M. E., Santer, R., Stuecker, R., Vettorazzi, E., & Breyer, S. R. (2020). Hip Morphology in Mucolipidosis Type II. Journal of Clinical Medicine, 9(3), 728. https://doi.org/10.3390/jcm9030728





