MSC Based Therapies—New Perspectives for the Injured Lung
Abstract
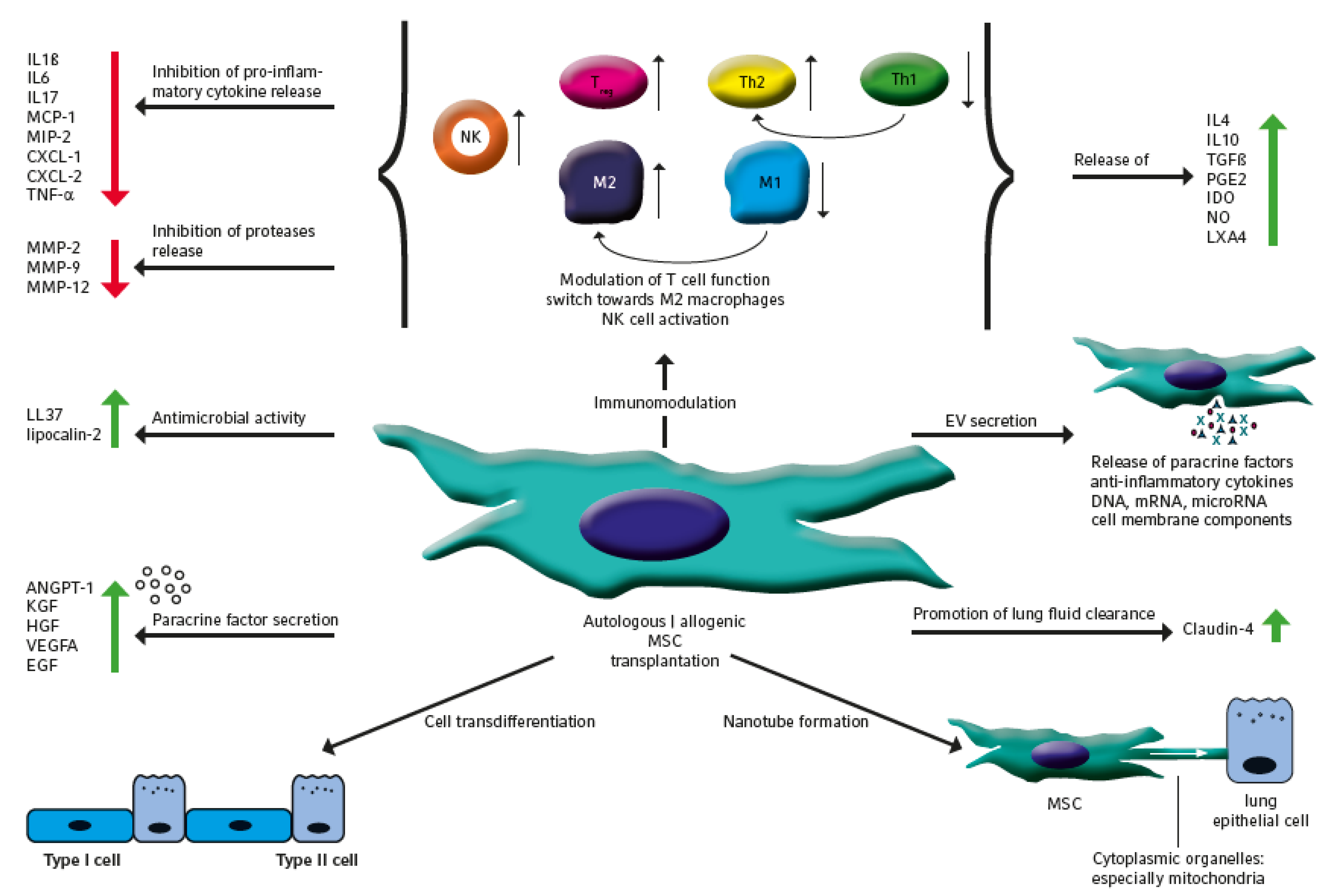
1. Introduction
2. The Therapeutic Potential of MSC
3. Therapeutic Efficacy of MSC Application in Preclinical Lung Disease Models
3.1. Bronchopulmonary Dysplasia
3.2. Asthma
3.3. Acute Lung Injury
3.4. Chronic Obstructive Pulmonary Disease
3.5. Idiopathic Pulmonary Fibrosis
| Experimental Lung Disease Model | Species | Cell Source | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| cigarette smoke | rat | BM | rat | 6 × 106 | i.t. | beginning 7th week | yes (2x/week for 5 weeks) | alleviated airway inflammation and edema | downregulated COX2 and prostaglandin E production suggested to be mediated by inhibition of p38 and ERK MAP kinase activity in macrophages | [85] |
| elastase | mice | BM | mice | 1 × 105 vs. 2 × 105 | i.t. | 3 h p.i. (second application after 7 days) | yes | reduced inflammation and collagen fiber content, improved VEGFA secretion and lung mechanics | only the repeated application one week apart reduced neutrophil counts and T cell pathology resulting in attenuation of pulmonary arterial hypertension | [90] |
| cigarette smoke | rat | BMC vs. BM | rat | 6 × 106 vs. 6 × 105 | i.v. | 6 month a.t. | no | BMC and MSC increased pulmonary vascularity, cell proliferation and number of small vessels. BMC reduce apoptotic cell death, attenuate mean pulmonary arterial pressure and muscularization | BMC better induced proliferation of AT2 cells and pulmonal vascular endothelial cells, BM MSC and BMC both alleviate emphysema. | [81] |
| elastase | rat | AT | rat | 5 × 107 | local (PGAF) | 1 week a.t. | no | enhancement of compensatory growth, restoration of pulmonary function, alveolar and vascular regeneration | selective delivery of HGF by AT MSC with alveolar regenerative and angiogenic effects | [79] |
| papain | rat | BM | rat | 4 × 106 | i.v. | 2 h a.t. | no | protective effect on pulmonary emphysema by secretion of reparative growth factors | BM MSC increase VEGF-A expression by TNF-α release with inhibition of apoptosis | [80] |
| elastase | mice | WJ | human | 5 × 104 | i.v. | 7 d a.t. | no | reduced degree of alveolar emphysema | WJ MSC deliver pulmonary regenerative effect, pathomechanism not investigated | [91] |
| elastase | mice | LT | mice | 5 × 104 | i.t. | 21 d a.t. | no | partially restored lung elasticity and alveolar architecture | activation of HGF/c-Met system, by promoting survival and proliferation of alveolar epithelial cells | [92] |
| cigarette smoke | mice | AT | human vs mice | 3 × 105 | i.v. | during last 8 weeks of treatment | yes (4x/last 8 weeks of treatment, biweekly) | reduced inflammatory infiltration, decreased cell death and airspace enlargement and restored weight loss | AT MSC abrogated the phosphorylation of p38 MAPK and attenuated JNK1 and AKT1 activities, murine and human ASC have same effects | [93] |
| elastase | mice | BM vs AT vs LT | mice | 1 × 105 | i.v. vs. i.t. | 3 h a.t. | no | BM, AT and LT MSCs decreased mean linear intercept, neutrophil infiltration, and cell apoptosis, increased elastic fiber content, reduced alveolar epithelial and endothelial cell damage | decreased keratinocyte-derived chemokine and TGF-β levels in all sources, i.v. administration of BM MSC with better cardiovascular function and phenotype change from M1 to M2 | [88] |
| elastase or cigarette smoke | mice | AT | human | 1 × 105 | i.v. | 7 d a.t. vs. 6-month a.t. | no | improved alveolar regeneration | AT MSC decrease mean linear intercept and reduce caspase-3 activity | [109] |
| Experimental Lung Disease Model | Species | Cell Source | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| bleomycin | mice | BM | mice | 5 × 105 | i.v. | 1 d vs. 3 d vs. 6 d a.t. | no | lung fibrosis and inflammation inhibited to greater degree in day 3 and 6, later administration of BM MSC engraftment more effective | decreased MMP9, TIMP-1, IFN-γ and TGF-β activity | [104] |
| bleomycin | mice | AT | mice | 5 × 105 | i.v. | 1 d a.t. | no | AT MSC from aged mice are inefficient to attenuate disease pathology | AT MSC from young mice inhibit MMP-2, IGF receptor and AKT activation | [102] |
| bleomycin | mice | AT | human | 4 × 107/kg BW | i.v. | 3,6,9 d a.t. | yes | increased survival, reduced collagen deposition, immunomodulation and anti-fibrotic effect in early stage of disease | suppression of profibrotic and inflammatory gene transcripts | [98] |
| bleomycin | mice | BM | human | 5 × 105 | i.v. | 2 d a.t. | no | reduced pulmonary fibrosis and improved lung function | suppression of total T cell and CD4+ T cell infiltration, pro-inflammatory cytokine production and fibrotic changes | [95] |
| bleomycin | mice | AT | mice | 5 × 105 | i.v. | 1 d a.t. | no | attenuation of lung fibrosis | inhibition of mir-199-3p and AKT phosphorylation, preservation of caveolin-1 expression | [101] |
| bleomycin | mice | BM | mice | 5 × 105 | i.v. | 7 d a.t. | no | reduction of inflammation and collagen deposition | response to injury, adopt an epithelium-like phenotype, and reduce inflammation and collagen deposition, replacing alveolar epithelial type II cells, reduced expression of MMP2 and MMP9 | [96] |
| bleomycin | rat | BM | rat | 1 × 106 | i.v. | 4 d a.t. | no | reduced lung injury and fibrosis, lower neutrophilic infiltration and collagen deposition | down-regulation of IL-1β, TGF-β VEGF, IL-6, TNF-α, and NOS | [97] |
| bleomycin | mice | BM | mice | 5 × 105 | i.v. | n.a. | no | protection of lung injury | Secretion of high levels of IL1RN, to antagonize the function of IL-1α and block production of TNF-α from activated macrophages | [99] |
4. Summary of Important Clinical Phase I and Phase II Studies
5. Safety Issues and Obstacles to Therapeutic MSC Application
6. Strategies to Improve Safety and Therapeutic Efficiency of MSC Based Therapies
| Disease Entity | Experimental Lung Disease Model | Cell Source | MSC Modification | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALI | LPS | BM | FGF2 overexpression | mice | 5 × 106 | i.v. | 1 h p.i. | no | FGF2 overexpression better preserves lung structure and pulmonary edema | MSC overexpressing FGF2 better attenuate pro-inflammatory cytokine and MPO secretion and neutrophil infiltration | [153] |
| ALI | LPS | BM | CXCR4 overexpression | rat | 1 × 106 | i.v. | 1 h p.i. | no | reduced lung injury score and lung edema | enhanced mobilization and chemotaxis of MSC, increased VEGFA secretion and reduced lung inflammation | [154] |
| ALI | LPS | BM | β-catenin overexpression | mice | 5 × 105 | i.t. | 4 h p.i. | no | improvements in alveolar epithelial barrier integrity and lung structure impairment | better MSC retention in the lung and AEC II transdifferentiation with higher levels of KGF and IL-10 and reduced IL-1β | [155] |
| ALI | LPS | BM | siRNA against claudin-4 | human | 1 × 106 | i.p. | n.a. | no | Claudin-4 promotes alveolar fluid clearance | hypoxic MSC preconditioning stipulates claudin-4 secretion | [28] |
| ALI | LPS | BM | MSC transfected with shRNA against VEGFA | rat | 5 × 106 | i.v. | 5 h p.i. | no | attenuated anti-inflammatory properties and beneficial effects on lung injury | transfected MSC reduced the proinflammatory cytokine IL-1β levels and elevated the anti-inflammatory cytokine IL-10 levels | [151] |
| ALI | LPS | BM | shRNA HGF transfection | rat | 5 × 106 | i.v. | 5 h p.i. | no | partial abrogation of MSC effects, MSC retention in the lung was not influenced, MSC restores lung permeability and lung injury | HGF-expressing character is required for MSC to protect the injured lung | [148] |
| ALI | LPS | AM | Nrf2 transfected MSC | human | 1 × 106 | i.v. | 4 h p.i. | no | reduced inflammation, epithelial cell injury and fibrosis | increased cell retention in the lung, more efficient differentiation into type II cells with higher SPC content | [162] |
| ALI | E. coli | UCB | IL-10 transgentic MSC | human | 1 × 107/kg | i.v. | 1 h p.i. | no | increased therapeutic efficiency of transgenic MSC which only prohibited all aspects of lung injury including gas exchange | enhanced macrophage function via prostaglandin E2 and lipoxygenase A4 | [158] |
| ALI | LPS | BM | transduction with heme oxygenase-1 | rat | 5 × 105 | i.v. | 2 h p.i. | no | improved survival rates, reduced lung inflammation and structural changes | superior prosurvival, antiapoptotic and paracrine functions | [150] |
| BPD | hyperoxia | BM | shRNA stromal cell-derived factor-1 | rat | 1 × 106 | i.t. | d7 | no | reduction of beneficial MSC effects on alveolarization and angiogenesis | SDF-1 from MSC exerts anti-inflammatory and angiogenesis promoting activities | [159] |
| asthma | ovalbumin | BM | erythropoietin gene modified MSC | mice | n.a. | i.v. | d20 | no | more efficient inhibition of all disease driving pathologies | maybe related with the downregulation of TGF-β1-TAK1-p38MAPK pathway activity | [173] |
| COPD | elastase | BM | VEGFA overexpression | mice | n.a. | i.v. | 14 d a.t. | no | Improved attenuation of emphysema compared to naïve MSC | Increased tissue expression of VEGFA, Nrf 2 and superoxide dismutase | [84] |
| COPD | elastase | BM | shRNA HGF knockdown | human | 0,1 vs. 5 vs. 25 vs. 125 × 103/g | i.v. | 6 h vs. d7 vs. d14 a.t. | no | MSC cell therapy more efficient than conditioned medium, higher doses and mid to delayed application better reduces collagen deposition and anti-inflammatory effects | anti-inflammatory, antifibrotic and antiapoptotic effects mediated partially through HGF | [187] |
| Disease Entity | Experimental Lung Disease Model | Cell Source | MSC Modification | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALI | endotoxin | BM | hypoxia | human | 5 × 104 cells/g | i.v. | n.a. | no | ischemic preconditioning potentiates the protective effect of through the secretion of exosome | less neutrophil influx and pro-inflammatory cytokine dysbalance with upregulation of IL-10 | [74] |
| ALI | CLP | BM | preconditioning with carbon monoxide | mice | 5 × 105(2 h) 2.5 × 105 (24 h/48 h) | i.v. | 2 h, 24 h, 48 h p.i. | yes | increased survival and alleviated lung injury | preconditioning stipulates the production of proresolving lipid mediators, especially resolvins | [166] |
| ALI | E. coli | BM | MSC pretreatment with Toll-like receptor-3 agonist | human | 2 × 107 vs. 4 × 107 | i.v. | 1 h p.i. | no | reduced pulmonary edema and bacterial load | increased antimicrobial activity of macrophages after application of pretreated EV | [167] |
| asthma | dust mite | BM | pretreatment of MSC with eicosapentaenoic acid | mice | 1 × 105 | i.t. | 1 d a.t. | no | reduced bronchoconstriction and lung tissue remodeling | reduced influx of eosinophils, macrophages, neutrophils and lymphocytes, shift towards anti-inflammatory macrophages and increased release of inflammation resolving and anti-inflammatory mediators | [171] |
| COPD | elastase, cigarette smoke | AT | preconditioning with pioglitazone | human | 1 × 105 | i.v. | 7 d a.t. | no | more efficient repair of lung injury | increased VEGFA production | [109] |
| COPD | elastase | AM | MSC predifferentiation to lung epithelial like progenitor cells | mice | 1 × 105 | i.t. | 2 weeks a.t. | no | improved lung regeneration, reduced presence of inflammatory and lung remodeling factors | integration of predifferentiated cells into lung alveolar structures | [168] |
| IPF | bleomycin | BM | oncostatin M preconditioning | mice | 2 × 105 | i.t. | 3 d a.t. | no | improved attenuation of inflammation, TGF-β1 and OSM induced extracellular matrix production, release of fibrotic factors | upregulation of paracrine HGF | [165] |
| Disease Entity | Experimental Lung Disease Model | Cell Source | MSC Modification | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BPD | hyperoxia | BM | combined treatment with erythropoetin | mice | 1 × 106 | i.v. | 1 h before + d7 | yes | improved airway structures and body weight | augmented cytoprotection | [172] |
| BPD | hyperoxia | PD | MSC plus surfactant | human | 1 × 105 | i.t. | d5 | no | application of surfactant partially attenuates the beneficial impact on lung histology | surfactant inhibits MSC viability and impairs the reduction of hyperoxia induced increase in mean linear intercept | [174] |
| asthma | ovalbumin | BM | erythropoietin gene modified MSC | mice | n.a. | i.v. | d20 | no | more efficient inhibition of all disease driving pathologies | maybe related with the downregulation of TGF-β1-TAK1-p38MAPK pathway activity | [173] |
| Disease Entity | Experimental Lung Disease Model | Source of EV | EV Modification | EV Species | EV dose (MSC Cell Equivalent) | Application Route | Time Point of EV Application | Repeated EV Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALI | E. coli | BM vs LF | - | human | 3 × 106 vs. 6 × 106 vs. 9 × 106 | i.t. i.t. i.v. | 4 h p.i. | no | increased survival, MV as effective as MSC | increased KGF release, decreased influx of inflammatory cells, pro-inflammatory cytokine release and pulmonary edema, attenuation of bacterial load | [184] |
| ALI | LPS (E. coli) | AT (young vs aged) | - | human | 1 × 106 | i.v. | 30 min p.i. | no | only EV from young donor MSC alleviated lung injury | macrophages are less prone to internalization of EV from aged MSC | [194] |
| ALI | E. coli | BM | antagomir of miR-145 | human | 9 × 106 | i.v. | 4 h p.i. | no | EV and MSC decrease lung injury, efficient bacterial clearance depends on high levels of leukotriene B4 | controlled by miR-145 enhanced LTB4 production and antimicrobial activity through LTB4/BLT1 signaling | [185] |
| BPD | hyperoxia | WJ vs BM | - | human | 0.5 × 106 | i.v. | d4 | no | reduction of alveolar and vascular remodeling and lung fibrosis same for WJ and BM EV | shift from pro-inflammatory M1 macrophage status to anti-inflammatory M2 status | [176] |
| BPD | hyperoxia | UCB | siRNA VEGF-knockdown | human | 5 × 105 | i.t. | d14 | no | EV similar effective in reduction of impairments in alveolarization and vasculogenesis | knockdown of VEGFA abolished the beneficial effects, EV were internalized into type II cells, fibroblasts and pericytes, but into endothelial cells | [178] |
| BPD | hyperoxia | UCB + WJ | TSG-6 siRNA knockdown | human | 0.7x106 | i.p. | d2 + d4 | yes | EV similar effectiveness in reduction of lung, heart and brain pathology | knockdown of TSG-6 abrogated the beneficial effects | [180] |
| BPD | hyperoxia | UCB | - | human | 6 × 106 | i.t. | d3, d7, d10 | yes | only EV, but not MSC prevented an increase in medial thickness in small pulmonary arteries | further studies required for mechanism of action | [186] |
| asthma | ovalbumin | AT | - | human | 1 × 105 | i.v. | d47 (1d after last challenge) | no | superiority of EV compared to MSC with respect to pro-inflammatory mediators and inflammatory cell infiltration, but similarly reduced eosinophils, collagen fiber content and levels of TGF-β | further studies required for mechanism of action | [188] |
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cote, C. Pharmacoeconomics and the Burden of Chronic Obstructive Pulmonary Disease. Clin. Pulm. Med. 2005, 12, S19–S21. [Google Scholar] [CrossRef]
- Global Surveillance, Prevention and Control of Chronic Respiratory Diseases. A Comprehensive Approach; Bousquet, J., Ed.; WHO: Geneva, Switzerland, 2007; ISBN 9789241563468. [Google Scholar]
- Yang, J.; Jia, Z. Cell-based therapy in lung regenerative medicine. Regen. Med. Res. 2014, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Reicherzer, T.; Häffner, S.; Shahzad, T.; Gronbach, J.; Mysliwietz, J.; Hübener, C.; Hasbargen, U.; Gertheiss, J.; Schulze, A.; Bellusci, S.; et al. Activation of the NF-κB pathway alters the phenotype of MSCs in the tracheal aspirates of preterm infants with severe BPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L87–L101. [Google Scholar] [CrossRef] [PubMed]
- Gronbach, J.; Shahzad, T.; Radajewski, S.; Chao, C.-M.; Bellusci, S.; Morty, R.E.; Reicherzer, T.; Ehrhardt, H. The Potentials and Caveats of Mesenchymal Stromal Cell-Based Therapies in the Preterm Infant. Stem Cells Int. 2018, 2018, 9652897. [Google Scholar] [CrossRef]
- Akram, K.M.; Patel, N.; Spiteri, M.A.; Forsyth, N.R. Lung Regeneration: Endogenous and Exogenous Stem Cell Mediated Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 128. [Google Scholar] [CrossRef]
- Garcia, O.; Carraro, G.; Navarro, S.; Bertoncello, I.; McQualter, J.; Driscoll, B.; Jesudason, E.; Warburton, D. Cell-based therapies for lung disease. Br. Med. Bull. 2012, 101, 147–161. [Google Scholar] [CrossRef]
- Foronjy, R.F.; Majka, S.M. The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: Understanding microenvironmental cues. Cells 2012, 1, 874–885. [Google Scholar] [CrossRef]
- Tropea, K.A.; Leder, E.; Aslam, M.; Lau, A.N.; Raiser, D.M.; Lee, J.-H.; Balasubramaniam, V.; Fredenburgh, L.E.; Alex Mitsialis, S.; Kourembanas, S.; et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L829–L837. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Najar, M.; Bouhtit, F.; Melki, R.; Afif, H.; Hamal, A.; Fahmi, H.; Merimi, M.; Lagneaux, L. Mesenchymal Stromal Cell-Based Therapy: New Perspectives and Challenges. J. Clin. Med. 2019, 8, 626. [Google Scholar] [CrossRef]
- Jiang, J.; Song, Z.; Zhang, L. miR-155-5p Promotes Progression of Acute Respiratory Distress Syndrome by Inhibiting Differentiation of Bone Marrow Mesenchymal Stem Cells to Alveolar Type II Epithelial Cells. Med. Sci. Monit. 2018, 24, 4330–4338. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Q.; Ding, Y.-J. Overexpressed microRNA-615-3p promotes progression of neonatal acute respiratory distress syndrome by inhibiting differentiation of mesenchymal stem cells to alveolar type II epithelial cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4625–4633. [Google Scholar] [PubMed]
- Zhen, G.; Liu, H.; Gu, N.; Zhang, H.; Xu, Y.; Zhang, Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front. Biosci. 2008, 13, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Bernard, O.; Jeny, F.; Uzunhan, Y.; Dondi, E.; Terfous, R.; Label, R.; Sutton, A.; Larghero, J.; Vanneaux, V.; Nunes, H.; et al. Mesenchymal stem cells reduce hypoxia-induced apoptosis in alveolar epithelial cells by modulating HIF and ROS hypoxic signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L360–L371. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Abbott, J.; Cheng, L.; Colby, J.K.; Lee, J.W.; Levy, B.D.; Matthay, M.A. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J. Immunol. 2015, 195, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Katsha, A.M.; Ohkouchi, S.; Xin, H.; Kanehira, M.; Sun, R.; Nukiwa, T.; Saijo, Y. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol. Ther. 2011, 19, 196–203. [Google Scholar] [CrossRef]
- Lee, J.W.; Gupta, N.; Serikov, V.; Matthay, M.A. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin. Biol. Ther. 2009, 9, 1259–1270. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffin, M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011, 2, 34. [Google Scholar] [CrossRef]
- Pedrazza, L.; Cunha, A.A.; Luft, C.; Nunes, N.K.; Schimitz, F.; Gassen, R.B.; Breda, R.V.; Donadio, M.V.F.; de Souza Wyse, A.T.; Pitrez, P.M.C.; et al. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J. Cell. Physiol. 2017, 232, 3552–3564. [Google Scholar] [CrossRef]
- Zheng, G.; Ge, M.; Qiu, G.; Shu, Q.; Xu, J. Mesenchymal Stromal Cells Affect Disease Outcomes via Macrophage Polarization. Stem Cells Int. 2015, 2015, 989473. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-X.; Xu, J.-f.; Seeley, E.J.; Tang, X.-D.; Xu, L.-L.; Zhu, Y.-G.; Song, Y.-L.; Qu, J.-M. Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Pulmonary Infection Caused by Pseudomonas aeruginosa via Inhibiting Overproduction of Prostaglandin E2. Stem Cells 2015, 33, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Krasnodembskaya, A.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; Matthay, M.A. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.H.J.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Uzunhan, Y.; Bernard, O.; Marchant, D.; Dard, N.; Vanneaux, V.; Larghero, J.; Gille, T.; Clerici, C.; Valeyre, D.; Nunes, H.; et al. Mesenchymal stem cells protect from hypoxia-induced alveolar epithelial-mesenchymal transition. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L439–L451. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, W.; Zhou, S.; Xu, L.; Jiang, C. Protective effect of bone marrow derived mesenchymal stem cells in lipopolysaccharide-induced acute lung injury mediated by claudin-4 in a rat model. Am. J. Transl. Res. 2016, 8, 3769–3779. [Google Scholar]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; Wani, M.R.; et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2019, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Baveja, R.; Liang, O.D.; Fernandez-Gonzalez, A.; Lee, C.; Mitsialis, S.A.; Kourembanas, S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 2009, 180, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Van Haaften, T.; Byrne, R.; Bonnet, S.; Rochefort, G.Y.; Akabutu, J.; Bouchentouf, M.; Rey-Parra, G.J.; Galipeau, J.; Haromy, A.; Eaton, F.; et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am. J. Respir. Crit. Care Med. 2009, 180, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, G.; Fernandez-Gonzalez, A.; Aslam, M.; Vitali, S.H.; Martin, T.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm. Circ. 2012, 2, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Sammour, I.; Somashekar, S.; Huang, J.; Batlahally, S.; Breton, M.; Valasaki, K.; Khan, A.; Wu, S.; Young, K.C. The Effect of Gender on Mesenchymal Stem Cell (MSC) Efficacy in Neonatal Hyperoxia-Induced Lung Injury. PLoS ONE 2016, 11, e0164269. [Google Scholar] [CrossRef]
- Al-Rubaie, A.; Wise, A.F.; Sozo, F.; de Matteo, R.; Samuel, C.S.; Harding, R.; Ricardo, S.D. The therapeutic effect of mesenchymal stem cells on pulmonary myeloid cells following neonatal hyperoxic lung injury in mice. Respir. Res. 2018, 19, 114. [Google Scholar] [CrossRef]
- Augustine, S.; Avey, M.T.; Harrison, B.; Locke, T.; Ghannad, M.; Moher, D.; Thébaud, B. Mesenchymal Stromal Cell Therapy in Bronchopulmonary Dysplasia: Systematic Review and Meta-Analysis of Preclinical Studies. Stem Cells Transl. Med. 2017, 6, 2079–2093. [Google Scholar] [CrossRef]
- Pierro, M.; Ionescu, L.; Montemurro, T.; Vadivel, A.; Weissmann, G.; Oudit, G.; Emery, D.; Bodiga, S.; Eaton, F.; Péault, B.; et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 2013, 68, 475–484. [Google Scholar] [CrossRef]
- Kim, Y.E.; Park, W.S.; Sung, D.K.; Ahn, S.Y.; Sung, S.I.; Yoo, H.S.; Chang, Y.S. Intratracheal transplantation of mesenchymal stem cells simultaneously attenuates both lung and brain injuries in hyperoxic newborn rats. Pediatr. Res. 2016, 80, 415–424. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Park, W.S. Cell type-dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy 2015, 17, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.K.; Chang, Y.S.; Ahn, S.Y.; Sung, S.I.; Yoo, H.S.; Choi, S.J.; Kim, S.Y.; Park, W.S. Optimal Route for Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation to Protect Against Neonatal Hyperoxic Lung Injury: Gene Expression Profiles and Histopathology. PLoS ONE 2015, 10, e0135574. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Deng, M.-X.; He, J.; Zeng, Q.-X.; Wen, W.; Wong, D.S.H.; Tse, H.-F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Gorham, J.D.; Bundoc, V.G.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef]
- Cho, K.-S.; Park, M.-K.; Kang, S.-A.; Park, H.-Y.; Hong, S.-L.; Park, H.-K.; Yu, H.-S.; Roh, H.-J. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediat. Inflamm. 2014, 2014, 436476. [Google Scholar] [CrossRef]
- Dai, R.; Yu, Y.; Yan, G.; Hou, X.; Ni, Y.; Shi, G. Intratracheal administration of adipose derived mesenchymal stem cells alleviates chronic asthma in a mouse model. BMC Pulm. Med. 2018, 18, 131. [Google Scholar] [CrossRef]
- Duong, K.M.; Arikkatt, J.; Ullah, M.A.; Lynch, J.P.; Zhang, V.; Atkinson, K.; Sly, P.D.; Phipps, S. Immunomodulation of airway epithelium cell activation by mesenchymal stromal cells ameliorates house dust mite-induced airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2015, 53, 615–624. [Google Scholar] [CrossRef]
- Zeng, S.-L.; Wang, L.-H.; Li, P.; Wang, W.; Yang, J. Mesenchymal stem cells abrogate experimental asthma by altering dendritic cell function. Mol. Med. Rep. 2015, 12, 2511–2520. [Google Scholar] [CrossRef]
- Mohammadian, M.; Sadeghipour, H.R.; Kashani, I.R.; Jahromi, G.P.; Omidi, A.; Nejad, A.K.; Golchoobian, R.; Boskabady, M.H. Evaluation of Simvastatin and Bone Marrow-Derived Mesenchymal Stem Cell Combination Therapy on Airway Remodeling in a Mouse Asthma Model. Lung 2016, 194, 777–785. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Park, D.-E.; Song, W.-J.; Bae, B.-R.; Lee, J.-W.; Sohn, K.-H.; Lee, H.-S.; Kang, H.-R.; Park, H.-W.; Chang, Y.-S.; et al. Immunologic regulatory effects of human umbilical cord blood-derived mesenchymal stem cells in a murine ovalbumin asthma model. Clin. Exp. Allergy 2017, 47, 937–945. [Google Scholar] [CrossRef]
- Takeda, K.; Webb, T.L.; Ning, F.; Shiraishi, Y.; Regan, D.P.; Chow, L.; Smith, M.J.; Ashino, S.; Guth, A.M.; Hopkins, S.; et al. Mesenchymal Stem Cells Recruit CCR2+ Monocytes to Suppress Allergic Airway Inflammation. J. Immunol. 2018, 200, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.H.; Kwon, H.-S.; Lee, K.Y.; Ha, E.H.; Moon, K.-A.; Kim, S.W.; Oh, W.; Kim, T.-B.; Moon, H.-B.; Cho, Y.S. hMSCs suppress neutrophil-dominant airway inflammation in a murine model of asthma. Exp. Mol. Med. 2017, 49, e288. [Google Scholar] [CrossRef] [PubMed]
- Kitoko, J.Z.; de Castro, L.L.; Nascimento, A.P.; Abreu, S.C.; Cruz, F.F.; Arantes, A.C.; Xisto, D.G.; Martins, M.A.; Morales, M.M.; Rocco, P.R.M.; et al. Therapeutic administration of bone marrow-derived mesenchymal stromal cells reduces airway inflammation without up-regulating Tregs in experimental asthma. Clin. Exp. Allergy 2018, 48, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Braza, F.; Dirou, S.; Forest, V.; Sauzeau, V.; Hassoun, D.; Chesné, J.; Cheminant-Muller, M.-A.; Sagan, C.; Magnan, A.; Lemarchand, P. Mesenchymal Stem Cells Induce Suppressive Macrophages Through Phagocytosis in a Mouse Model of Asthma. Stem Cells 2016, 34, 1836–1845. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Xu, L.; Xie, S.-S.; Yu, F.; Hu, H.-Y.; Song, X.-L.; Wang, C.-H. Mesenchymal stem cells suppress lung inflammation and airway remodeling in chronic asthma rat model via PI3K/Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8958–8967. [Google Scholar]
- Li, Y.; Qu, T.; Tian, L.; Han, T.; Jin, Y.; Wang, Y. Human placenta mesenchymal stem cells suppress airway inflammation in asthmatic rats by modulating Notch signaling. Mol. Med. Rep. 2018, 17, 5336–5343. [Google Scholar] [CrossRef]
- Abreu, S.C.; Antunes, M.A.; Xisto, D.G.; Cruz, F.F.; Branco, V.C.; Bandeira, E.; Zola Kitoko, J.; de Araújo, A.F.; Dellatorre-Texeira, L.; Olsen, P.C.; et al. Bone Marrow, Adipose, and Lung Tissue-Derived Murine Mesenchymal Stromal Cells Release Different Mediators and Differentially Affect Airway and Lung Parenchyma in Experimental Asthma. Stem Cells Transl. Med. 2017, 6, 1557–1567. [Google Scholar] [CrossRef]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef]
- Frank, J.A.; Briot, R.; Lee, J.W.; Ishizaka, A.; Uchida, T.; Matthay, M.A. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L52–L59. [Google Scholar] [CrossRef]
- Martínez-González, I.; Roca, O.; Masclans, J.R.; Moreno, R.; Salcedo, M.T.; Baekelandt, V.; Cruz, M.J.; Rello, J.; Aran, J.M. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 2013, 49, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lu, X.; Zou, L.; Xu, X.; Qiu, H. E-Prostanoid 2 Receptor Overexpression Promotes Mesenchymal Stem Cell Attenuated Lung Injury. Hum. Gene Ther. 2016, 27, 621–630. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.D.; Mei, S.H.J.; Lai, P.F.H.; Zhang, Q.W.; Parker, C.H.; Suen, R.S.; Hood, R.D.; Zhao, Y.D.; Deng, Y.; Han, R.N.N.; et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am. J. Respir. Crit. Care Med. 2007, 175, 1014–1026. [Google Scholar] [CrossRef]
- Li, J.-W.; Wu, X. Mesenchymal stem cells ameliorate LPS-induced acute lung injury through KGF promoting alveolar fluid clearance of alveolar type II cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2368–2378. [Google Scholar] [PubMed]
- Gupta, N.; Sinha, R.; Krasnodembskaya, A.; Xu, X.; Nizet, V.; Matthay, M.A.; Griffin, J.H. The TLR4-PAR1 Axis Regulates Bone Marrow Mesenchymal Stromal Cell Survival and Therapeutic Capacity in Experimental Bacterial Pneumonia. Stem Cells 2018, 36, 796–806. [Google Scholar] [CrossRef]
- Devaney, J.; Horie, S.; Masterson, C.; Elliman, S.; Barry, F.; O’Brien, T.; Curley, G.F.; O’Toole, D.; Laffey, J.G. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015, 70, 625–635. [Google Scholar] [CrossRef]
- Jackson, M.V.; Morrison, T.J.; Doherty, D.F.; McAuley, D.F.; Matthay, M.A.; Kissenpfennig, A.; O’Kane, C.M.; Krasnodembskaya, A.D. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016, 34, 2210–2223. [Google Scholar] [CrossRef]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Masterson, C.; Devaney, J.; Horie, S.; O’Flynn, L.; Deedigan, L.; Elliman, S.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Syndecan-2-positive, Bone Marrow-derived Human Mesenchymal Stromal Cells Attenuate Bacterial-induced Acute Lung Injury and Enhance Resolution of Ventilator-induced Lung Injury in Rats. Anesthesiology 2018, 129, 502–516. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Liu, X.; Tang, S.; Wei, F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J. Inflamm. 2012, 9, 33. [Google Scholar] [CrossRef]
- McIntyre, L.A.; Moher, D.; Fergusson, D.A.; Sullivan, K.J.; Mei, S.H.J.; Lalu, M.; Marshall, J.; Mcleod, M.; Griffin, G.; Grimshaw, J.; et al. Efficacy of Mesenchymal Stromal Cell Therapy for Acute Lung Injury in Preclinical Animal Models: A Systematic Review. PLoS ONE 2016, 11, e0147170. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.W.; Kuok, D.I.T.; Leung, C.Y.H.; Hui, K.P.Y.; Valkenburg, S.A.; Lau, E.H.Y.; Nicholls, J.M.; Fang, X.; Guan, Y.; Lee, J.W.; et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Shi, W.; Chen, C.; Shao, Y.; Zhu, L.; Lu, W.; Han, X. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res. Ther. 2016, 7, 159. [Google Scholar] [CrossRef]
- Loy, H.; Kuok, D.I.T.; Hui, K.P.Y.; Choi, M.H.L.; Yuen, W.; Nicholls, J.M.; Peiris, J.S.M.; Chan, M.C.W. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury. J. Infect. Dis. 2019, 219, 186–196. [Google Scholar] [CrossRef]
- Laffey, J.G.; Matthay, M.A. Fifty Years of Research in ARDS. Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am. J. Respir. Crit. Care Med. 2017, 196, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Hackstein, H.; Lippitsch, A.; Krug, P.; Schevtschenko, I.; Kranz, S.; Hecker, M.; Dietert, K.; Gruber, A.D.; Bein, G.; Brendel, C.; et al. Prospectively defined murine mesenchymal stem cells inhibit Klebsiella pneumoniae-induced acute lung injury and improve pneumonia survival. Respir. Res. 2015, 16, 123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef]
- Shigemura, N.; Okumura, M.; Mizuno, S.; Imanishi, Y.; Matsuyama, A.; Shiono, H.; Nakamura, T.; Sawa, Y. Lung tissue engineering technique with adipose stromal cells improves surgical outcome for pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2006, 174, 1199–1205. [Google Scholar] [CrossRef]
- Zhen, G.; Xue, Z.; Zhao, J.; Gu, N.; Tang, Z.; Xu, Y.; Zhang, Z. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy 2010, 12, 605–614. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, S.-Y.; Lee, J.H.; Lee, J.-S.; Van Ta, Q.; Kim, M.; Oh, Y.-M.; Lee, Y.-S.; Lee, S.-D. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L255–L266. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Liu, A.-R.; Qiu, H.-B.; Yang, Y. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem Cell Res. Ther. 2015, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.-J.; Song, L.; Han, F.-F.; Cui, Z.-L.; Chen, X.; Guo, X.-J.; Xu, W.-G. Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J. Cell. Biochem. 2013, 114, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Lan, Y.-W.; Chen, L.-G.; Huang, T.-T.; Choo, K.-B.; Cheng, W.T.K.; Lee, H.-S.; Chong, K.-Y. Mesenchymal stem cell-based HSP70 promoter-driven VEGFA induction by resveratrol alleviates elastase-induced emphysema in a mouse model. Cell Stress Chaperones 2015, 20, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Song, L.; Li, X.-M.; Wang, D.; Guo, X.-J.; Xu, W.-G. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci. Rep. 2015, 5, 8733. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Lee, J.-H.; Kim, H.J.; Park, M.K.; Huh, J.W.; Ro, J.Y.; Oh, Y.-M.; Lee, S.-D.; Lee, Y.-S. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L891–L908. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Yeung, S.C.; Liang, Y.; Liang, X.; Ding, Y.; Ip, M.S.M.; Tse, H.-F.; Mak, J.C.W.; Lian, Q. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am. J. Respir. Cell Mol. Biol. 2014, 51, 455–465. [Google Scholar] [CrossRef]
- Antunes, M.A.; Abreu, S.C.; Cruz, F.F.; Teixeira, A.C.; Lopes-Pacheco, M.; Bandeira, E.; Olsen, P.C.; Diaz, B.L.; Takyia, C.M.; Freitas, I.P.R.G.; et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir. Res. 2014, 15, 118. [Google Scholar] [CrossRef]
- Khedoe, P.P.S.J.; de Kleijn, S.; van Oeveren-Rietdijk, A.M.; Plomp, J.J.; de Boer, H.C.; van Pel, M.; Rensen, P.C.N.; Berbée, J.F.P.; Hiemstra, P.S. Acute and chronic effects of treatment with mesenchymal stromal cells on LPS-induced pulmonary inflammation, emphysema and atherosclerosis development. PLoS ONE 2017, 12, e0183741. [Google Scholar] [CrossRef]
- Poggio, H.A.; Antunes, M.A.; Rocha, N.N.; Kitoko, J.Z.; Morales, M.M.; Olsen, P.C.; Lopes-Pacheco, M.; Cruz, F.F.; Rocco, P.R.M. Impact of one versus two doses of mesenchymal stromal cells on lung and cardiovascular repair in experimental emphysema. Stem Cell Res. Ther. 2018, 9, 296. [Google Scholar] [CrossRef]
- Cho, J.W.; Park, K.S.; Bae, J.Y. Effects of Wharton’s jelly-derived mesenchymal stem cells on chronic obstructive pulmonary disease. Regen. Ther. 2019, 11, 207–211. [Google Scholar] [CrossRef]
- Cappetta, D.; de Angelis, A.; Spaziano, G.; Tartaglione, G.; Piegari, E.; Esposito, G.; Ciuffreda, L.P.; Liparulo, A.; Sgambato, M.; Russo, T.P.; et al. Lung Mesenchymal Stem Cells Ameliorate Elastase-Induced Damage in an Animal Model of Emphysema. Stem Cells Int. 2018, 2018, 9492038. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.S.; Johnstone, B.H.; Garrison, J.; Rush, N.I.; Cooper, S.; Traktuev, D.O.; Feng, D.; Adamowicz, J.J.; van Demark, M.; Fisher, A.J.; et al. Adipose Stem Cell Treatment in Mice Attenuates Lung and Systemic Injury Induced by Cigarette Smoking. Am. J. Respir. Crit. Care Med. 2010, 183, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Tesei, A. The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2019, 20, 3876. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Liu, M.; Zheng, J.; Wen, L.; Chen, Q.; Xiang, Z.; Lam, K.-T.; Liu, Y.; Chan, G.C.-F.; Lau, Y.-L.; et al. PD-1/PD-L1 Pathway Mediates the Alleviation of Pulmonary Fibrosis by Human Mesenchymal Stem Cells in Humanized Mice. Am. J. Respir. Cell Mol. Biol. 2018, 58, 684–695. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jang, A.-S.; Kim, Y.-E.; Cha, J.-Y.; Kim, T.-H.; Jung, S.; Park, S.-K.; Lee, Y.-K.; Won, J.-H.; Kim, Y.-H.; et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir. Res. 2010, 11, 16. [Google Scholar] [CrossRef]
- Reddy, M.; Fonseca, L.; Gowda, S.; Chougule, B.; Hari, A.; Totey, S. Human Adipose-derived Mesenchymal Stem Cells Attenuate Early Stage of Bleomycin Induced Pulmonary Fibrosis: Comparison with Pirfenidone. Int. J. Stem Cells 2016, 9, 192–206. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef]
- Cao, H.; Wang, C.; Chen, X.; Hou, J.; Xiang, Z.; Shen, Y.; Han, X. Inhibition of Wnt/β-catenin signaling suppresses myofibroblast differentiation of lung resident mesenchymal stem cells and pulmonary fibrosis. Sci. Rep. 2018, 8, 13644. [Google Scholar] [CrossRef]
- Rubio, G.A.; Elliot, S.J.; Wikramanayake, T.C.; Xia, X.; Pereira-Simon, S.; Thaller, S.R.; Glinos, G.D.; Jozic, I.; Hirt, P.; Pastar, I.; et al. Mesenchymal stromal cells prevent bleomycin-induced lung and skin fibrosis in aged mice and restore wound healing. J. Cell. Physiol. 2018, 233, 5503–5512. [Google Scholar] [CrossRef]
- Tashiro, J.; Elliot, S.J.; Gerth, D.J.; Xia, X.; Pereira-Simon, S.; Choi, R.; Catanuto, P.; Shahzeidi, S.; Toonkel, R.L.; Shah, R.H.; et al. Therapeutic Benefits of Young, But Not Old, Adipose-Derived Mesenchymal Stem Cells in a Chronic Mouse Model of Bleomycin-Induced Pulmonary Fibrosis. Transl. Res. 2015, 166, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yang, Y.; Zhang, J.; Ding, G.; Chen, S.; Peng, C.; Lavin, M.F.; Yeo, A.J.; Du, Z.; Shao, H. Efficacy of bone marrow mesenchymal stem cell transplantation in animal models of pulmonary fibrosis after exposure to bleomycin: A meta-analysis. Exp. Ther. Med. 2019, 17, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-h.; Liu, L.-j.; Lv, B.; Che, C.-l.; Fan, D.-p.; Wang, L.-f.; Zhang, Y.-m. Inhibition of bleomycin-induced pulmonary fibrosis by bone marrow-derived mesenchymal stem cells might be mediated by decreasing MMP9, TIMP-1, INF-γ and TGF-β. Cell Biochem. Funct. 2015, 33, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, Y.; Chang, P.; Gong, S.; Shao, L.; Dong, L. Mesenchymal stem cell-based therapy for radiation-induced lung injury. Stem Cell Res. Ther. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Rosso, L.; Zanella, A.; Righi, I.; Barilani, M.; Lazzari, L.; Scotti, E.; Gori, F.; Mendogni, P. Lung transplantation, ex-vivo reconditioning and regeneration: State of the art and perspectives. J. Thorac. Dis. 2018, 10, S2423–S2430. [Google Scholar] [CrossRef] [PubMed]
- Tiono, J.; Surate Solaligue, D.E.; Mižíková, I.; Nardiello, C.; Vadász, I.; Böttcher-Friebertshäuser, E.; Ehrhardt, H.; Herold, S.; Seeger, W.; Morty, R.E. Mouse genetic background impacts susceptibility to hyperoxia-driven perturbations to lung maturation. Pediatr. Pulmonol. 2019, 54, 1060–1077. [Google Scholar] [CrossRef] [PubMed]
- Srour, N.; Thébaud, B. Mesenchymal Stromal Cells in Animal Bleomycin Pulmonary Fibrosis Models: A Systematic Review. Stem Cells Transl. Med. 2015, 4, 1500–1510. [Google Scholar] [CrossRef]
- Li, L.; Jin, S.; Zhang, Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int. J. Clin. Exp. Med. 2015, 8, 3825–3832. [Google Scholar]
- Zheng, G.; Huang, L.; Tong, H.; Shu, Q.; Hu, Y.; Ge, M.; Deng, K.; Zhang, L.; Zou, B.; Cheng, B.; et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: A randomized, placebo-controlled pilot study. Respir. Res. 2014, 15, 39. [Google Scholar] [CrossRef]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Paspaliaris, V.; Koliakos, G.; Ntolios, P.; Bouros, E.; Oikonomou, A.; Zissimopoulos, A.; Boussios, N.; Dardzinski, B.; Gritzalis, D.; et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Ntolios, P.; Manoloudi, E.; Tzouvelekis, A.; Bouros, E.; Steiropoulos, P.; Anevlavis, S.; Bouros, D.; Froudarakis, M.E. Longitudinal outcomes of patients enrolled in a phase Ib clinical trial of the adipose-derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Clin. Respir. J. 2018, 12, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients with Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017, 151, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Enever, D.; Ilic, N.; Sparks, L.; Whitelaw, K.; Ayres, J.; Yerkovich, S.T.; Khalil, D.; Atkinson, K.M.; Hopkins, P.M.A. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 2014, 19, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.E.; Kim, G.-H.J.; Kyeong, N.-Y.; Goldin, J.G.; Glassberg, M.K. Intravenous stem cell dose and changes in quantitative lung fibrosis and DLCO in the AETHER trial: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7568–7572. [Google Scholar]
- Ribeiro-Paes, J.T.; Bilaqui, A.; Greco, O.T.; Ruiz, M.A.; Marcelino, M.Y.; Stessuk, T.; de Faria, C.A.; Lago, M.R. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 63–71. [Google Scholar] [CrossRef]
- Weiss, D.J.; Casaburi, R.; Flannery, R.; LeRoux-Williams, M.; Tashkin, D.P. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 2013, 143, 1590–1598. [Google Scholar] [CrossRef]
- Stolk, J.; Broekman, W.; Mauad, T.; Zwaginga, J.J.; Roelofs, H.; Fibbe, W.E.; Oostendorp, J.; Bajema, I.; Versteegh, M.I.M.; Taube, C.; et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM 2016, 109, 331–336. [Google Scholar] [CrossRef]
- De Oliveira, H.G.; Cruz, F.F.; Antunes, M.A.; de Macedo Neto, A.V.; Oliveira, G.A.; Svartman, F.M.; Borgonovo, T.; Rebelatto, C.L.K.; Weiss, D.J.; Brofman, P.R.S.; et al. Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. Stem Cells Transl. Med. 2017, 6, 962–969. [Google Scholar] [CrossRef]
- Armitage, J.; Tan, D.B.A.; Troedson, R.; Young, P.; Lam, K.-V.; Shaw, K.; Sturm, M.; Weiss, D.J.; Moodley, Y.P. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: A phase I pilot study. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J. Pediatr. 2014, 164, 966.e6–972.e6. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Kim, J.H.; Sung, S.I.; Park, W.S. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J. Pediatr. 2017, 185, 49.e2–54.e2. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Enever, D.; Lawrence, S.; Sturm, M.J.; Herrmann, R.; Yerkovich, S.; Musk, M.; Hopkins, P.M.A. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl. Med. 2017, 6, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, K.M.; Karagiannis, K.; Tsitoura, E.; Bibaki, E.; Lasithiotaki, I.; Proklou, A.; Spandidos, D.A.; Tzanakis, N. Clinical applications of mesenchymal stem cells in chronic lung diseases. Biomed. Rep. 2018, 8, 314–318. [Google Scholar] [CrossRef]
- Kadyk, L.C.; DeWitt, N.D.; Gomperts, B. Proceedings: Regenerative Medicine for Lung Diseases: A CIRM Workshop Report. Stem Cells Transl. Med. 2017, 6, 1823–1828. [Google Scholar] [CrossRef]
- Geiger, S.; Hirsch, D.; Hermann, F.G. Cell therapy for lung disease. Eur. Respir. Rev. 2017, 26. [Google Scholar] [CrossRef]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Giuliani, M.; Bennaceur-Griscelli, A.; Nanbakhsh, A.; Oudrhiri, N.; Chouaib, S.; Azzarone, B.; Durrbach, A.; Lataillade, J.-J. TLR ligands stimulation protects MSC from NK killing. Stem Cells 2014, 32, 290–300. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Youn Oh, J. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol. Ther. 2011, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bustos, M.L.; Huleihel, L.; Meyer, E.M.; Donnenberg, A.D.; Donnenberg, V.S.; Sciurba, J.D.; Mroz, L.; McVerry, B.J.; Ellis, B.M.; Kaminski, N.; et al. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl. Med. 2013, 2, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Broekman, W.; Khedoe, P.P.S.J.; Schepers, K.; Roelofs, H.; Stolk, J.; Hiemstra, P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax 2018, 73, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.; Paredes, B.D.; Araújo, I.M.; Lopes-Pacheco, M.; Oliveira, M.V.; Suhett, G.D.; Faccioli, L.A.P.; Assis, E.; Castro-Faria-Neto, H.C.; Goldenberg, R.C.S.; et al. Effects of bone marrow-derived mononuclear cells from healthy or acute respiratory distress syndrome donors on recipient lung-injured mice. Crit. Care Med. 2014, 42, e510–e524. [Google Scholar] [CrossRef]
- Antebi, B.; Walker, K.P.; Mohammadipoor, A.; Rodriguez, L.A.; Montgomery, R.K.; Batchinsky, A.I.; Cancio, L.C. The effect of acute respiratory distress syndrome on bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 251. [Google Scholar] [CrossRef]
- Islam, D.; Huang, Y.; Fanelli, V.; Delsedime, L.; Wu, S.; Khang, J.; Han, B.; Grassi, A.; Li, M.; Xu, Y.; et al. Identification and Modulation of Microenvironment Is Crucial for Effective Mesenchymal Stromal Cell Therapy in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2019, 199, 1214–1224. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Chiang, C.-H.; Hung, S.-C.; Chian, C.-F.; Tsai, C.-L.; Chen, W.-C.; Zhang, H. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS ONE 2017, 12, e0187637. [Google Scholar] [CrossRef]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics 2017, 7, 106–116. [Google Scholar] [CrossRef]
- Moodley, Y.; Vaghjiani, V.; Chan, J.; Baltic, S.; Ryan, M.; Tchongue, J.; Samuel, C.S.; Murthi, P.; Parolini, O.; Manuelpillai, U. Anti-inflammatory effects of adult stem cells in sustained lung injury: A comparative study. PLoS ONE 2013, 8, e69299. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Wang, J.; Fu, Z.; Wang, J.; Liu, M.; Ren, D.; Yu, B.; Zheng, L.; Hu, X.; et al. Immunomodulation by mesenchymal stem cells in treating human autoimmune disease-associated lung fibrosis. Stem Cell Res. Ther. 2016, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, A.; Toonkel, R.; Karampitsakos, T.; Medapalli, K.; Ninou, I.; Aidinis, V.; Bouros, D.; Glassberg, M.K. Mesenchymal Stem Cells for the Treatment of Idiopathic Pulmonary Fibrosis. Front. Med. 2018, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef] [PubMed]
- Bustos, M.L.; Huleihel, L.; Kapetanaki, M.G.; Lino-Cardenas, C.L.; Mroz, L.; Ellis, B.M.; McVerry, B.J.; Richards, T.J.; Kaminski, N.; Cerdenes, N.; et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am. J. Respir. Crit. Care Med. 2014, 189, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Chen, M.; Ren, S.; Cheng, P.; Zhang, H. miR-301b~miR-130b-PPARγ axis underlies the adipogenic capacity of mesenchymal stem cells with different tissue origins. Sci. Rep. 2017, 7, 1160. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Copland, I.B.; Yuan, S.; Romieu-Mourez, R.; Waller, E.K.; Galipeau, J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 2012, 14, 147–152. [Google Scholar] [CrossRef]
- Xu, J.; Qu, J.; Cao, L.; Sai, Y.; Chen, C.; He, L.; Yu, L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J. Pathol. 2008, 214, 472–481. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Gao, X.; Li, C.; Liang, Z.; Yu, L.; Li, Y.; Xiao, X.; Chen, L. Keratinocyte growth factor gene delivery via mesenchymal stem cells protects against lipopolysaccharide-induced acute lung injury in mice. PLoS ONE 2013, 8, e83303. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Xu, X.; Liu, A.; He, H.; Xu, J.; Chen, Q.; Liu, S.; Liu, L.; Qiu, H.; et al. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res. Ther. 2016, 7, 66. [Google Scholar] [CrossRef]
- Mei, S.H.J.; McCarter, S.D.; Deng, Y.; Parker, C.H.; Liles, W.C.; Stewart, D.J. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007, 4, e269. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, S.; Tang, L.; Ma, L.; Wang, F.; Feng, H.; Meng, J.; Han, Z. Mesenchymal stem cells overexpressing heme oxygenase-1 ameliorate lipopolysaccharide-induced acute lung injury in rats. J. Cell. Physiol. 2019, 234, 7301–7319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, S.; Xu, X.; Li, J.; Liu, A.; Han, J.; Liu, S.; Liu, L.; Qiu, H. The Vascular Endothelial Growth Factors-Expressing Character of Mesenchymal Stem Cells Plays a Positive Role in Treatment of Acute Lung Injury In Vivo. Mediat. Inflamm. 2016, 2016, 2347938. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mo, M.; Wang, J.; Sadia, S.; Shi, B.; Fu, X.; Yu, L.; Tredget, E.E.; Wu, Y. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell. Mol. Life Sci. 2018, 75, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Luo, Y.-M.; Xiong, W.; Ding, W.; Li, Y.-R.; Zhao, W.; Zeng, H.-Z.; Gao, H.-C.; Wu, X.-L. Mesenchymal stem cell-based FGF2 gene therapy for acute lung injury induced by lipopolysaccharide in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 857–865. [Google Scholar]
- Yang, J.-X.; Zhang, N.; Wang, H.-W.; Gao, P.; Yang, Q.-P.; Wen, Q.-P. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J. Biol. Chem. 2015, 290, 1994–2006. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-x.; Liu, A.-R.; Chen, S.; He, H.-l.; Chen, Q.-H.; Xu, J.-y.; Pan, C.; Yang, Y.; Guo, F.-m.; Huang, Y.-z.; et al. Activation of Wnt/β-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res. Ther. 2015, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-x.; Liu, A.-R.; Chen, S.; He, H.-l.; Chen, Q.-H.; Xu, J.-y.; Pan, C.; Yang, Y.; Guo, F.-m.; Huang, Y.-z.; et al. The Orphan Receptor Tyrosine Kinase ROR2 Facilitates MSCs to Repair Lung Injury in ARDS Animal Model. Cell Transplant. 2016, 25, 1561–1574. [Google Scholar] [CrossRef]
- Wang, C.; Lv, D.; Zhang, X.; Ni, Z.-A.; Sun, X.; Zhu, C. Interleukin-10-Overexpressing Mesenchymal Stromal Cells Induce a Series of Regulatory Effects in the Inflammatory System and Promote the Survival of Endotoxin-Induced Acute Lung Injury in Mice Model. DNA Cell Biol. 2018, 37, 53–61. [Google Scholar] [CrossRef]
- Jerkic, M.; Masterson, C.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Otulakowski, G.; Zhang, H.; Kavanagh, B.P.; Laffey, J.G. Overexpression of IL-10 Enhances the Efficacy of Human Umbilical-Cord-Derived Mesenchymal Stromal Cells in E. coli Pneumosepsis. J. Clin. Med. 2019, 8, 847. [Google Scholar] [CrossRef]
- Reiter, J.; Drummond, S.; Sammour, I.; Huang, J.; Florea, V.; Dornas, P.; Hare, J.M.; Rodrigues, C.O.; Young, K.C. Stromal derived factor-1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir. Res. 2017, 18, 137. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Steens, J.; Wiesemann, A.; Schulz, F.; Kaschani, F.; Röck, K.; Yamaguchi, M.; Wirsdörfer, F.; Kaiser, M.; Fischer, J.W.; et al. Mesenchymal Stem Cell Therapy Protects Lungs from Radiation-Induced Endothelial Cell Loss by Restoring Superoxide Dismutase 1 Expression. Antioxid. Redox Signal. 2017, 26, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-X.; Xiang, H.; Xu, W.-H.; Li, M.; Yuan, J.; Liu, J.; Sun, W.-J.; Zhang, R.; Li, J.; Ren, Z.-Q.; et al. Manganese Superoxide Dismutase Gene-Modified Mesenchymal Stem Cells Attenuate Acute Radiation-Induced Lung Injury. Hum. Gene Ther. 2017, 28, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, W.; Ma, L.; Liu, Y.; Zhang, X.; Wang, S. Nrf2 transfection enhances the efficacy of human amniotic mesenchymal stem cells to repair lung injury induced by lipopolysaccharide. J. Cell. Biochem. 2018, 119, 1627–1636. [Google Scholar] [CrossRef]
- Tsoyi, K.; Hall, S.R.R.; Dalli, J.; Colas, R.A.; Ghanta, S.; Ith, B.; Coronata, A.; Fredenburgh, L.E.; Baron, R.M.; Choi, A.M.K.; et al. Carbon Monoxide Improves Efficacy of Mesenchymal Stromal Cells During Sepsis by Production of Specialized Proresolving Lipid Mediators*. Crit. Care Med. 2016, 44, e1236–e1245. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Lim, H.; Liu, A.; Hu, S.; Lee, J.; Zhuo, H.; Hao, Q.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell Microvesicles in an Ex Vivo Perfused Human Lung Injured with Severe E. coli Pneumonia. Thorax 2018, 74, 43–50. [Google Scholar] [CrossRef]
- Lan, Y.-W.; Yang, J.-C.; Yen, C.-C.; Huang, T.-T.; Chen, Y.-C.; Chen, H.-L.; Chong, K.-Y.; Chen, C.-M. Predifferentiated amniotic fluid mesenchymal stem cells enhance lung alveolar epithelium regeneration and reverse elastase-induced pulmonary emphysema. Stem Cell Res. Ther. 2019, 10, 163. [Google Scholar] [CrossRef]
- Lan, Y.-W.; Choo, K.-B.; Chen, C.-M.; Hung, T.-H.; Chen, Y.-B.; Hsieh, C.-H.; Kuo, H.-P.; Chong, K.-Y. Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res. Ther. 2015, 6, 97. [Google Scholar] [CrossRef]
- Lan, Y.-W.; Theng, S.-M.; Huang, T.-T.; Choo, K.-B.; Chen, C.-M.; Kuo, H.-P.; Chong, K.-Y. Oncostatin M-Preconditioned Mesenchymal Stem Cells Alleviate Bleomycin-Induced Pulmonary Fibrosis Through Paracrine Effects of the Hepatocyte Growth Factor. Stem Cells Transl. Med. 2017, 6, 1006–1017. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, Y.-S.; Hong, S.-H.; Oh, Y.-M. Therapeutic effects of adipose-derived stem cells pretreated with pioglitazone in an emphysema mouse model. Exp. Mol. Med. 2016, 48, e266. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, H.; Zhou, W.-G.; Guo, X.-C.; Wu, M.-J.; Xu, Z.-Y.; Jiang, J.-f.; Shen, C.; Liu, H.-Q. N-acetylcysteine-pretreated human embryonic mesenchymal stem cell administration protects against bleomycin-induced lung injury. Am. J. Med. Sci. 2013, 346, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, D.; Gong, W.; Zhao, G.; Liu, L.; Yang, L.; Hou, Y. The toll-like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem Cells 2014, 32, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.C.; Lopes-Pacheco, M.; da Silva, A.L.; Xisto, D.G.; de Oliveira, T.B.; Kitoko, J.Z.; de Castro, L.L.; Amorim, N.R.; Martins, V.; Silva, L.H.A.; et al. Eicosapentaenoic Acid Enhances the Effects of Mesenchymal Stromal Cell Therapy in Experimental Allergic Asthma. Front. Immunol. 2018, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Pan, Y.-Y.; Jing, R.-S.; Luan, Y.; Zhang, L.; Sun, C.; Kong, F.; Li, K.-L.; Wang, Y.-B. Protective effects of BMSCs in combination with erythropoietin in bronchopulmonary dysplasia-induced lung injury. Mol Med Report 2016, 14, 1302–1308. [Google Scholar] [CrossRef][Green Version]
- Han, X.-P.; Zhang, F.-Q.; Tan, X.-S.; Liu, L.; Ma, W.-X.; Ou-Yang, H.-F.; Wu, C.-G. EPO modified MSCs can inhibit asthmatic airway remodeling in an animal model. J. Cell. Biochem. 2018, 119, 1008–1016. [Google Scholar] [CrossRef]
- Chen, C.-M.; Chou, H.-C.; Lin, W.; Tseng, C. Surfactant effects on the viability and function of human mesenchymal stem cells: In vitro and in vivo assessment. Stem Cell Res. Ther. 2017, 8, 180. [Google Scholar] [CrossRef][Green Version]
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J. Clin. Med. 2018, 7, 355. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl. Med. 2015, 4, 1302–1316. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 26. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Antebi, B.; Batchinsky, A.I.; Cancio, L.C. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir. Res. 2018, 19, 218. [Google Scholar] [CrossRef]
- Chaubey, S.; Thueson, S.; Ponnalagu, D.; Alam, M.A.; Gheorghe, C.P.; Aghai, Z.; Singh, H.; Bhandari, V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res. Ther. 2018, 9, 173. [Google Scholar] [CrossRef]
- Ionescu, L.I.; Alphonse, R.S.; Arizmendi, N.; Morgan, B.; Abel, M.; Eaton, F.; Duszyk, M.; Vliagoftis, H.; Aprahamian, T.R.; Walsh, K.; et al. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am. J. Respir. Cell Mol. Biol. 2012, 46, 207–216. [Google Scholar] [CrossRef]
- Waszak, P.; Alphonse, R.; Vadivel, A.; Ionescu, L.; Eaton, F.; Thébaud, B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012, 21, 2789–2797. [Google Scholar] [CrossRef]
- Shentu, T.-P.; Huang, T.-S.; Cernelc-Kohan, M.; Chan, J.; Wong, S.S.; Espinoza, C.R.; Tan, C.; Gramaglia, I.; van der Heyde, H.; Chien, S.; et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci. Rep. 2017, 7, 18052. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.-G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.-J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef]
- Hao, Q.; Gudapati, V.; Monsel, A.; Park, J.H.; Hu, S.; Kato, H.; Lee, J.H.; Zhou, L.; He, H.; Lee, J.W. Mesenchymal Stem Cell-Derived Extracellular Vesicles Decrease Lung Injury in Mice. J. Immunol. 2019, 203, 1961–1972. [Google Scholar] [CrossRef]
- Porzionato, A.; Zaramella, P.; Dedja, A.; Guidolin, D.; van Wemmel, K.; Macchi, V.; Jurga, M.; Perilongo, G.; de Caro, R.; Baraldi, E.; et al. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L6–L19. [Google Scholar] [CrossRef]
- Kennelly, H.; Mahon, B.P.; English, K. Human mesenchymal stromal cells exert HGF dependent cytoprotective effects in a human relevant pre-clinical model of COPD. Sci. Rep. 2016, 6, 38207. [Google Scholar] [CrossRef]
- De Castro, L.L.; Xisto, D.G.; Kitoko, J.Z.; Cruz, F.F.; Olsen, P.C.; Redondo, P.A.G.; Ferreira, T.P.T.; Weiss, D.J.; Martins, M.A.; Morales, M.M.; et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017, 8, 151. [Google Scholar] [CrossRef]
- Gennai, S.; Monsel, A.; Hao, Q.; Park, J.; Matthay, M.A.; Lee, J.W. Microvesicles Derived from Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transplant 2015, 15, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R.M. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 2020, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Sun, F.; Liu, J.; Ding, T.; She, J.; Mao, F.; Xu, W.; Qian, H.; Yan, Y. Emerging Role of Mesenchymal Stem Cell-derived Exosomes in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2019, 14, 482–494. [Google Scholar] [CrossRef]
- Tang, X.-D.; Shi, L.; Monsel, A.; Li, X.-Y.; Zhu, H.-L.; Zhu, Y.-G.; Qu, J.-M. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells 2017, 35, 1849–1859. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, J.-Y.; Cho, R.; Shin, D.-M.; Lee, S.W.; Oh, Y.-M. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp. Mol. Med. 2017, 49, e284. [Google Scholar] [CrossRef]
- Huang, R.; Qin, C.; Wang, J.; Hu, Y.; Zheng, G.; Qiu, G.; Ge, M.; Tao, H.; Shu, Q.; Xu, J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY) 2019, 11, 7996–8014. [Google Scholar] [CrossRef]
- Koritzinsky, E.H.; Street, J.M.; Star, R.A.; Yuen, P.S.T. Quantification of Exosomes. J. Cell. Physiol. 2017, 232, 1587–1590. [Google Scholar] [CrossRef]
- Lignelli, E.; Palumbo, F.; Myti, D.; Morty, R.E. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L832–L887. [Google Scholar] [CrossRef]
- Caverly, L.J.; Huang, Y.J.; Sze, M.A. Past, Present, and Future Research on the Lung Microbiome in Inflammatory Airway Disease. Chest 2019, 156, 376–382. [Google Scholar] [CrossRef]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Göpel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The Microbiome and Preterm Birth: A Change in Paradigm with Profound Implications for Pathophysiologic Concepts and Novel Therapeutic Strategies. Biomed Res. Int. 2018, 2018, 7218187. [Google Scholar] [CrossRef]
| Experimental Lung Disease Model | Species | Cell Source | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| hyperoxia | rat | UCB vs. AT vs. MNC | human | 5 × 105 | i.t. | d5 | no | UC MSC better preserve lung structure than AT or MNC MSC | higher HGF and VEGFA production in BM MSC, only BM MSC attenuate impaired angiogenesis, cell death induction, macrophage influx and pro-inflammatory cytokine production | [42] |
| hyperoxia | rat | UCB | human | 5 × 105 2 × 106 | i.t. i.v. | d5 | no | reduction of lung inflammation | intratracheal application more efficient than intravenous injection | [43] |
| hyperoxia | rat | BM | rat | 1 × 106 | i.t. | d7 | no | improved alveolarization and vascular density, reduced pulmonary hypertension | MSC from female donors stronger impact on vascular development, maybe the most potent MSC population for lung repair in severe BPD | [37] |
| hyperoxia | mice | BM | human | 2.5 × 105 | i.t. | d4 | no | attenuated structural damage and lung fibrosis until adulthood | shift in macrophage populations towards an anti-inflammatory phenotype | [38] |
| hyperoxia | mice | BM | mice | 5 × 104 | i.v. | d4 | no | cytoprotective effects and attenuation of lung injury | paracrine MSC reaction via the release of immunomodulatory factors to ameliorate the parenchymal and vascular injury | [34] |
| hyperoxia | rat | BM | rat | 1 × 105 | i.t. | d4 | no | improved survival and exercise tolerance while attenuating alveolar and lung vascular injury and pulmonary hypertension | BM MSC prevent arrested alveolar and vascular growth in part through paracrine activity | [35] |
| Experimental lung Disease model | Species | Cell Source | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| house dust mite | mice | BM | mice | 1 × 106 | i.v. | d14 | no | reduced airway hyperresponsiveness | reduction of eosinophilia, Th2 response and activated dendritic cells | [48] |
| ovalbumin | mice | BM | mice | 1 × 106 | i.v. | d19 | no | reduced pulmonary inflammation | reduced attraction of lymphocytes and eosinophils to the lung, suppression of lung dendritic cell maturation and Th2 response | [49] |
| ovalbumin | mice | BM | mice | 1 × 106 | i.v. | d67 | no | combination therapy of MSC plus simvastatin potentiates anti-inflammatory effects and suppression of lung airway remodeling | reduced recruitment of neutrophils and eosinophils, goblet cell hyperplasia and lung fibrosis | [50] |
| house dust mite | mice | BM | mice | 5 × 105 | i.v. | d27 | no | decreased airway responsiveness, reduced eosinophil and neutrophil influx, normalized lung function | MSC express high levels of COX, M2 macrophages high levels of IL-10 and TGF-β and low level of IL-6 | [55] |
| ovalbumin | mice | UCB | human | 5 × 105 | i.v. | d14 | no | decreased airway hyperresponsiveness, inflammatory cell infiltration and Th2 cytokine production while percentage of Tregs was increased | MSC treatment reduces allergic inflammation, which could be mediated by regulatory T cells | [51] |
| ovalbumin | mice | BM vs. AT | mice | 1 × 105 | i.t. | d22 | no | reduction of lung parenchymal inflammation and inflammatory profile of alveolar macrophages | therapeutic efficiency only after treatment with BM MSC | [54] |
| ovalbumin | rat | PD | human | 1 × 106/kg | i.v. | d7 | no | inflammatory cell infiltration and goblet cell hyperplasia were markedly decreased | shift from Notch-1, -2 and jagged-1 to Notch-3, -4 and delta-like ligand-4 signaling | [57] |
| ovalbumin | mice | iPSC vs. BM | human | 1 × 106 | i.v. | d0 (iMR90-iPSC) + d20 (all) | yes | inhibition of inflammatory cell infiltration and mucus production, reduction in eosinophil infiltration, and a decrease in inflammatory cell infiltration | iPSC-MSC same therapeutic effect as BM MSC | [44] |
| ragweed | mice | BM | mice | n.a. | i.v. | d14 | no | inhibition of eosinophil infiltration, decreased levels of Th2 cytokines and immunoglobulins, IL-4 and/or IL-13 activate the STAT6 pathway in the BMSCs resulting in an increase of their TGF-β production | BM MSC suppress Th2-driven allergic responses by TFG- β production | [45] |
| house dust mite | mice | BM | mice | 1 × 105 | i.t. | d22 | no | reducing levels of IL-4, IL-13, and eotaxin, increased mRNA expressions of TGF-β1, IFN-γ, IL-10, TSG-6, IDO-1, and IL-1RN and induced M2 macrophage polarization | reduction of inflammation and remodeling, as well as improvement in lung function | [58] |
| Experimental lung Disease Model | Species | Cell Source | MSC Species | Dose (Cells) | Application Route | Time Point of Application | Repeated Application | Biological Function In Vivo | Molecular Changes/Results | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| LPS | rat | BM | rat | 5 × 105 | i.v. | 4 h p.i. | no | reduction of lung edema | RNA interference against KGF abrogated the MSC effect | [65] |
| Klebsiella | mice | (PαS) BM | mice | 1 × 106 | i.v. | 4 h p.i. | no | reduced alveolitis and lung edema | attenuated neutrophil, T cell and dendritic cell influx | [77] |
| Pseudomonas aeruginosa | mice | AT | mice | 1 × 105 vs. 1 × 106 | i.t. | 1 h p.i. | no | reduced inflammation, neutrophil accumulation, bacterial burden and lung injury | protective effects only be achieved at high dose instillation, inhibition of prostaglandin E2 production by IGF-1, improved bacterial properties and phagocytosis activity in macrophages | [23] |
| E. coli | rat | BM | human | 1 × 107 vs. 2 × 107 vs. 5 × 106 vs. 2 × 106/kg | i.v. vs. i.t. | 30 min p.i. | no | intratracheal application as effective as intravenous application, reduced efficiency of cryopreserved cells, best balance between efficacy and dose was seen at 1 × 107 hMSCs/kg | increased macrophage phagocytosis capacity and LL-37 secretion | [67] |
| Influenza | mice | BM | human | 5 × 105 | i.v. | 5 d p.i. | no | reduced impairment of alveolar fluid clearance and attenuated lung injury | effects were mediated by infected cells’ release of soluble factors that down-regulate the sodium and chloride transporters | [73] |
| Influenza | mice | BM | mice | 1 × 105 | i.v. | 30 min p.i. | no | reduced lung injury, pro-inflammatory cytokine production, inflammatory cell recruitment and lung edema | reduction of JNK and ERK phosphorylation | [74] |
| E. coli | mice | BM | human | 1 × 106 | i.v. vs. i.n. | 4 h p.i. | no | beneficial effects centrally exerted by enhanced alveolar macrophage phagocytosis which is stipulated by mitochondria transfer via nanotube structures | macrophage depletion abolished the beneficial MSC effects, i.v. route could be more beneficial | [68] |
| E. coli | rat | BM | human | 1 × 107/kg | i.v. | 30 min p.i. | no | reduction in bacterial load and lung inflammation, attenuated lung injury, potential to enhance epithelial wound repair | CD362+ MSC account for the therapeutic efficiency | [70] |
| influenza | mice | UCB vs. BM | human | 5 × 105 | i.v. | 5 d p.i. | no | improved outcome for umbilical cord MSC compared to BM MSC | umbilical cord MSC improved growth factor release of ANGPT-1 and HGF | [75] |
| E. coli | mice | BM | mice | 75 × 104 | i.t. | 4 h p.i. | no | down-regulation of proinflammatory responses, reducing TNF-α and MIP-2 and increasing the anti-inflammatory cytokine IL-10 | BM MSC decrease the severity of endotoxin-induced ALI and improve survival | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.-M.; Böttcher-Friebertshäuser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies—New Perspectives for the Injured Lung. J. Clin. Med. 2020, 9, 682. https://doi.org/10.3390/jcm9030682
Behnke J, Kremer S, Shahzad T, Chao C-M, Böttcher-Friebertshäuser E, Morty RE, Bellusci S, Ehrhardt H. MSC Based Therapies—New Perspectives for the Injured Lung. Journal of Clinical Medicine. 2020; 9(3):682. https://doi.org/10.3390/jcm9030682
Chicago/Turabian StyleBehnke, Judith, Sarah Kremer, Tayyab Shahzad, Cho-Ming Chao, Eva Böttcher-Friebertshäuser, Rory E. Morty, Saverio Bellusci, and Harald Ehrhardt. 2020. "MSC Based Therapies—New Perspectives for the Injured Lung" Journal of Clinical Medicine 9, no. 3: 682. https://doi.org/10.3390/jcm9030682
APA StyleBehnke, J., Kremer, S., Shahzad, T., Chao, C.-M., Böttcher-Friebertshäuser, E., Morty, R. E., Bellusci, S., & Ehrhardt, H. (2020). MSC Based Therapies—New Perspectives for the Injured Lung. Journal of Clinical Medicine, 9(3), 682. https://doi.org/10.3390/jcm9030682







