Current and Future Aspects of Multimodal Imaging, Diagnostic, and Treatment Strategies in Bicuspid Aortic Valve and Associated Aortopathies
Abstract
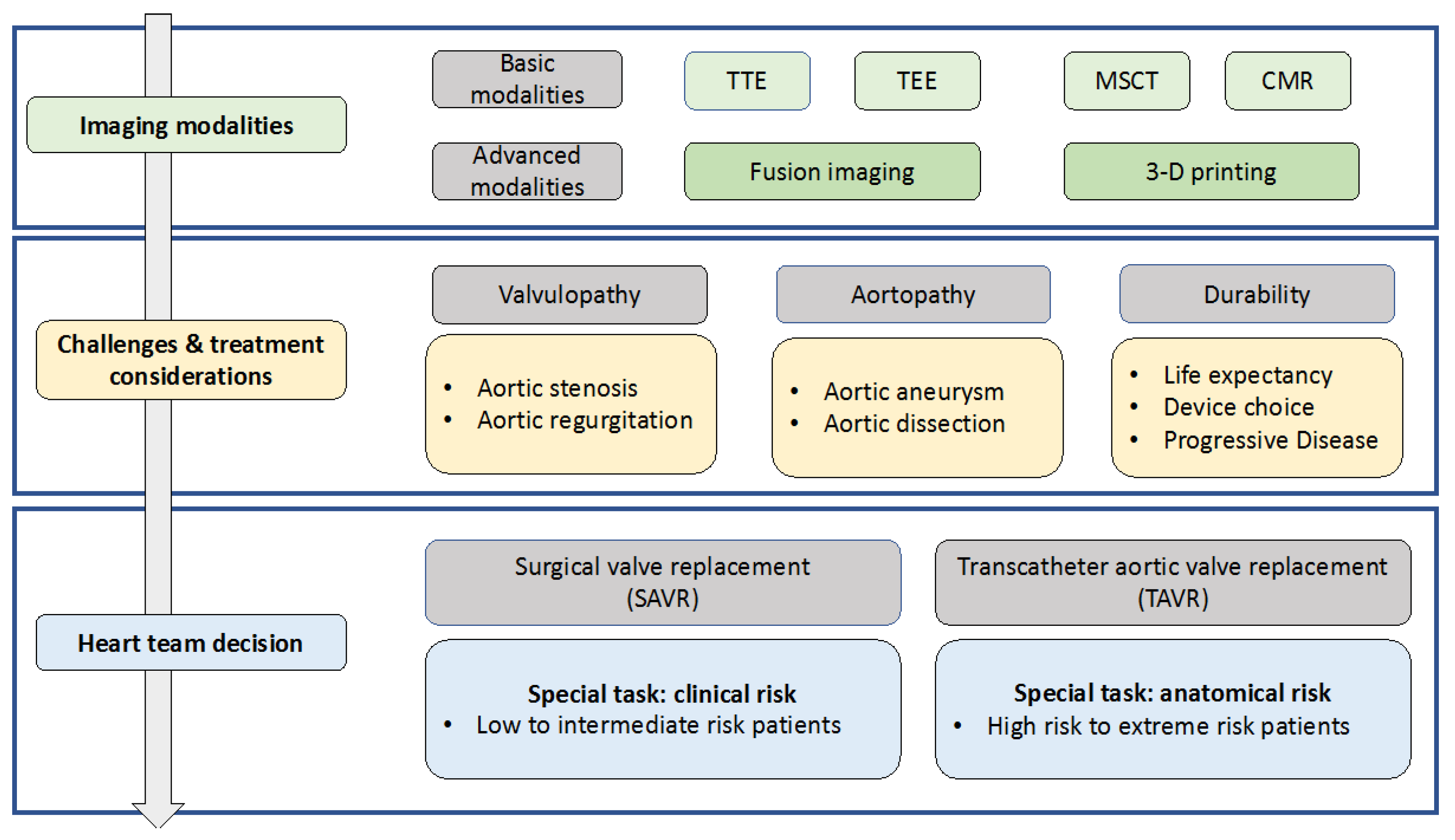
1. Introduction
2. Diagnostic Approaches
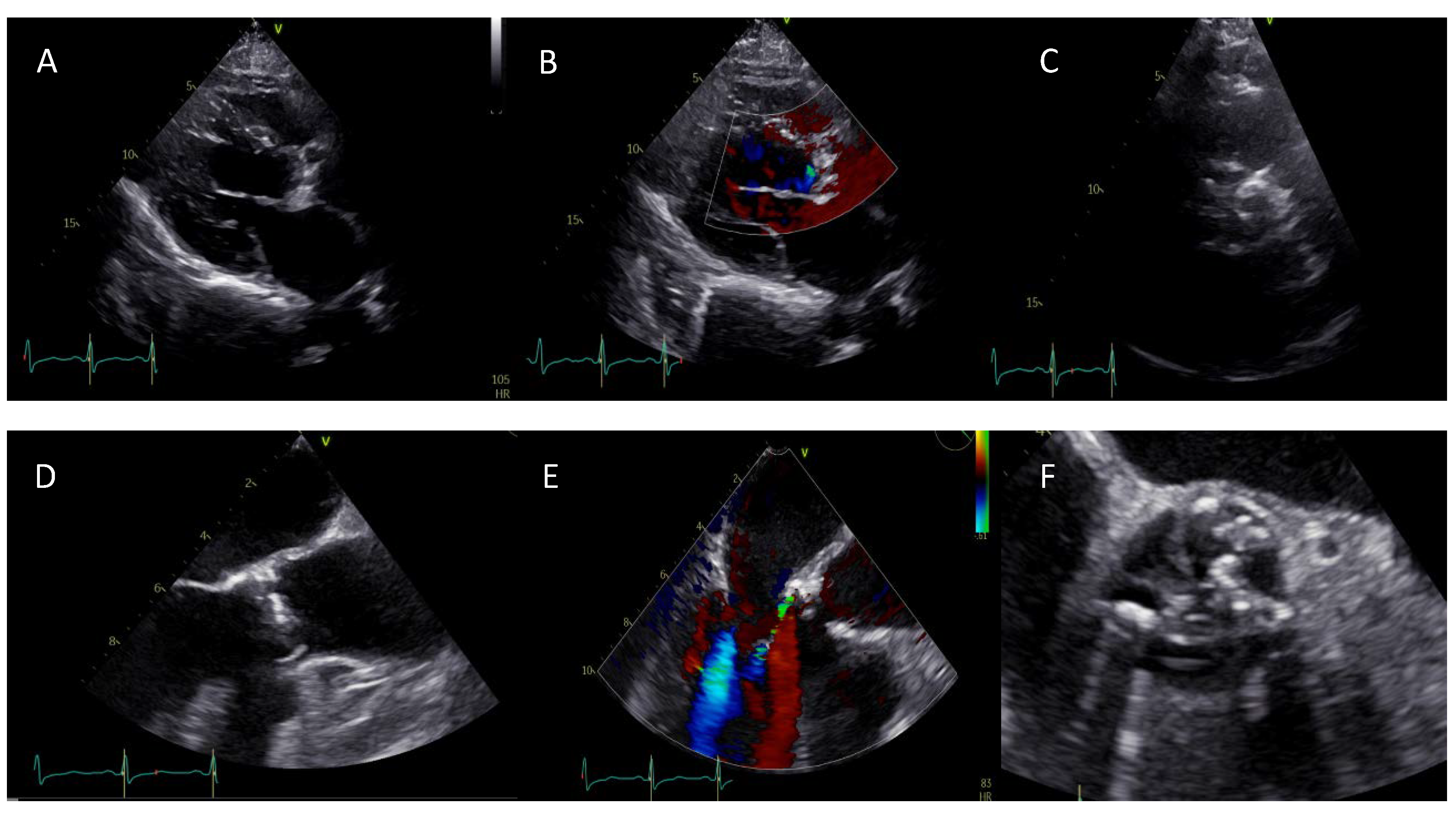
2.1. Aortic Valve Morphology and Function
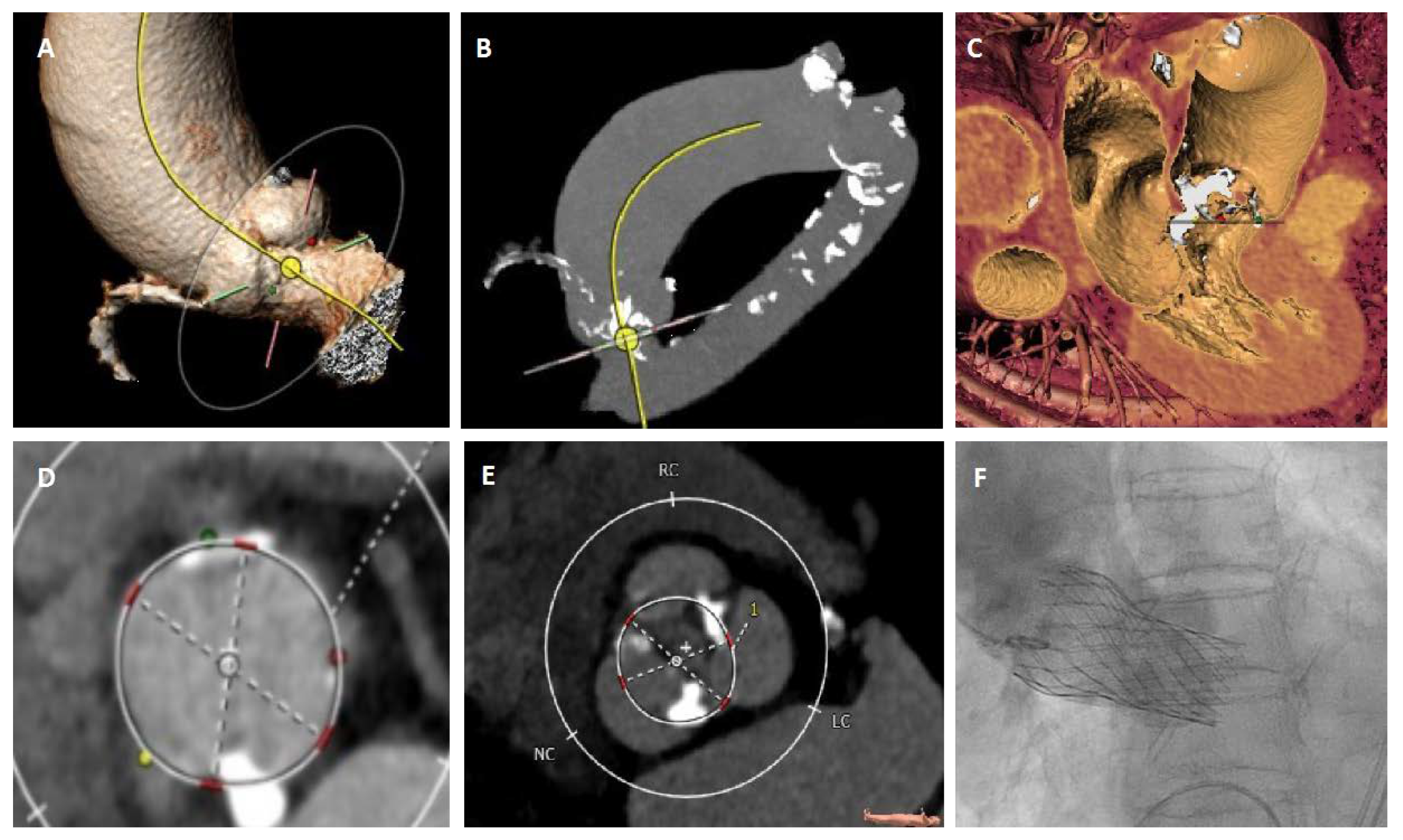
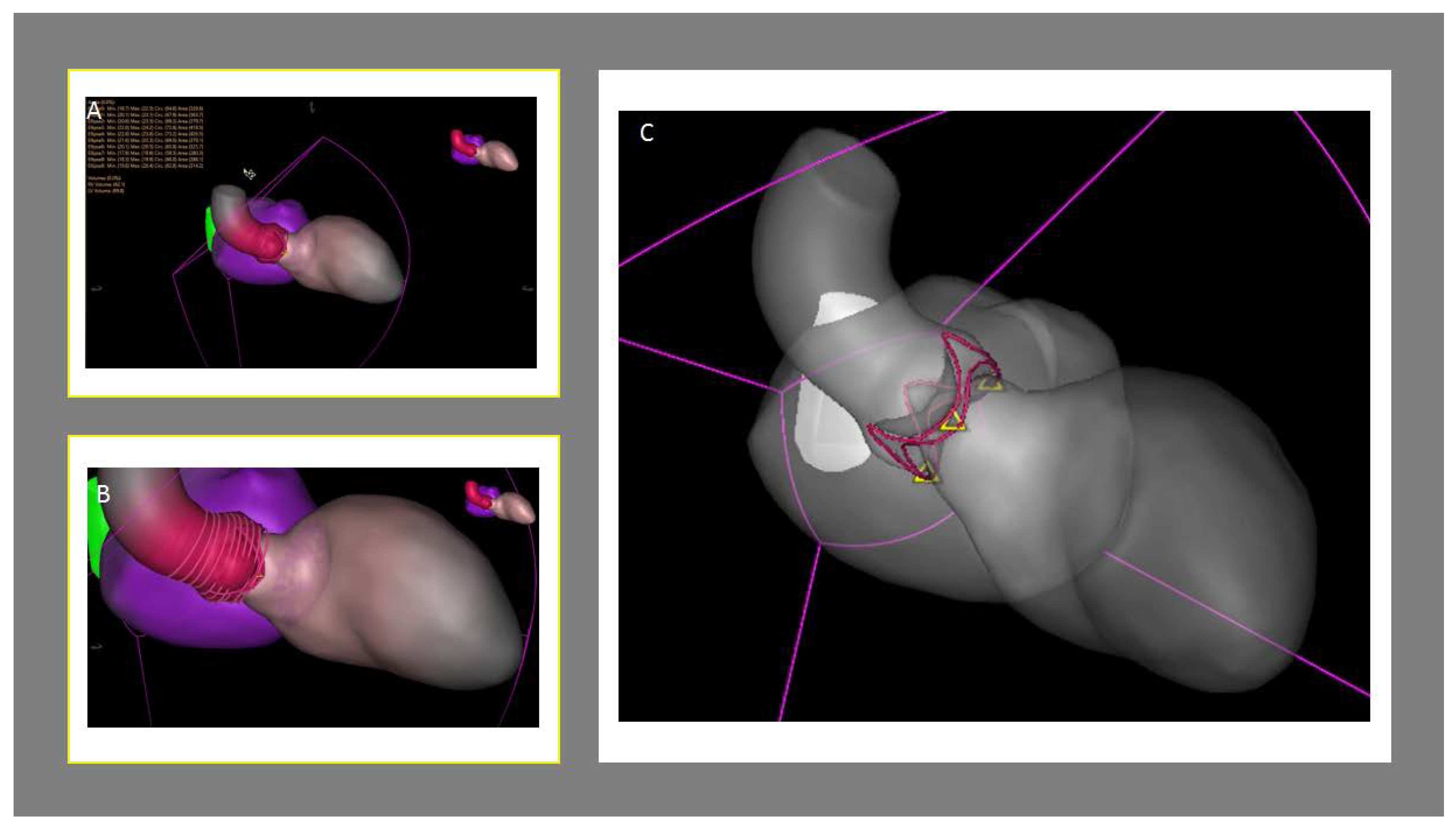
2.2. Aortic Valve Sizing
2.3. BAV-Associated Aortopathy
3. Treatment Options
3.1. Surgery
3.2. Transcatheter Aortic Valve Replacement (TAVR)
4. Future Perspectives
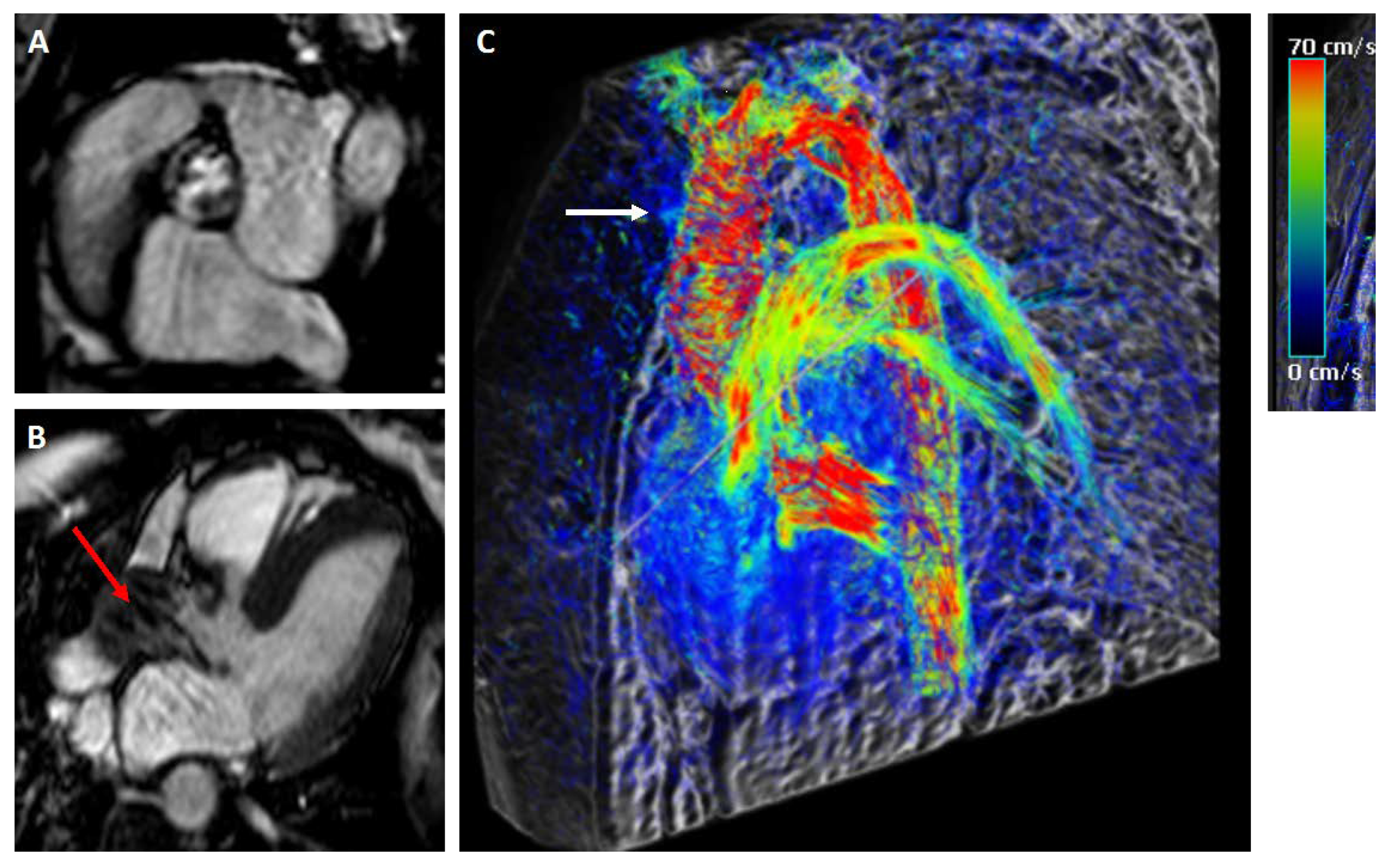
4.1. Diagnostic Modalities
4.2. Treatment Options
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borger, M.A. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J. Thorac. Cardiovasc. Surg. 2018, 156, e41–e74. [Google Scholar] [CrossRef]
- Verma, S.; Siu, S.C. Aortic dilatation in patients with bicuspid aortic valve. N. Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Sherrah, A.G.; Andvik, S.; van der Linde, D.; Davies, L.; Bannon, P.G.; Padang, R.; Vallely, M.P.; Wilson, M.K.; Keech, A.C.; Jeremy, R.W. Nonsyndromic Thoracic Aortic Aneurysm and Dissection: Outcomes With Marfan Syndrome Versus Bicuspid Aortic Valve Aneurysm. J. Am. Coll. Cardiol. 2016, 67, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.M.; Verma, S.; David, T.E.; Leask, R.L.; Weisel, R.D.; Butany, J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002, 106, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Jilaihawi, H.; Chen, M. A bicuspid aortic valve imaging classification for the TAVR era. JACC Cardiovasc. Imaging. 2016, 9, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Veulemans, V.; Hellhammer, K.; Polzin, A.; Bönner, F.; Zeus, T.; Kelm, M. Current and future aspects of multimodal and fusion imaging in structural and coronary heart disease. Clin. Res. Cardiol. 2018, 107 (Suppl. 2), 49–54. [Google Scholar] [CrossRef]
- Mediratta, A.; Addetia, K.; Medvedofsky, D.; Schneider, R.J.; Kruse, E.; Shah, A.P.; Nathan, S.; Paul, J.D.; Blair, J.E.; Ota, T.; et al. 3D echocardiographic analysis of aortic annulus for transcatheter aortic valve replacement using novel aortic valve quantification software: Comparison with computed tomography. Echocardiography 2017, 34, 690–699. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- Warmerdam, E.; Krings, G.J.; Leiner, T.; Grotenhuis, H.B. Three-dimensional and four-dimensional flow assessment in congenital heart disease. Heart 2019. [Google Scholar] [CrossRef]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reson. 2015, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Barker, A.J.; Markl, M. The Role of Imaging of Flow Patterns by 4D Flow MRI in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J. Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.Q.; Hameed, I.; Salemi, A.; Rahouma, M.; Khan, F.M.; Wijeysundera, H.C.; Angiolillo, D.J.; Shore-Lesserson, L.; Biondi-Zoccai, G.; Girardi, L.N.; et al. Three-Dimensional Echocardiography for Transcatheter Aortic Valve Replacement Sizing: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e013463. [Google Scholar] [CrossRef]
- Kim, W.K.; Renker, M.; Rolf, A.; Fischer-Rasokat, U.; Wiedemeyer, J.; Doss, M.; Möllmann, H.; Walther, T.; Nef, H.; Hamm, C.W.; et al. Annular versus supra-annular sizing for TAVI in bicuspid aortic valve stenosis. EuroIntervention 2019, 15, e231–e238. [Google Scholar] [CrossRef]
- Frangieh, A.H.; Michel, J.; Deutsch, O.; Joner, M.; Pellegrini, C.; Rheude, T.; Bleiziffer, S.; Kasel, A.M. Aortic annulus sizing in stenotic bicommissural non-raphe-type bicuspid aortic valves: Reconstructing a three-dimensional structure using only two hinge points. Clin. Res. Cardiol. 2019, 108, 6–15. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Zhu, Q.; Gao, F.; He, W.; Yu, L.; Zhou, Q.; Kong, M.; Wang, J. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter. Cardiovasc. Interv. 2018, 91, 986–994. [Google Scholar] [CrossRef]
- Michelena, H.I.; Prakash, S.K.; Della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.W.; et al. Bicuspid aortic valve: Identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Lazkani, M.; Moualla, S.; Orazio, A.; Tasset, M.; Morris, M.; Fang, K.; Pershad, A. Impact of aortic aneurysms in trans-catheter aortic valve replacement: A single center experience. Indian Heart J. 2018, 70 (Suppl. 3), S303–S308. [Google Scholar] [CrossRef]
- Das, R.; Puri, R. Transcatheter Treatment of Bicuspid Aortic Valve Disease: Imaging and Interventional Considerations. Front. Cardiovasc. Med. 2018, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Mylotte, D.; Lefevre, T.; Søndergaard, L.; Watanabe, Y.; Modine, T.; Dvir, D.; Bosmans, J.; Tchetche, D.; Kornowski, R.; Sinning, J.M.; et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2014, 64, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Leipsic, J.; Perlman, G.; Stub, D.; Dvir, D.; Hansson, N.C.; Norgaard, B.L.; Blanke, P.; Cheung, A.; Ye, J.; et al. A strategy of underexpansion and ad hoc post-dilation of balloonexpandable transcatheter aortic valves in patients at risk of annular injury: Favorable mid-term outcomes. JACC Cardiovasc. Interv. 2015, 8, 1727–1732. [Google Scholar] [CrossRef][Green Version]
- Yousef, A.; Simard, T.; Webb, J.; Rodés-Cabau, J.; Costopoulos, C.; Kochman, J.; Hernández-Garcia, J.M.; Chiam, P.T.; Welsh, R.C.; Wijeysundera, H.C.; et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve: A patient level multi-center analysis. Int. J. Cardiol. 2015, 189, 282–288. [Google Scholar] [CrossRef]
- Kochman, J.; Huczek, Z.; Scisło, P.; Dabrowski, M.; Chmielak, Z.; Szymański, P.; Witkowski, A.; Parma, R.; Ochala, A.; Chodór, P.; et al. Comparison of one- and 12-month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am. J. Cardiol. 2014, 114, 757–762. [Google Scholar] [CrossRef]
- Yoon, S.H.; Bleiziffer, S.; De Backer, O.; Delgado, V.; Arai, T.; Ziegelmueller, J.; Barbanti, M.; Sharma, R.; Perlman, G.Y.; Khalique, O.K.; et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2017, 69, 2579–2589. [Google Scholar] [CrossRef]
- Perlman, G.Y.; Blanke, P.; Dvir, D.; Pache, G.; Modine, T.; Barbanti, M.; Holy, E.W.; Treede, H.; Ruile, P.; Neumann, F.-J.; et al. Bicuspid aortic valve stenosis: Favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc. Interv. 2016, 9, 817–824. [Google Scholar] [CrossRef]
- Kochman, J.; Zbroński, K.; Kołtowski, Ł.; Parma, R.; Ochała, A.; Huczek, Z.; Rymuza, B.; Wilimski, R.; Dąbrowski, M.; Witkowski, A.; et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis utilizing the next-generation fully retrievable and repositionable valve system: Mid-term results from a prospective multicentre registry. Clin. Res. Cardiol. 2019. [Google Scholar] [CrossRef]
- Yoon, S.H.; Sharma, R.; Chakravarty, T.; Miyasaka, M.; Ochiai, T.; Nomura, T.; Gellada, N.; Nemanpour, S.; Nakamura, M.; Chen, W.; et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis: Where do we stand? J. Cardiovasc. Surg. (Torino) 2018, 59, 381–391. [Google Scholar]
- Astudillo, P.; Mortier, P.; Bosmans, J.; De Backer, O.; de Jaegere, P.; De Beule, M.; Dambre, J. Enabling Automated Device Size Selection for Transcatheter Aortic Valve Implantation. J. Interv. Cardiol. 2019, 2019, 3591314. [Google Scholar] [CrossRef] [PubMed]
- Veulemans, V.; Afzal, S.; Papadopoulos, G.; Maier, O.; Kelm, M.; Zeus, T.; Hellhammer, K. TAVR-related echocardiographic assessment—Status quo, challenges and perspectives. Acta Cardiol. 2019, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bruckheimer, E.; Rotschild, C. Holography for imaging in structural heart disease. EuroIntervention 2016, 12 (Suppl. X), X81–X84. [Google Scholar] [CrossRef]
- Rymuza, B.; Grodecki, K.; Kamiński, J.; Scisło, P.; Huczek, Z. Holographic imaging during transcatheter aortic valve implantation procedure in bicuspid aortic valve stenosis. Kardiol. Pol. 2017, 75, 10.5603. [Google Scholar] [CrossRef]
- Jone, P.N.; Khoo, N. Innovation in 3D Echocardiographic Imaging. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.; Bavo, A.M.; El Faquir, N.; Mortier, P.; de Jaegere, P.; De Backer, O.; Sondergaard, L.; Ruile, P.; Mylotte, D.; McConkey, H.; et al. Patient-Specific Computer Simulation of Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Morphology. Circ. Cardiovasc. Imaging 2019, 12, e009178. [Google Scholar] [CrossRef]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. Cardiac 3D Printing and its Future Directions. JACC Cardiovasc. Imaging 2017, 10, 171–184. [Google Scholar] [CrossRef]
- Elbadawi, A.; Saad, M.; Elgendy, I.Y.; Barssoum, K.; Omer, M.A.; Soliman, A.; Almahmoud, M.F.; Ogunbayo, G.O.; Mentias, A.; Gilani, S.; et al. Temporal Trends and Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement for Bicuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2019, 12, 1811–1822. [Google Scholar] [CrossRef]
- Aicher, D.; Kunihara, T.; Abou Issa, O.; Brittner, B.; Gräber, S.; Schäfers, H.J. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011, 123, 178–185. [Google Scholar] [CrossRef]
- Svensson, L.G.; Al Kindi, A.H.; Vivacqua, A.; Pettersson, G.B.; Gillinov, A.M.; Mihaljevic, T.; Roselli, E.E.; Sabik, J.F., 3rd; Griffin, B.; Hammer, D.F.; et al. Long-term durability of bicuspid aortic valve repair. Ann. Thorac. Surg. 2014, 97, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; El-Hamamsy, I.; Verma, S.; Peterson, M.D.; Bonow, R.O.; Yacoub, M.H.; David, T.E.; Bhatt, D.L. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2761–2777. [Google Scholar] [CrossRef] [PubMed]
- Ouzounian, M.; Mazine, A.; David, T.E. The Ross procedure is the best operation to treat aortic stenosis in young and middle-aged adults. J. Thorac. Cardiovasc. Surg. 2017, 154, 778–782. [Google Scholar] [PubMed]
- Carrel, T.; Kadner, A. Long-Term Clinical and Imaging Follow-Up after Reinforced Pulmonary Autograft Ross Procedure. Semin. Thorac. Cardiovasc. Surg. Pediatric Card. Surg. Annu. 2016, 19, 59–62. [Google Scholar] [CrossRef]
- Poh, C.L.; Buratto, E.; Larobina, M.; Wynne, R.; O’Keefe, M.; Goldblatt, J.; Tatoulis, J.; Skillington, P.D. The Ross procedure in adults presenting with bicuspid aortic valve and pure aortic regurgitation: 85% freedom from reoperation at 20 years. Eur. J. Cardiothorac. Surg. 2018, 54, 420–426. [Google Scholar] [CrossRef]
- Zacek, P.; Holubec, T.; Vobornik, M.; Dominik, J.; Takkenberg, J.; Harrer, J.; Vojacek, J. Quality of life after aortic valve repair is similar to Ross patients and superior to mechanical valve replacement: A cross-sectional study. BMC Cardiovasc. Disord. 2016, 16, 63. [Google Scholar] [CrossRef]
- Ozaki, S.; Kawase, I.; Yamashita, H.; Uchida, S.; Nozawa, Y.; Takatoh, M.; Hagiwara, S.; Kiyohara, N. Reconstruction of bicuspid aortic valve with autologous pericardium—Usefulness of tricuspidization. Circ. J. 2014, 78, 1144–1151. [Google Scholar] [CrossRef]
- Prêtre, R.; Kadner, A.; Dave, H.; Bettex, D.; Genoni, M. Tricuspidisation of the aortic valve with creation of a crown-like annulus is able to restore a normal valve function in bicuspid aortic valves. Eur. J. Cardiothorac. Surg. 2006, 29, 1001–1006. [Google Scholar] [CrossRef]
- Khan, J.M.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Eng, M.H.; Paone, G.; Leshnower, B.G.; Reisman, M.; Satler, L.; Waksman, R.; et al. The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction. JACC Cardiovasc. Interv. 2019, 12, 1240–1252. [Google Scholar] [CrossRef]
- Arias, E.A.; Bhan, A.; Lim, Z.Y.; Mullen, M. TAVI for Pure Native Aortic Regurgitation: Are We There Yet? Interv. Cardiol. Rev. 2019, 14, 26–30. [Google Scholar] [CrossRef]
| Modality | Indication | Advantages | Disadvantages |
|---|---|---|---|
| TTE |
|
|
|
| TEE |
|
|
|
| MSCT |
|
|
|
| CMR |
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, S.; Piayda, K.; Maier, O.; Goh, S.; Hellhammer, K.; Cramer, M.; Bönner, F.; Polzin, A.; Nijhof, N.; Kelm, M.; et al. Current and Future Aspects of Multimodal Imaging, Diagnostic, and Treatment Strategies in Bicuspid Aortic Valve and Associated Aortopathies. J. Clin. Med. 2020, 9, 662. https://doi.org/10.3390/jcm9030662
Afzal S, Piayda K, Maier O, Goh S, Hellhammer K, Cramer M, Bönner F, Polzin A, Nijhof N, Kelm M, et al. Current and Future Aspects of Multimodal Imaging, Diagnostic, and Treatment Strategies in Bicuspid Aortic Valve and Associated Aortopathies. Journal of Clinical Medicine. 2020; 9(3):662. https://doi.org/10.3390/jcm9030662
Chicago/Turabian StyleAfzal, Shazia, Kerstin Piayda, Oliver Maier, Shouheng Goh, Katharina Hellhammer, Mareike Cramer, Florian Bönner, Amin Polzin, Niels Nijhof, Malte Kelm, and et al. 2020. "Current and Future Aspects of Multimodal Imaging, Diagnostic, and Treatment Strategies in Bicuspid Aortic Valve and Associated Aortopathies" Journal of Clinical Medicine 9, no. 3: 662. https://doi.org/10.3390/jcm9030662
APA StyleAfzal, S., Piayda, K., Maier, O., Goh, S., Hellhammer, K., Cramer, M., Bönner, F., Polzin, A., Nijhof, N., Kelm, M., Zeus, T., & Veulemans, V. (2020). Current and Future Aspects of Multimodal Imaging, Diagnostic, and Treatment Strategies in Bicuspid Aortic Valve and Associated Aortopathies. Journal of Clinical Medicine, 9(3), 662. https://doi.org/10.3390/jcm9030662





