Robust Association between Acute Kidney Injury after Radical Nephrectomy and Long-term Renal Function
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Patient Data and Outcome Measurements
2.3. Statistical Methods
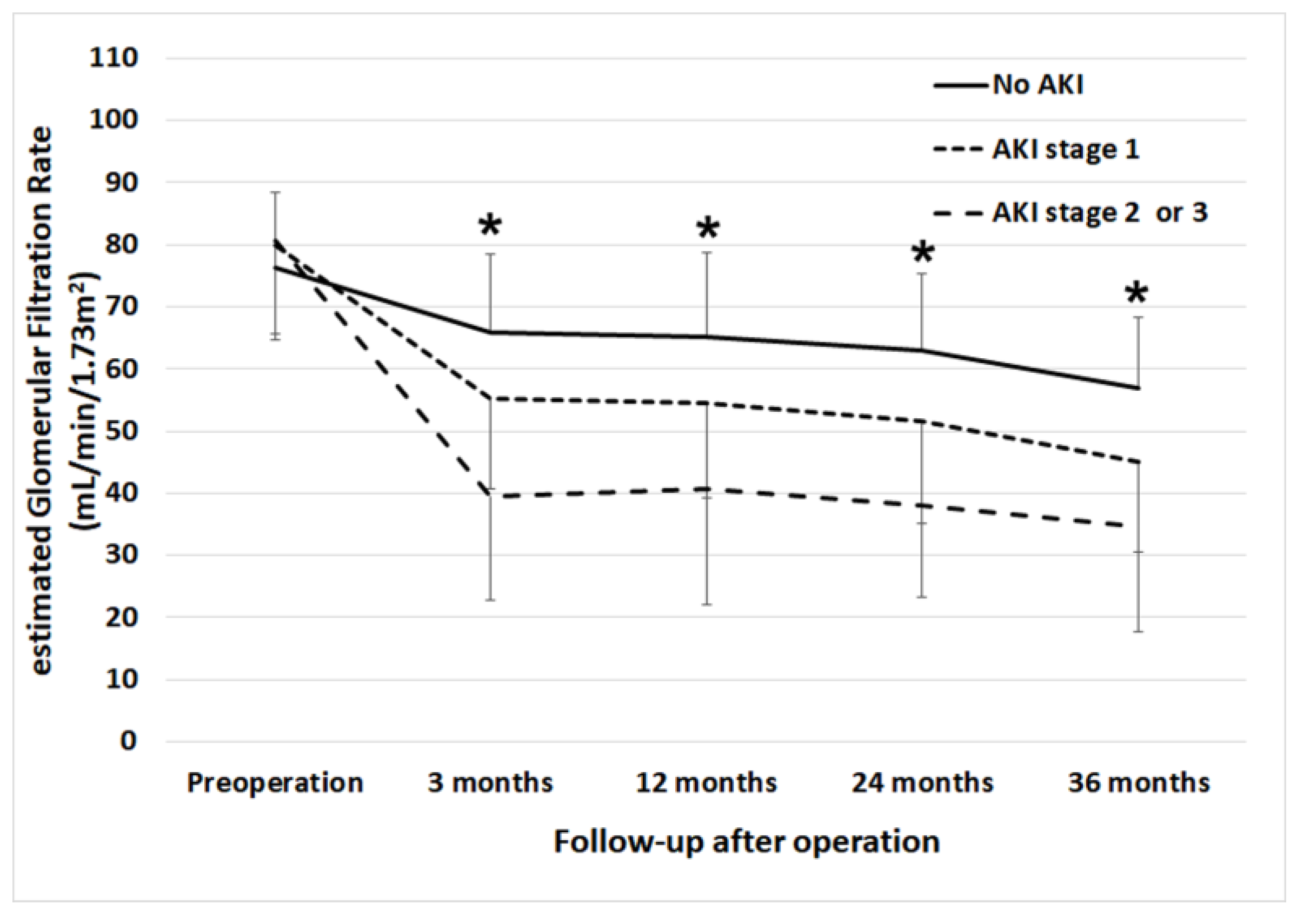
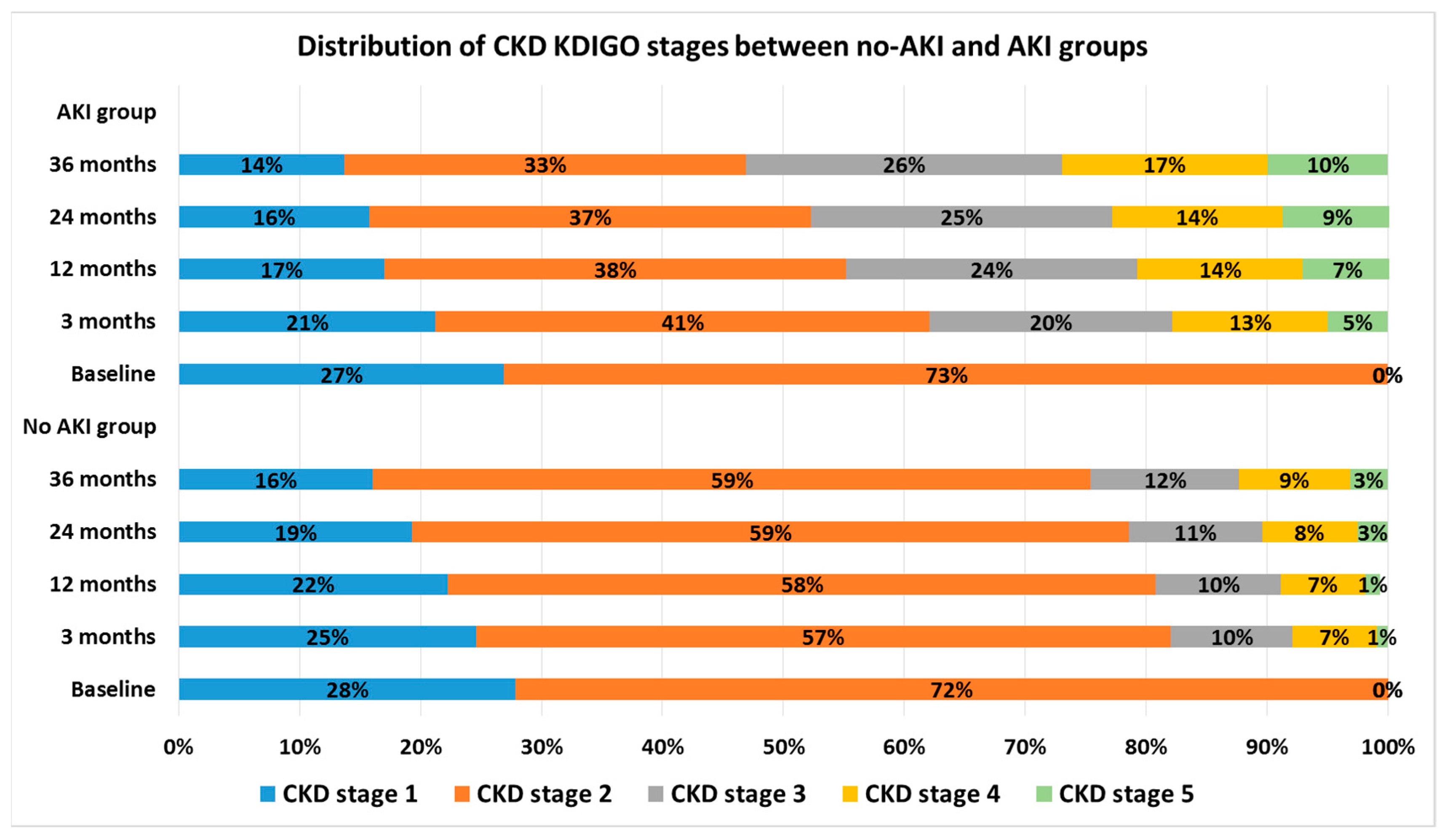
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cho, A.; Lee, J.E.; Kwon, G.Y.; Huh, W.; Lee, H.M.; Kim, Y.G.; Kim, D.J.; Oh, H.Y.; Choi, H.Y. Post-operative acute kidney injury in patients with renal cell carcinoma is a potent risk factor for new-onset chronic kidney disease after radical nephrectomy. Nephrol. Dial. Transpl. 2011, 26, 3496–3501. [Google Scholar] [CrossRef]
- Garofalo, C.; Liberti, M.E.; Russo, D.; Russo, L.; Fuiano, G.; Cianfrone, P.; Conte, G.; De Nicola, L.; Minutolo, R.; Borrelli, S. Effect of post-nephrectomy acute kidney injury on renal outcome: A retrospective long-term study. World J. Urol. 2018, 36, 59–63. [Google Scholar] [CrossRef]
- Shin, S.; Han, Y.; Park, H.; Chung, Y.S.; Ahn, H.; Kim, C.S.; Cho, Y.P.; Kwon, T.W. Risk factors for acute kidney injury after radical nephrectomy and inferior vena cava thrombectomy for renal cell carcinoma. J. Vasc. Surg. 2013, 58, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The Environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Zabell, J.; Isharwal, S.; Dong, W.; Abraham, J.; Wu, J.; Suk-Ouichai, C.; Palacios, D.A.; Remer, E.; Li, J.; Campbell, S.C. Acute Kidney Injury after Partial Nephrectomy of Solitary Kidneys: Impact on Long-Term Stability of Renal Function. J. Urol. 2018, 200, 1295–1301. [Google Scholar] [CrossRef]
- Miller, I.J.; Suthanthiran, M.; Riggio, R.R.; Williams, J.J.; Riehle, R.A.; Vaughan, E.D.; Stubenbord, W.T.; Mouradian, J.; Cheigh, J.S.; Stenzel, K.H. Impact of renal donation. Long-term clinical and biochemical follow-up of living donors in a single center. Am. J. Med. 1985, 79, 201–208. [Google Scholar] [CrossRef]
- Ibrahim, H.N.; Foley, R.; Tan, L.; Rogers, T.; Bailey, R.F.; Guo, H.; Gross, C.R.; Matas, A.J. Long-term consequences of kidney donation. N. Engl. J. Med. 2009, 360, 459–469. [Google Scholar] [CrossRef]
- Lee, H.J.; Bae, J.; Kwon, Y.; Jang, H.S.; Yoo, S.; Jeong, C.W.; Kim, J.T.; Kim, W.H. General Anesthetic Agents and Renal Function after Nephrectomy. J. Clin. Med. 2019, 8, 1530. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef]
- KDIGO working group. KDIGO 2012 clinical practice guidelines for evaluation and management of chronic kidney disease. Kidney Int. 2013, 3, 1–150. [Google Scholar]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef]
- Shin, S.R.; Kim, W.H.; Kim, D.J.; Shin, I.W.; Sohn, J.T. Prediction and Prevention of Acute Kidney Injury after Cardiac Surgery. Biomed. Res. Int. 2016, 2016, 2985148. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, U.S.; Spiegelman, D.; Thurston, S.W.; Ganguli, B.; Eisen, E.A. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat. Med. 2007, 26, 3735–3752. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Demler, O.V.; Paynter, N.P.; Cook, N.R. Tests of calibration and goodness-of-fit in the survival setting. Stat. Med. 2015, 34, 1659–1680. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Yokoyama, M.; Fujii, Y.; Iimura, Y.; Saito, K.; Koga, F.; Masuda, H.; Kawakami, S.; Kihara, K. Longitudinal change in renal function after radical nephrectomy in Japanese patients with renal cortical tumors. J. Urol. 2011, 185, 2066–2071. [Google Scholar] [CrossRef]
- Barlow, L.J.; Korets, R.; Laudano, M.; Benson, M.; McKiernan, J. Predicting renal functional outcomes after surgery for renal cortical tumours: A multifactorial analysis. BJU Int. 2010, 106, 489–492. [Google Scholar] [CrossRef]
- Huang, W.C.; Levey, A.S.; Serio, A.M.; Snyder, M.; Vickers, A.J.; Raj, G.V.; Scardino, P.T.; Russo, P. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 2006, 7, 735–740. [Google Scholar] [CrossRef]
- Li, L.; Lau, W.L.; Rhee, C.M.; Harley, K.; Kovesdy, C.P.; Sim, J.J.; Jacobsen, S.; Chang, A.; Landman, J.; Kalantar-Zadeh, K. Risk of chronic kidney disease after cancer nephrectomy. Nat. Rev. Nephrol. 2014, 10, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.D.; Pierorazio, P.M.; Johnson, M.H.; Sharma, R.; Iyoha, E.; Allaf, M.E.; Bass, E.B.; Sozio, S.M. Renal Functional Outcomes after Surgery, Ablation, and Active Surveillance of Localized Renal Tumors: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.G.; Parker, D.C.; Egleston, B.L.; Uzzo, R.G.; Haseebuddin, M.; Joshi, S.S.; Viterbo, R.; Greenberg, R.E.; Chen, D.Y.T.; Smaldone, M.C.; et al. Prediction of significant estimated glomerular filtration rate decline after renal unit removal to aid in the clinical choice between radical and partial nephrectomy in patients with a renal mass and normal renal function. BJU Int. 2019, 124, 999–1005. [Google Scholar] [CrossRef]
- Fergany, A.F.; Hafez, K.S.; Novick, A.C. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J. Urol. 2000, 163, 442–445. [Google Scholar] [CrossRef]
- Patard, J.J.; Shvarts, O.; Lam, J.S.; Pantuck, A.J.; Kim, H.L.; Ficarra, V.; Cindolo, L.; Han, K.R.; De La Taille, A.; Tostain, J.; et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J. Urol. 2004, 171, 2181–2185. [Google Scholar] [CrossRef]
- Klarenbach, S.; Moore, R.B.; Chapman, D.W.; Dong, J.; Braam, B. Adverse renal outcomes in subjects undergoing nephrectomy for renal tumors: A population-based analysis. Eur. Urol. 2011, 59, 333–339. [Google Scholar] [CrossRef]
- Sun, M.; Bianchi, M.; Hansen, J.; Trinh, Q.D.; Abdollah, F.; Tian, Z.; Sammon, J.; Shariat, S.F.; Graefen, M.; Montorsi, F.; et al. Chronic kidney disease after nephrectomy in patients with small renal masses: A retrospective observational analysis. Eur. Urol. 2012, 62, 696–703. [Google Scholar] [CrossRef]
- Alam, R.; Patel, H.D.; Osumah, T.; Srivastava, A.; Gorin, M.A.; Johnson, M.H.; Trock, B.J.; Chang, P.; Wagner, A.A.; McKiernan, J.M.; et al. Comparative effectiveness of management options for patients with small renal masses: A prospective cohort study. BJU Int. 2019, 123, 42–50. [Google Scholar] [CrossRef]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for management of the clinical T1 renal mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Ishani, A.; Xue, J.L.; Himmelfarb, J.; Eggers, P.W.; Kimmel, P.L.; Molitoris, B.A.; Collins, A.J. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009, 20, 223–228. [Google Scholar] [CrossRef]
- Lo, L.J.; Go, A.S.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordonez, J.D.; Hsu, C.Y. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009, 76, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, Y.J.; Ryoo, S.M.; Sohn, C.H.; Seo, D.W.; Ahn, S.; Lim, K.S.; Kim, W.Y. One—Year Progression and Risk Factors for the Development of Chronic Kidney Disease in Septic Shock Patients with Acute Kidney Injury: A Single-Centre Retrospective Cohort Study. J. Clin. Med. 2018, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Hori, D.; Katz, N.M.; Fine, D.M.; Ono, M.; Barodka, V.M.; Lester, L.C.; Yenokyan, G.; Hogue, C.W. Defining oliguria during cardiopulmonary bypass and its relationship with cardiac surgery-associated acute kidney injury. Br. J. Anaesth. 2016, 117, 733–740. [Google Scholar] [CrossRef]
- Mizota, T.; Yamamoto, Y.; Hamada, M.; Matsukawa, S.; Shimizu, S.; Kai, S. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br. J. Anaesth. 2017, 119, 1127–1134. [Google Scholar] [CrossRef]
- Gaffney, A.M.; Sladen, R.N. Acute kidney injury in cardiac surgery. Curr. Opin. Anaesthesiol. 2015, 28, 50–59. [Google Scholar] [CrossRef]
- Anderson, R.J.; Linas, S.L.; Berns, A.S.; Henrich, W.L.; Miller, T.R.; Gabow, P.A.; Schrier, R.W. Nonoliguric acute renal failure. N. Engl. J. Med. 1977, 296, 1134–1138. [Google Scholar] [CrossRef]
- Anderson, R.G.; Bueschen, A.J.; Lloyd, L.K.; Dubovsky, E.V.; Burns, J.R. Short-term and long-term changes in renal function after donor nephrectomy. J. Urol. 1991, 145, 11–13. [Google Scholar] [CrossRef]
- Vincenti, F.; Amend, W.J., Jr.; Kaysen, G.; Feduska, N.; Birnbaum, J.; Duca, R.; Salvatierra, O. Long-term renal function in kidney donors. Sustained compensatory hyperfiltration with no adverse effects. Transplantation 1983, 36, 626–629. [Google Scholar] [CrossRef]
- Talseth, T.; Fauchald, P.; Skrede, S.; Djoseland, O.; Berg, K.J.; Stenstrom, J.; Heilo, A.; Brodwall, E.K.; Flatmark, A. Long-term blood pressure and renal function in kidney donors. Kidney Int. 1986, 29, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Najarian, J.S.; Chavers, B.M.; McHugh, L.E.; Matas, A.J. 20 years or more of follow-up of living kidney donors. Lancet 1992, 340, 807–810. [Google Scholar] [CrossRef]
- Giglio, M.; Dalfino, L.; Puntillo, F.; Brienza, N. Hemodynamic goal-directed therapy and postoperative kidney injury: An updated meta-analysis with trial sequential analysis. Crit. Care 2019, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Hur, M.; Park, S.K.; Yoo, S.; Choi, S.N.; Jeong, C.W.; Kim, W.H.; Kim, J.T.; Kwak, C.; Bahk, J.H. The association between intraoperative urine output and postoperative acute kidney injury differs between partial and radical nephrectomy. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.G.; Jeong, I.G.; Lee, J.W.; Lee, S.E.; Lee, E. Prognostic factors for chronic kidney disease after curative surgery in patients with small renal tumors. Urology 2009, 74, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Hong, J.H.; Koh, D.H.; Lee, J.; Nam, H.J.; Kim, S.Y. Effect of Diabetes Mellitus on Acute Kidney Injury after Minimally Invasive Partial Nephrectomy: A Case-Matched Retrospective Analysis. J. Clin. Med. 2019, 8, 468. [Google Scholar] [CrossRef]
- Bijol, V.; Mendez, G.P.; Hurwitz, S.; Rennke, H.G.; Nose, V. Evaluation of the nonneoplastic pathology in tumor nephrectomy specimens: Predicting the risk of progressive renal failure. Am. J. Surg. Pathol. 2006, 30, 575–584. [Google Scholar] [CrossRef]
- Kil, H.K.; Kim, J.Y.; Choi, Y.D.; Lee, H.S.; Kim, T.K.; Kim, J.E. Effect of Combined Treatment of Ketorolac and Remote Ischemic Preconditioning on Renal Ischemia-Reperfusion Injury in Patients Undergoing Partial Nephrectomy: Pilot Study. J. Clin. Med. 2018, 7, 470. [Google Scholar] [CrossRef]
- Bravi, C.A.; Larcher, A.; Capitanio, U.; Mari, A.; Antonelli, A.; Artibani, W.; Barale, M.; Bertini, R.; Bove, P.; Brunocilla, E.; et al. Perioperative Outcomes of Open, Laparoscopic, and Robotic Partial Nephrectomy: A Prospective Multicenter Observational Study (The RECORd 2 Project). Eur. Urol. Focus 2019. [Google Scholar] [CrossRef]



| Variables | Patients | Proportion without Missing (%) |
|---|---|---|
| Demographic data | ||
| Age, year | 60 (51–68) | 100 |
| Female, n | 171 (30.6) | 100 |
| Body-mass index, kg/m2 | 24.4 (22.6–26.2) | 100 |
| Baseline medical status | ||
| Hypertension, n | 287 (51.4) | 100 |
| Diabetes mellitus, n | 98 (17.6) | 100 |
| Cerebrovascular accident, n | 11 (2.0) | 100 |
| Angina pectoris, n | 9 (1.6) | 100 |
| Preoperative hemoglobin, g/dL | 13.5 (12.0–14.6) | 100 |
| Preoperative serum albumin level, mg/dL | 4.3 (4.1–4.6) | 99.8 |
| Preoperative proteinuria, n | 67 (12.0) | 100 |
| Preoperative hematuria, n | 53 (9.5) | 100 |
| Preoperative eGFR, mL/min/1.73 m2 | 100 | |
| eGFR ≥ 90 mL/min/m2 | 153 (27.4) | |
| eGFR 60–89 mL/min/1.73m2 | 405 (72.6) | |
| Surgical parameters | ||
| Surgery type, n | 100 | |
| Laparoscopic | 223 (40.0) | |
| Robot-assisted | 9 (1.6) | |
| Open | 325 (58.2) | |
| Clinical stage, n | 100 | |
| T1a/ T1b | 141 (25.3)/152 (27.2) | |
| T2a/ T2b | 154 (27.6)/61 (10.9) | |
| T3a/T3b/T3c | 19 (3.4)/ 17 (3.0)/14 (2.5) | |
| N 0/1 | 514 (92.1)/44 (7.9) | |
| M 0/1 | 520 (93.2)/38 (6.8) | |
| R.E.N.A.L. score | 100 | |
| Low (4–6) | 225 (40.3) | |
| Intermediate (7–9) | 286 (51.3) | |
| High (10–12) | 47 (8.4) | |
| Tumor maximal diameter, cm | 5.5 (3.2–7.8) | 100 |
| Operation time, min | 130 (100–170) | 100 |
| Bleeding and transfusion amount | ||
| pRBC transfusion, n | 52 (9.3) | 100 |
| Estimated blood loss, mL | 200 (100–400) | 99.6 |
| Anesthesia-related parameters | ||
| Volatile anesthetics use, n | 494 (88.5) | |
| Total intravenous anesthesia, n | 64 (11.5) | |
| Crystalloid administration, mL | 1100 (750–1500) | 100 |
| Colloid administration, mL | 0 (0–300) | 100 |
| Vasopressor infusion during surgery | 29 (5.2) | 100 |
| Variable | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age, years | 1.05 | 1.00–1.09 | 0.043 |
| Female | 1.30 | 0.81–2.10 | 0.368 |
| Body-mass index, kg/m2 | 1.01 | 0.95–1.08 | 0.769 |
| History of hypertension | 1.70 | 1.07–2.78 | 0.022 |
| History of diabetes mellitus | 1.95 | 1.13–3.44 | 0.012 |
| Preoperative hemoglobin, g/dL | 1.14 | 0.99–1.30 | 0.064 |
| Preoperative albumin, g/dL | 0.63 | 0.33–1.12 | 0.077 |
| Preoperative proteinuria, n | 0.82 | 0.42–1.80 | 0.547 |
| Preoperative hematuria, n | 1.12 | 0.57–1.74 | 0.657 |
| Preoperative estimated glomerular filtration rate, mL/min/1.73m2 | 0.99 | 0.98–0.99 | 0.042 |
| Postoperative acute kidney injury | 2.46 | 1.70–3.63 | <0.001 |
| No acute kidney injury | baseline | ||
| Acute kidney injury stage 1 | 1.71 | 1.25–2.32 | <0.001 |
| Acute kidney injury stage 2 or 3 | 2.72 | 1.78–4.10 | <0.001 |
| Preoperative tumor maximal diameter, cm | 1.05 | 0.97–1.12 | 0.164 |
| Open surgery (vs. laparoscopic or robot-assisted) | 0.74 | 0.49–1.15 | 0.255 |
| Operation time, hour | 0.96 | 0.81–1.18 | 0.847 |
| Total intravenous anesthesia | 0.89 | 0.61–1.35 | 0.558 |
| Intraoperative crystalloid administration, per 100 mL | 0.87 | 0.62–1.28 | 0.415 |
| Intraoperative colloid administration, per 100 mL | 1.06 | 0.98–1.16 | 0.176 |
| Intraoperative vasopressor infusion, n | 0.94 | 0.92–1.17 | 0.514 |
| Red blood cell transfusion, n | 0.82 | 0.37–1.75 | 0.427 |
| Variable | β ± Standard Error | p-Value | VIF |
|---|---|---|---|
| Age, years | 0.012 ± 0.001 | 0.057 | 1.69 |
| Female | 0.037 ± 0.031 | 0.240 | 1.30 |
| Body-mass index, kg/m2 | 0.002 ± 0.005 | 0.647 | 1.14 |
| History of hypertension | −0.030 ± 0.011 | 0.047 | 1.52 |
| History of diabetes mellitus | −0.044 ± 0.018 | 0.044 | 1.24 |
| Preoperative hemoglobin concentration, g/dL | 0.007 ± 0.010 | 0.411 | 2.06 |
| Preoperative albumin level, mg/dL | 0.033 ± 0.041 | 0.481 | 1.82 |
| Preoperative proteinuria | −0.034 ± 0.047 | 0.470 | 1.45 |
| Preoperative estimated glomerular filtration rate, per 10 mL/min/1.73 m2 | 0.170 ± 0.122 | 0.002 | 1.26 |
| Postoperative acute kidney injury | −0.168 ± 0.322 | 0.011 | 1.16 |
| Maximal diameter of renal mass, cm | 0.002 ± 0.004 | 0.572 | 1.28 |
| Open surgery (vs. laparoscopic or robot-assisted) | −0.030 ± 0.028 | 0.228 | 1.19 |
| Operation time, hour | −0.016 ± 0.012 | 0.179 | 1.20 |
| Total intravenous anesthesia | 0.069 ± 0.056 | 0.375 | 1.10 |
| Intraoperative crystalloid administration, mL/kg | −0.011 ± 0.023 | 0.724 | 1.45 |
| Intraoperative colloid administration, mL/kg | −0.001 ± 0.004 | 0.717 | 1.37 |
| Intraoperative red cell transfusion, n | 0.096 ± 0.049 | 0.150 | 1.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.H.; Shin, K.W.; Ji, S.-H.; Jang, Y.-E.; Lee, J.-H.; Jeong, C.W.; Kwak, C.; Lim, Y.-J. Robust Association between Acute Kidney Injury after Radical Nephrectomy and Long-term Renal Function. J. Clin. Med. 2020, 9, 619. https://doi.org/10.3390/jcm9030619
Kim WH, Shin KW, Ji S-H, Jang Y-E, Lee J-H, Jeong CW, Kwak C, Lim Y-J. Robust Association between Acute Kidney Injury after Radical Nephrectomy and Long-term Renal Function. Journal of Clinical Medicine. 2020; 9(3):619. https://doi.org/10.3390/jcm9030619
Chicago/Turabian StyleKim, Won Ho, Kyung Won Shin, Sang-Hwan Ji, Young-Eun Jang, Ji-Hyun Lee, Chang Wook Jeong, Cheol Kwak, and Young-Jin Lim. 2020. "Robust Association between Acute Kidney Injury after Radical Nephrectomy and Long-term Renal Function" Journal of Clinical Medicine 9, no. 3: 619. https://doi.org/10.3390/jcm9030619
APA StyleKim, W. H., Shin, K. W., Ji, S.-H., Jang, Y.-E., Lee, J.-H., Jeong, C. W., Kwak, C., & Lim, Y.-J. (2020). Robust Association between Acute Kidney Injury after Radical Nephrectomy and Long-term Renal Function. Journal of Clinical Medicine, 9(3), 619. https://doi.org/10.3390/jcm9030619





