First Multi-Center All-Comers Study for the Aquablation Procedure
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Participants
2.2. Data and Study Monitoring
2.3. Study Endpoints and Statistical Analysis
2.4. Data Availability
2.5. Research Ethics
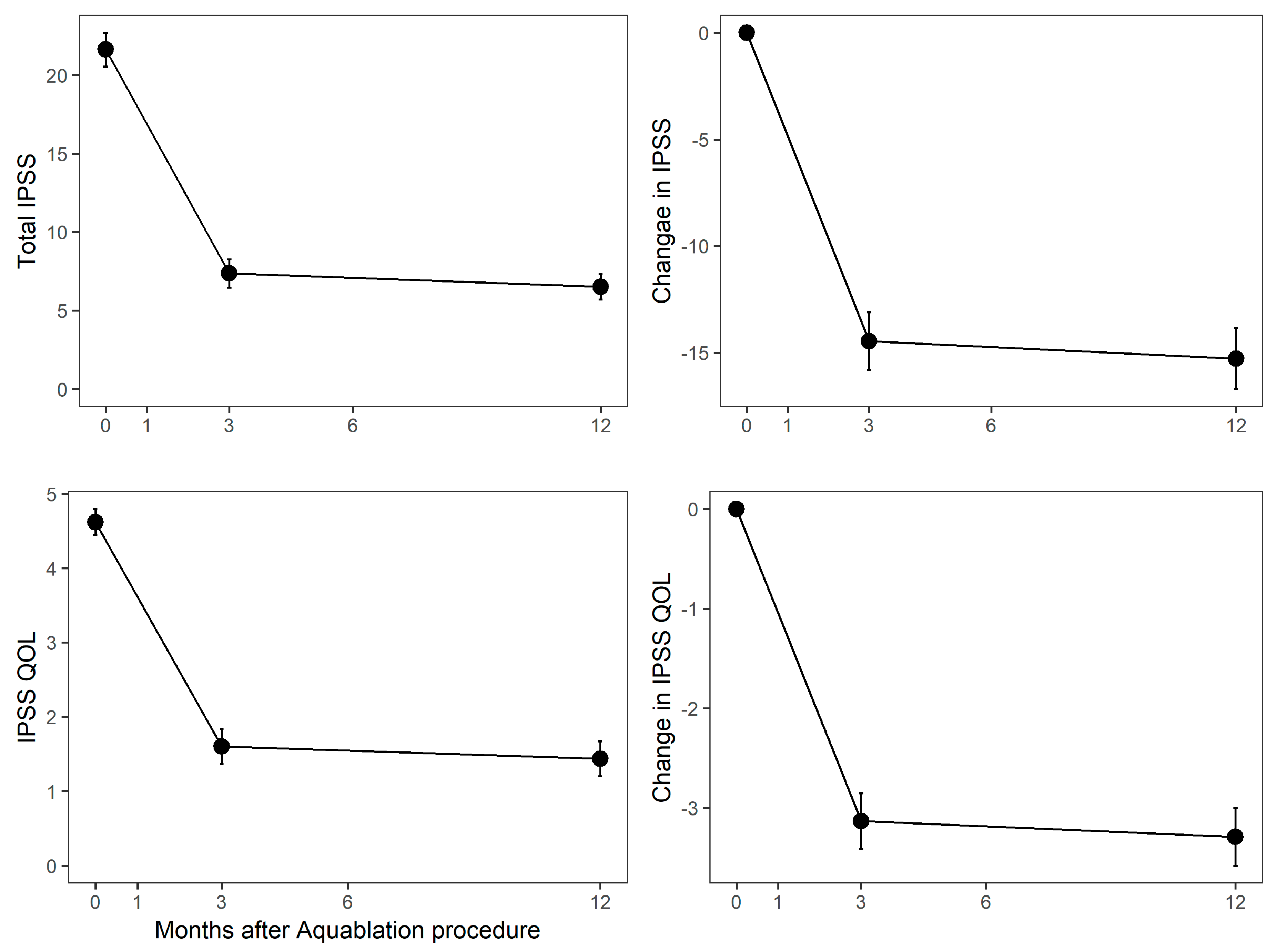
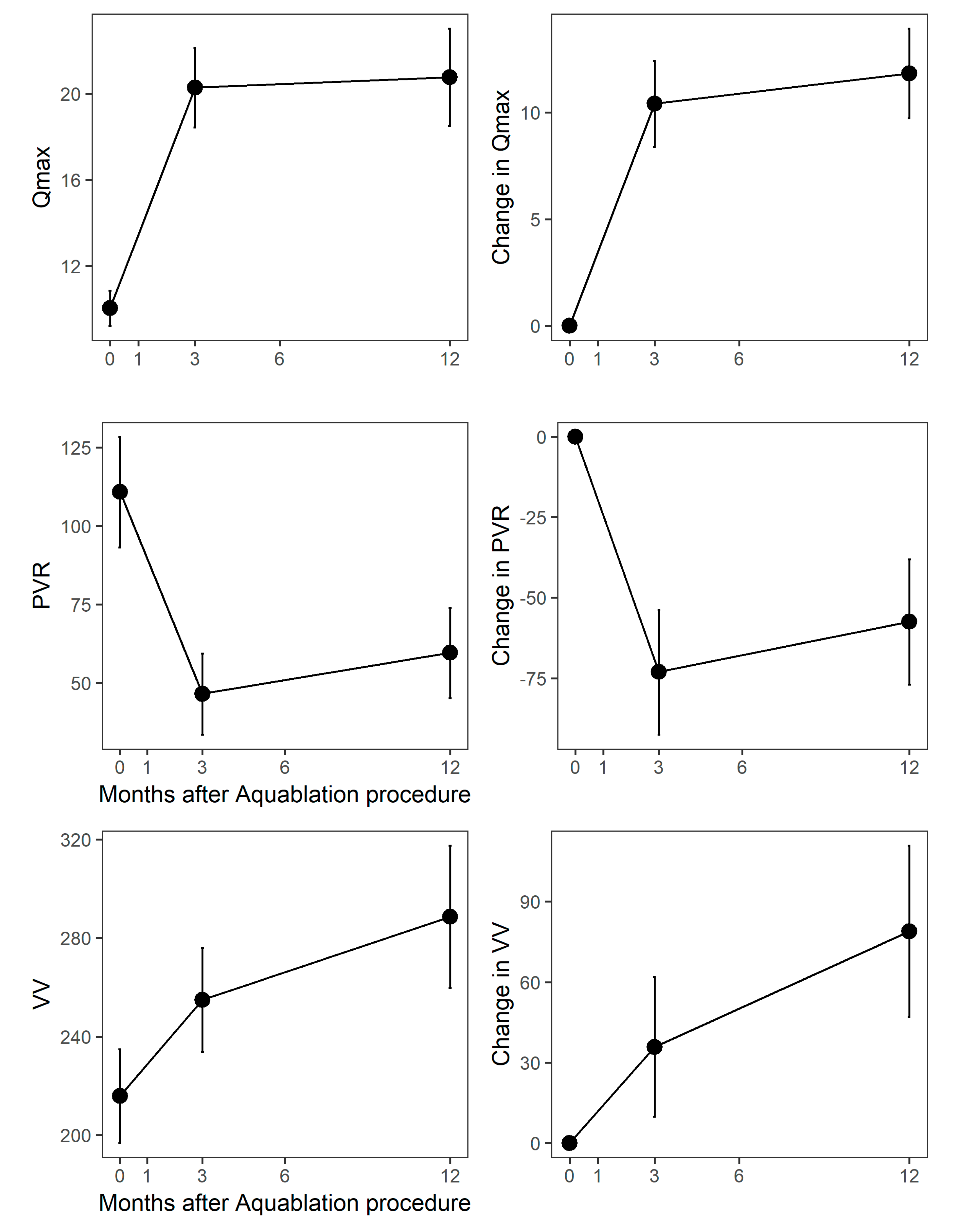
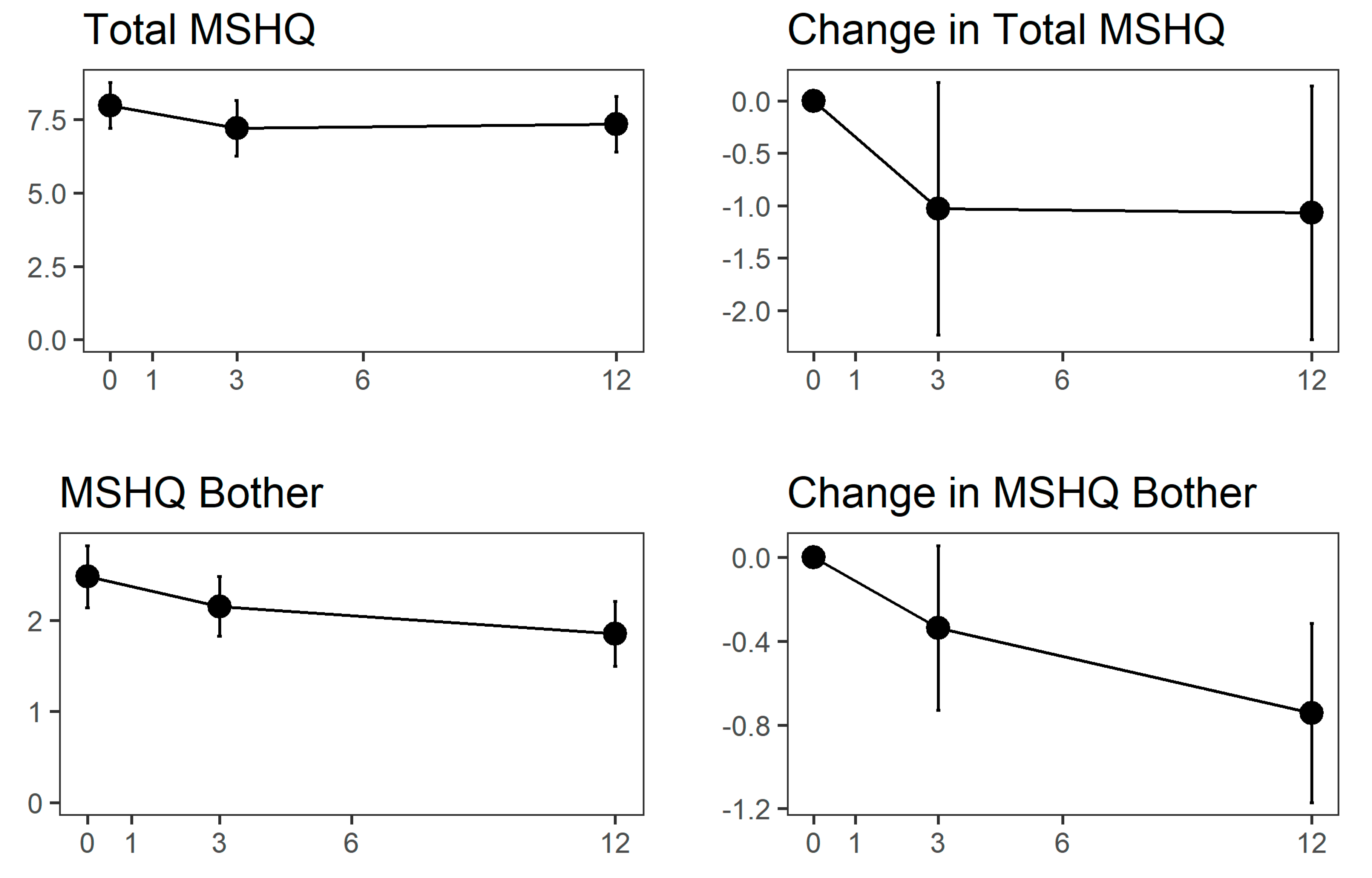
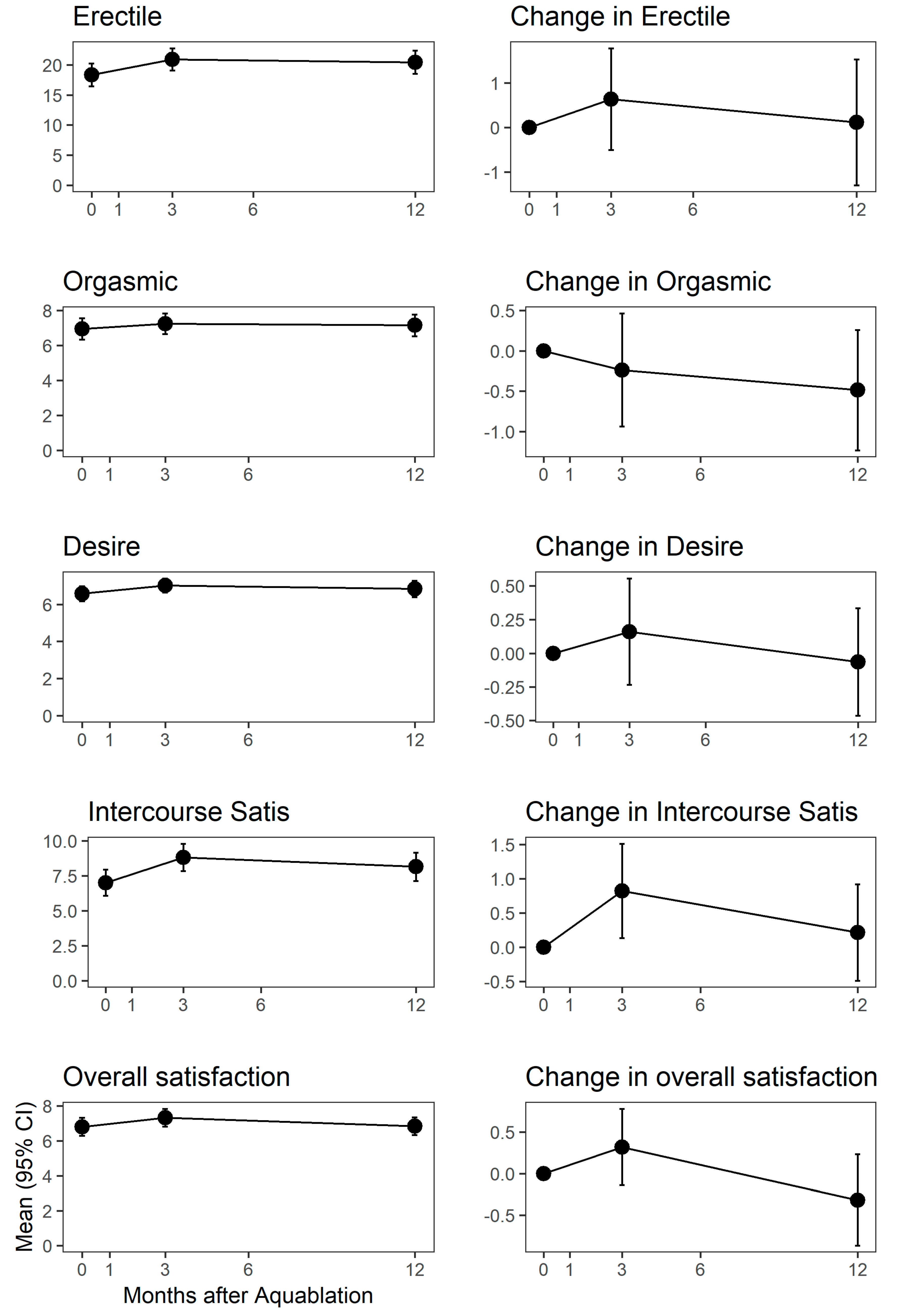
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Site No. | Institution | Primary Investigator | EC Information |
|---|---|---|---|
| 01 | Frimley Park Hospital | Mr. Neil Barber | NHS Health Research Authority (REC) |
| 02 | Asklepios Klinikum Harburg | Prof. Dr. med. Thorsten Bach | Ethics Committee of the Hamburg Medical Association |
| 04 | Tauranga Hospital | Prof. Peter Gilling | Health and Disability Ethics Committees Ministry of Health (Northern B) |
| 05 | American University of Beirut Medical Center | Dr. Albert El-Hajj | American University of Beirut Institutional Review Board |
| 06 | Royal Melbourne Hospital | Mr. Paul Anderson | Melbourne Health Human Research Ethics Committee |
References
- Ferretti, M.; Phillips, J. Prostatectomy for benign prostate disease: Open, laparoscopic and robotic techniques. Can. J. Urol. 2015, 22 (Suppl. 1), 60–66. [Google Scholar]
- Pariser, J.J.; Pearce, S.M.; Patel, S.G.; Bales, G.T. National Trends of Simple Prostatectomy for Benign Prostatic Hyperplasia With an Analysis of Risk Factors for Adverse Perioperative Outcomes. Urology 2015, 86, 721–726. [Google Scholar] [CrossRef] [PubMed]
- American Urological Association-Adult Urodynamics: AUA/SUFU Guideline. Available online: https://www.auanet.org/guidelines/urodynamics-(2012) (accessed on 3 July 2017).
- Zhong, J.; Feng, Z.; Peng, Y.; Liang, H. A Systematic Review and Meta-analysis of Efficacy and Safety Following Holmium Laser Enucleation of Prostate and Transurethral Resection of Prostate for Benign Prostatic Hyperplasia. Urology 2019, 131, 14–20. [Google Scholar] [CrossRef]
- Thangasamy, I.A.; Chalasani, V.; Bachmann, A.; Woo, H.H. Photoselective vaporisation of the prostate using 80-W and 120-W laser versus transurethral resection of the prostate for benign prostatic hyperplasia: A systematic review with meta-analysis from 2002 to 2012. Eur. Urol. 2012, 62, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Brunckhorst, O.; Ahmed, K.; Nehikhare, O.; Marra, G.; Challacombe, B.; Popert, R. Evaluation of the Learning Curve for Holmium Laser Enucleation of the Prostate Using Multiple Outcome Measures. Urology 2015, 86, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Cornu, J.-N.; Fourmarier, M.; Saussine, C.; Descazeaud, A.; Azzouzi, A.-R.; Vicaut, E.; Lukacs, B. Multicentre prospective evaluation of the learning curve of holmium laser enucleation of the prostate (HoLEP). BJU Int. 2016, 117, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Meskawi, M.; Hueber, P.-A.; Valdivieso, R.; Bruyere, F.; Misrai, V.; Fournier, G.; Munver, R.; Sivarajan, G.; Rutman, M.; Te, A.E.; et al. Multicenter international experience of 532 nm-laser photo-vaporization with Greenlight XPS in men with large prostates (prostate volume > 100 cc). World J. Urol. 2017, 35, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.; Reuther, R.; Kahokehr, A.; Fraundorfer, M. Aquablation - image-guided robot-assisted waterjet ablation of the prostate: Initial clinical experience. BJU Int. 2016, 117, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.; Tan, A.; Anderson, P. Aquablation of the Prostate for Symptomatic Benign Prostatic Hyperplasia: One-Year Results. J. Urol. 2017, 197, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.; Barber, N.; Bidair, M.; Anderson, P.; Sutton, M.; Aho, T.; Kramolowsky, E.; Thomas, A.; Cowan, B.; Kaufman, R.P.; et al. WATER: A Double-Blind, Randomized, Controlled Trial of Aquablation® vs Transurethral Resection of the Prostate in Benign Prostatic Hyperplasia. J. Urol. 2018, 199, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Allen, K.R.; Ni, X.; Araujo, A.B. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur. Urol. 2011, 60, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Catania, J.; Pollack, L.; Althof, S.; O’Leary, M.; Seftel, A.D. Male Sexual Health Questionnaire (MSHQ): Scale development and psychometric validation. Urology 2004, 64, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Mamoulakis, C.; Efthimiou, I.; Kazoulis, S.; Christoulakis, I.; Sofras, F. The modified Clavien classification system: A standardized platform for reporting complications in transurethral resection of the prostate. World J. Urol. 2011, 29, 205–210. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Kasivisvanathan, V.; Hussain, M. Aquablation versus transurethral resection of the prostate: 1 year United States—Cohort outcomes. Can. J. Urol. 2018, 25, 9317–9322. [Google Scholar]
- Sorokin, I.; Sundaram, V.; Singla, N.; Walker, J.; Margulis, V.; Roehrborn, C.; Gahan, J.C. Robot-Assisted Versus Open Simple Prostatectomy for Benign Prostatic Hyperplasia in Large Glands: A Propensity Score-Matched Comparison of Perioperative and Short-Term Outcomes. J. Endourol. 2017, 31, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Monn, M.F.; El Tayeb, M.; Bhojani, N.; Mellon, M.J.; Sloan, J.C.; Boris, R.S.; Lingeman, J.E. Predictors of Enucleation and Morcellation Time During Holmium Laser Enucleation of the Prostate. Urology 2015, 86, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, R.; Hueber, P.-A.; Meskawi, M.; Belleville, E.; Ajib, K.; Bruyere, F.; Te, A.E.; Chughtai, B.; Elterman, D.; Misrai, V.; et al. Multicentre international experience of 532-nm laser photoselective vaporization with GreenLight XPS in men with very large prostates. BJU Int. 2018, 122, 873–878. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Statistic * |
|---|---|
| Age, years, mean (SD, range) | 67.7 (8.5, 38–88) |
| Leakage of urine, n (%) | 122 (68.5%) |
| History of urinary retention | 36 (20.2%) |
| History of prostate cancer | 1 (0.6%) |
| Bladder outlet obstruction | 154 (86.5%) |
| Prostate size (TRUS, cc) | 59.3 (26.9, 20–148) |
| Middle lobe | 106 (59.6%) |
| Intravesical component | 77/106 (72.6%) |
| Baseline questionnaires ** | |
| IPSS score, (mean, SD) | 21.6 (7.2, 0–35) |
| IPSS QOL, (mean, SD) | 4.6 (1.2, 1–6) |
| Sexually active, n (%) [MSHQ-EjD] | 126 (70.8%) |
| MSHQ-EjD (mean, SD) *** | 8 (3.9, 1–15) |
| Laboratory | |
| Baseline hemoglobin | 14.7 (1.3, 10–18) |
| Prostate specific antigen, g/dL; (mean, SD) | 4.3 (3.9, 0.1–23) |
| Characteristic | Statistic * |
|---|---|
| Intraoperative, min | |
| Anesthesia duration | 50.5 (19.8, 22–115) |
| Operative time | 24.2 (11.3, 8–70) |
| Handpiece in/out time | 18.9 (8.5, 7–60) |
| Postoperative | |
| Discharge hemoglobin | 12.8 (1.8, 7.2–17) |
| Change in hemoglobin | −2 (1.7, −7.5–0.5) |
| Length of stay, mean (SD, range) | 2.2 (1.7, 0–12) |
| CD Grade | Term | Events | Subjects | Rate |
|---|---|---|---|---|
| 1 | Bleeding | 3 | 3 | 2% |
| Cardiovascular | 4 | 4 | 2% | |
| Ejaculatory dysfunction | 14 | 14 | 8% | |
| Erectile dysfunction | 1 | 1 | 1% | |
| Infection | 2 | 2 | 1% | |
| Other | 1 | 1 | 1% | |
| Paraphimosis | 1 | 1 | 1% | |
| Urinary incontinence | 1 | 1 | 1% | |
| Urinary retention | 6 | 6 | 3% | |
| 2 | Bleeding | 3 | 3 | 2% |
| Cardiovascular | 1 | 1 | 1% | |
| Infection | 8 | 7 | 4% | |
| Other | 1 | 1 | 1% | |
| Urinary retention | 2 | 2 | 1% | |
| 3a | Ejaculatory dysfunction (climacturia *) | 1 | 1 | 1% |
| Infection | 1 | 1 | 1% | |
| Meatal stenosis | 2 | 2 | 1% | |
| Poor urinary flow | 1 | 1 | 1% | |
| 3b | Bleeding | 14 | 13 | 7% |
| Rectal perforation | 1 | 1 | 1% | |
| Urethral stricture | 1 | 1 | 1% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bach, T.; Gilling, P.; El Hajj, A.; Anderson, P.; Barber, N. First Multi-Center All-Comers Study for the Aquablation Procedure. J. Clin. Med. 2020, 9, 603. https://doi.org/10.3390/jcm9020603
Bach T, Gilling P, El Hajj A, Anderson P, Barber N. First Multi-Center All-Comers Study for the Aquablation Procedure. Journal of Clinical Medicine. 2020; 9(2):603. https://doi.org/10.3390/jcm9020603
Chicago/Turabian StyleBach, Thorsten, Peter Gilling, Albert El Hajj, Paul Anderson, and Neil Barber. 2020. "First Multi-Center All-Comers Study for the Aquablation Procedure" Journal of Clinical Medicine 9, no. 2: 603. https://doi.org/10.3390/jcm9020603
APA StyleBach, T., Gilling, P., El Hajj, A., Anderson, P., & Barber, N. (2020). First Multi-Center All-Comers Study for the Aquablation Procedure. Journal of Clinical Medicine, 9(2), 603. https://doi.org/10.3390/jcm9020603





