Identification of Clonality through Genomic Profile Analysis in Multiple Lung Cancers
Abstract
1. Introduction
2. Methods
2.1. Patients and Sample Preparation
2.2. Targeted Deep Sequencing and Data Analysis
3. Results
3.1. Patient Characteristics
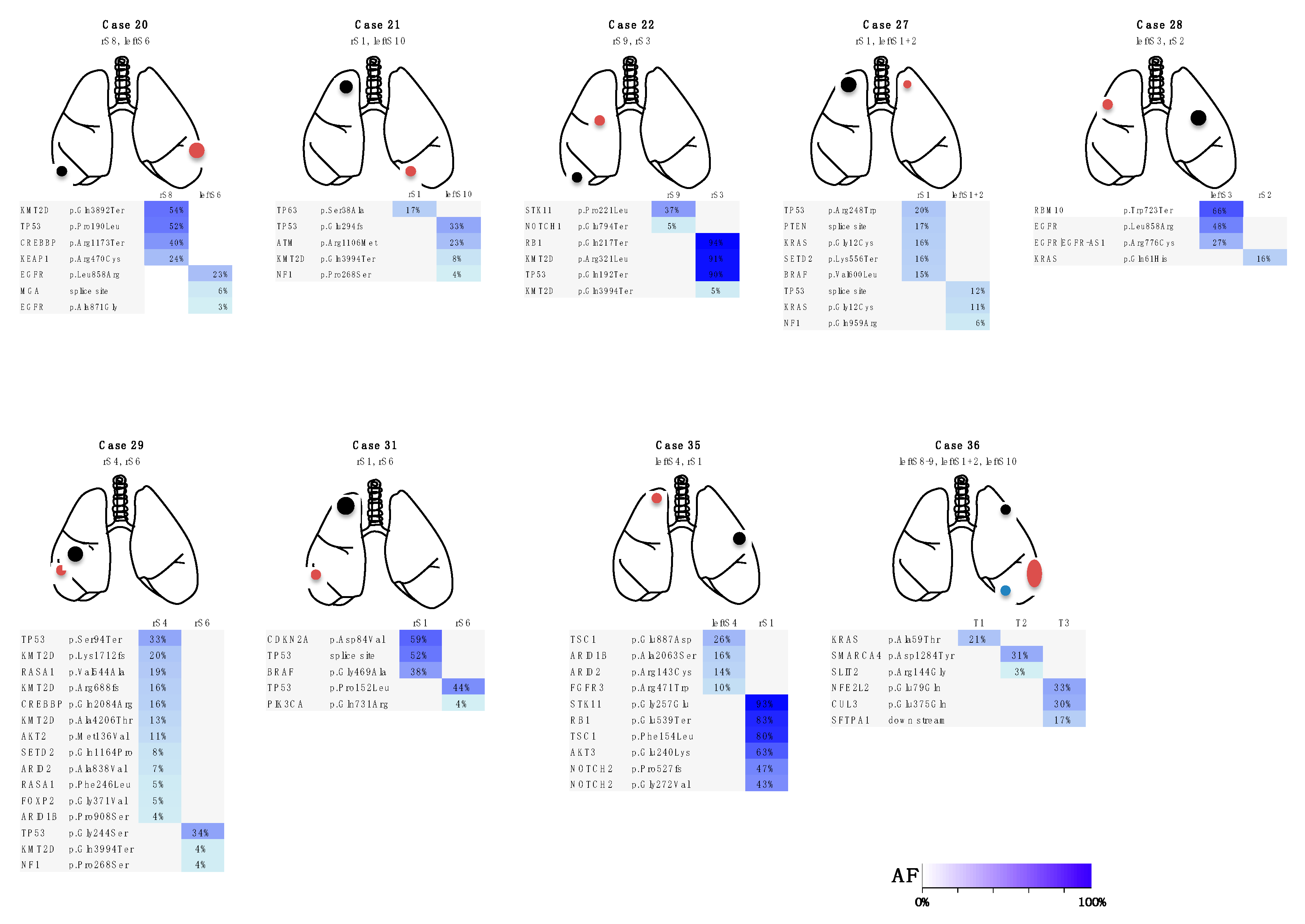
3.2. Targeted Sequencing Identified Somatic Mutations in the Lung Cancers
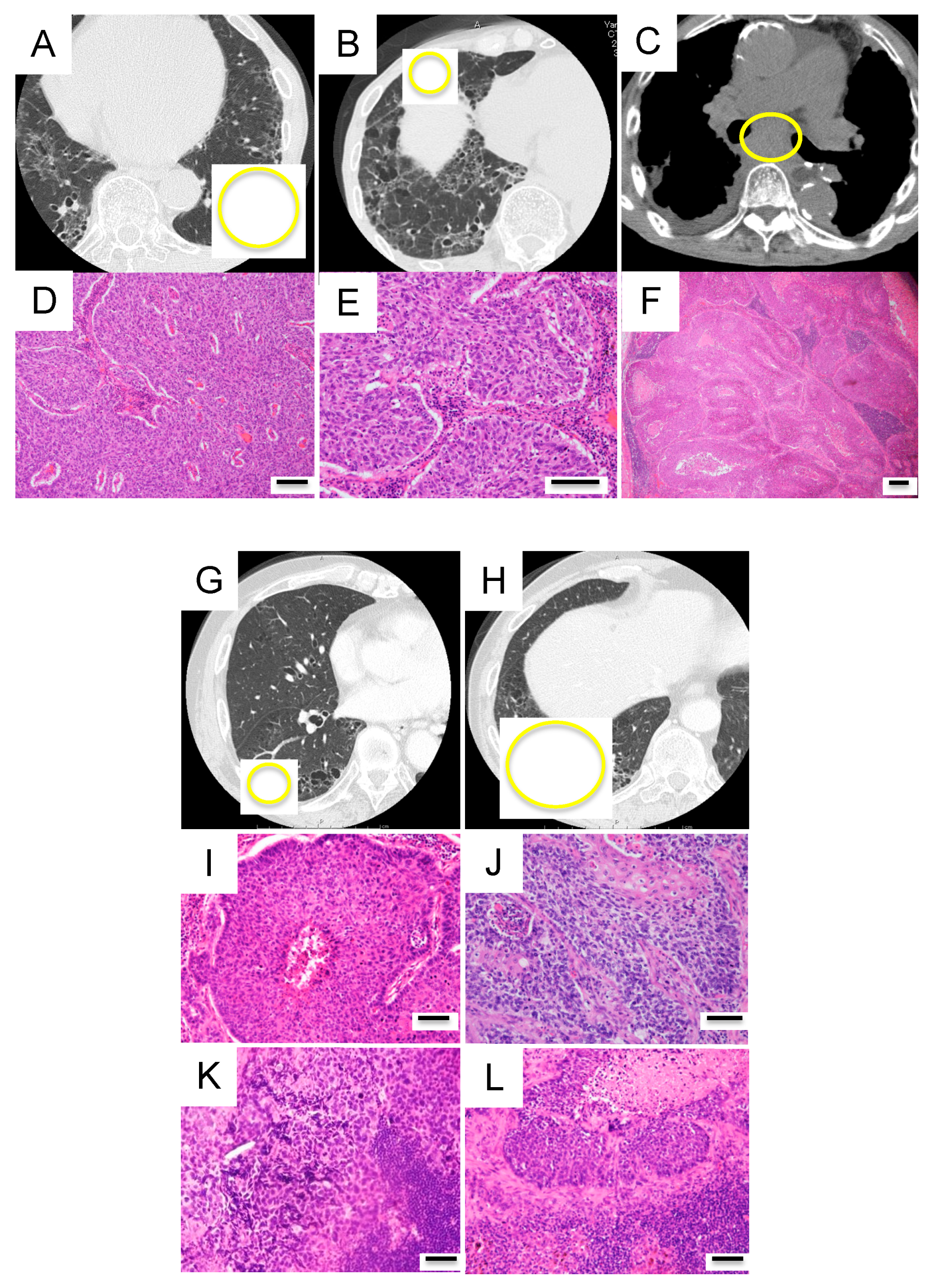
3.3. Case Presentations
Three Representative Cases are Described in Detail Below
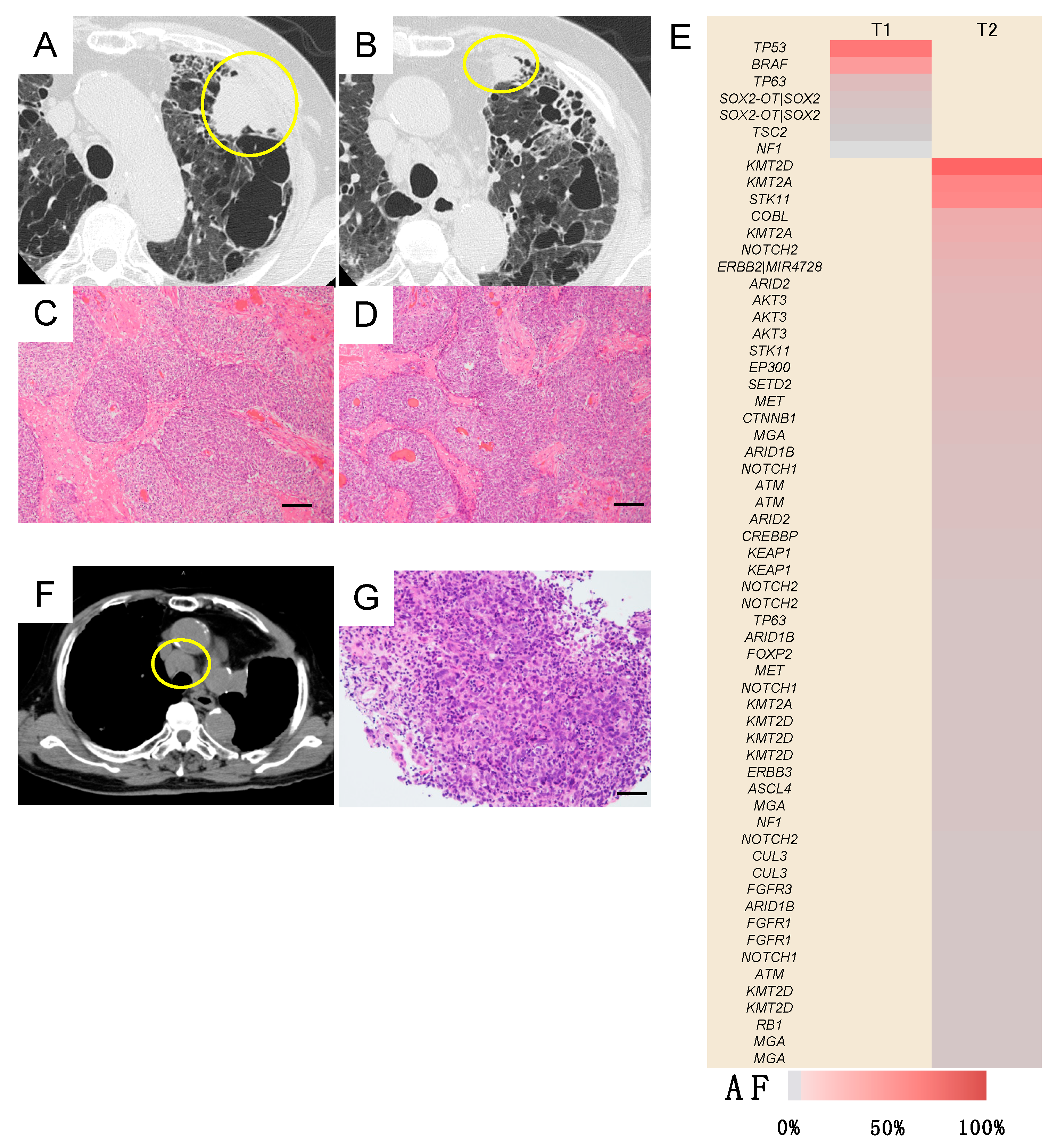
Case A (Case 30 in Table 1 and Table S2)
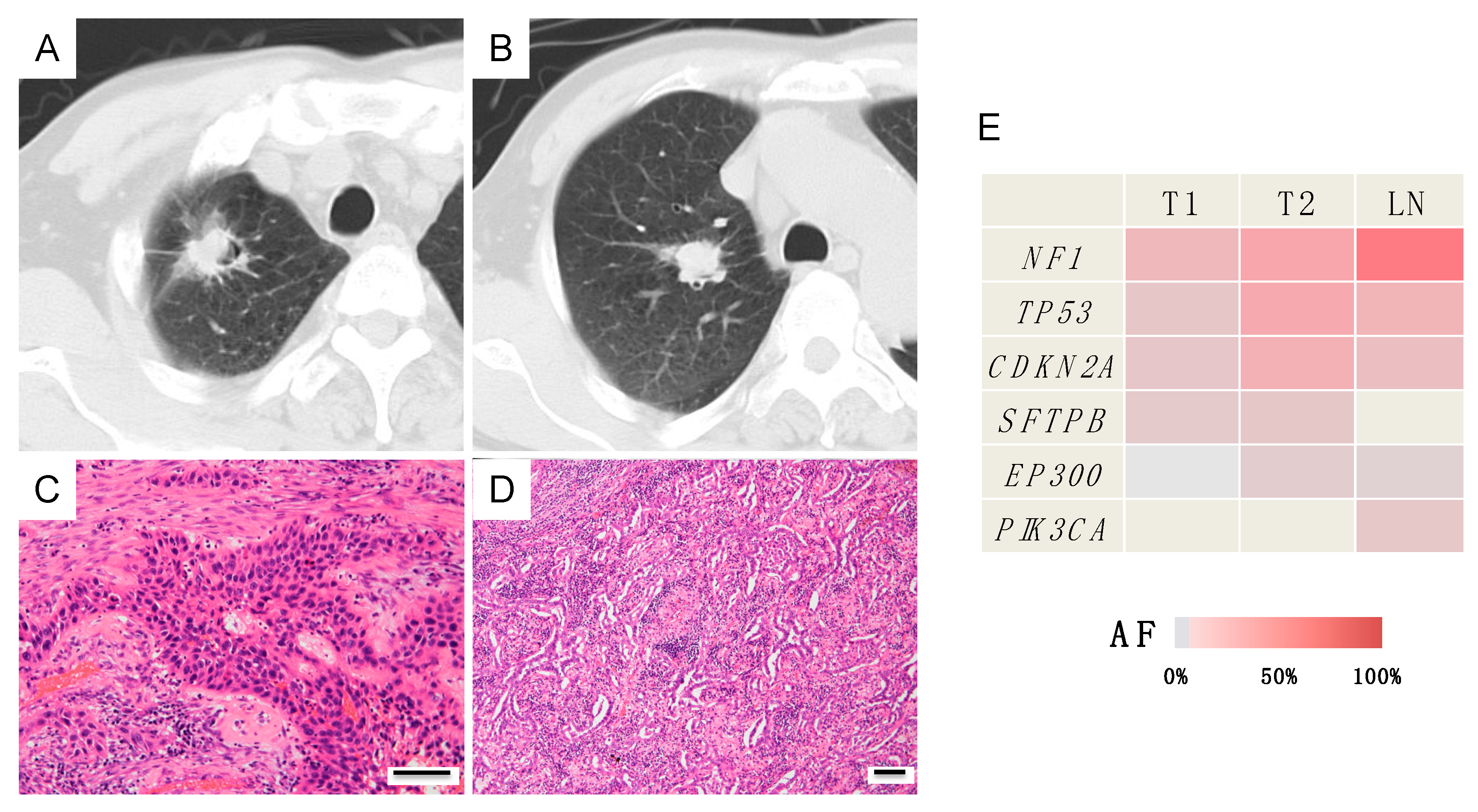
Case B (Case 10 in Table 1 and Table S2)
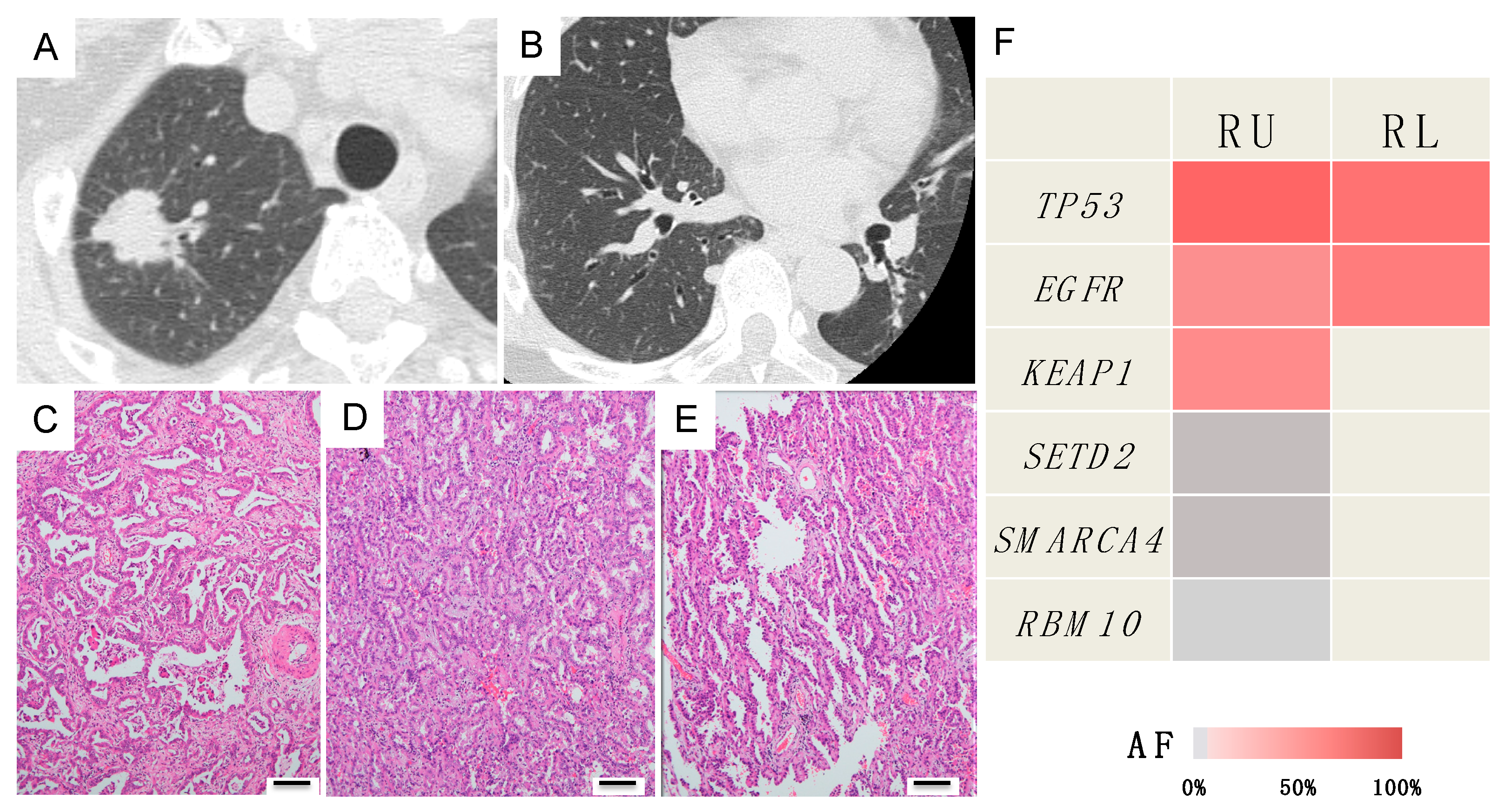
Case C (Case 18 in Table 1 and Table S2)
3.4. Investigation of the Discrepancies between the Clinical and/or Histopathological Diagnoses and Genetic Diagnosis
3.5. Genetic Diagnosis of Lymph Node Metastasis in Patients with Multiple Lung Cancers
3.6. Case Presentations
Three Representative Cases are Described in Detail Below
Case D (Case I in Table 2 and Figure 6)
Case E (Case II in Table 2 and Figure 6)
Case F (Case III in Table 2 and Figure 6)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goto, T.; Hirotsu, Y.; Mochizuki, H.; Nakagomi, T.; Shikata, D.; Yokoyama, Y.; Oyama, T.; Amemiya, K.; Okimoto, K.; Omata, M. Mutational analysis of multiple lung cancers: Discrimination between primary and metastatic lung cancers by genomic profile. Oncotarget 2017, 8, 31133–31143. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.R.; Thunnissen, F.B. Histological typing of lung and pleural tumours: Third edition. J. Clin. Pathol. 2001, 54, 498–499. [Google Scholar] [CrossRef]
- Chansky, K.; Detterbeck, F.C.; Nicholson, A.G.; Rusch, V.W.; Vallieres, E.; Groome, P.; Kennedy, C.; Krasnik, M.; Peake, M.; Shemanski, L.; et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2017, 12, 1109–1121. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012, 489, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Clinical Lung Cancer Genome Project (CLCGP); Network Genomic Medicine (NGM). A genomics-based classification of human lung tumors. Sci. Transl. Med. 2013, 5, 209ra153. [Google Scholar]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef]
- Peifer, M.; Fernandez-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef]
- Imielinski, M.; Berger, A.H.; Hammerman, P.S.; Hernandez, B.; Pugh, T.J.; Hodis, E.; Cho, J.; Suh, J.; Capelletti, M.; Sivachenko, A.; et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012, 150, 1107–1120. [Google Scholar] [CrossRef]
- Govindan, R.; Ding, L.; Griffith, M.; Subramanian, J.; Dees, N.D.; Kanchi, K.L.; Maher, C.A.; Fulton, R.; Fulton, L.; Wallis, J.; et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012, 150, 1121–1134. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Boutselakis, H.; Bamford, S.; Bindal, N.; Tate, J.; Cole, C.G.; Ward, S.; Dawson, E.; Ponting, L.; et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017, 45, D777–D783. [Google Scholar] [CrossRef]
- Goto, T.; Hirotsu, Y.; Oyama, T.; Amemiya, K.; Omata, M. Analysis of tumor-derived DNA in plasma and bone marrow fluid in lung cancer patients. Med. Oncol. 2016, 33, 29. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, T.; Goto, T.; Hirotsu, Y.; Shikata, D.; Yokoyama, Y.; Higuchi, R.; Otake, S.; Amemiya, K.; Oyama, T.; Mochizuki, H.; et al. Genomic Characteristics of Invasive Mucinous Adenocarcinomas of the Lung and Potential Therapeutic Targets of B7-H3. Cancers 2018, 10, 487. [Google Scholar] [CrossRef]
- Goto, T.; Hirotsu, Y.; Amemiya, K.; Mochizuki, H.; Omata, M. Understanding Intratumor Heterogeneity and Evolution in NSCLC and Potential New Therapeutic Approach. Cancers 2018, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.K.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef]
- Goto, T.; Hirotsu, Y.; Mochizuki, H.; Nakagomi, T.; Oyama, T.; Amemiya, K.; Omata, M. Stepwise addition of genetic changes correlated with histological change from “well-differentiated” to “sarcomatoid” phenotypes: A case report. BMC Cancer 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Matsuo, K.; Mitsudomi, T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J. Clin. Oncol. 2011, 29, 2972–2977. [Google Scholar] [CrossRef]
- Swanton, C. Intratumor heterogeneity: Evolution through space and time. Cancer Res. 2012, 72, 4875–4882. [Google Scholar] [CrossRef]
- Yap, T.A.; Gerlinger, M.; Futreal, P.A.; Pusztai, L.; Swanton, C. Intratumor heterogeneity: Seeing the wood for the trees. Sci. Transl. Med. 2012, 4, 127ps10. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Nicholson, A.G.; Franklin, W.A.; Marom, E.M.; Travis, W.D.; Girard, N.; Arenberg, D.A.; Bolejack, V.; Donington, J.S.; Mazzone, P.J.; et al. The IASLC Lung Cancer Staging Project: Summary of Proposals for Revisions of the Classification of Lung Cancers with Multiple Pulmonary Sites of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J. Thorac. Oncol. 2016, 11, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Goto, T. Radiation as an In Situ Auto-Vaccination: Current Perspectives and Challenges. Vaccines 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]



| Case | Occurrence of Tumors | Interval between the 1st and 2nd Tumors | Clinical Dx | Pathological Dx | Genomic Dx |
|---|---|---|---|---|---|
| 1 | Synchronous | - | Double | Double | Double |
| 2 | Synchronous | - | Double | Double | Double |
| 3 | Synchronous | - | Double | Double | Double |
| 4 | Synchronous | - | Metastasis | Metastasis | Double |
| 5 | Synchronous | - | Double | Double | Double |
| 6 | Synchronous | - | Double | Double | Double |
| 7 | Synchronous | - | Double | Double | Double |
| 8 | Synchronous | - | Double | Double | Double |
| 9 | Synchronous | - | Double | Double | Double |
| 10 | Metachronous | 14 months | Metastasis | Double | Metastasis |
| 11 | Synchronous | - | Double | Double | Double |
| 12 | Synchronous | - | Metastasis | Double | Double |
| 13 | Synchronous | - | Double | Double | Double |
| 14 | Synchronous | - | Double | Double | Double |
| 15 | Metachronous | 15 months | Double | Metastasis | Metastasis |
| 16 | Synchronous | - | Double | Double | Double |
| 17 | Synchronous | - | Double | Double | Double |
| 18 | Synchronous | - | Metastasis | Metastasis | Double |
| 19 | Synchronous | - | Double | Double | Double |
| 20 | Synchronous | - | Double | Double | Double |
| 21 | Metachronous | 17 months | Double | Double | Double |
| 22 | Metachronous | 28 months | Double | Double | Double |
| 23 | Metachronous | 23 months | Double | Metastasis | Metastasis |
| 24 | Metachronous | 37 months | Double | Double | Double |
| 25 | Metachronous | 37 months | Double | Double | Metastasis |
| 26 | Synchronous | - | Double | Double | Double |
| 27 | Synchronous | - | Double | Double | Double |
| 28 | Metachronous | 41 months | Double | Double | Double |
| 29 | Synchronous | - | Double | Double | Double |
| 30 | Synchronous | - | Double | Double | Metastasis |
| 31 | Synchronous | - | Double | Double | Double |
| 32 | Metachronous | 16 months | Double | Metastasis | Metastasis |
| 33 | Synchronous | - | Double | Metastasis | Metastasis |
| 34 | Metachronous | 13 months | Double | Double | Double |
| 35 | Synchronous | - | Double | Double | Double |
| 36 | Synchronous | - | triple | triple | triple |
| 37 | Metachronous | 46 months | Double | Double | Metastasis |
| Case | Case No. in Table 1 | Location and Histology of T1 | Location and Histology of T2 | Location and Histology of LN | LN Biopsy Method | Occurrence of LN Metastasis | Inconsistency between Clinical and Genomic Diagnoses |
|---|---|---|---|---|---|---|---|
| I | 18 | Left upper, Sq | Left upper, Sq | Right tracheobronchial, Sq | EBUS-TBNA | Postoperative | + |
| II | 34 | Left lower, Sq | Middle, Sq | Subcarinal, Sq | EBUS-TBNA | Postoperative | + |
| III | 26 | Right S9, Sq | Right S6, Sq | Interlobar and subcarinal, Sq | Surgery | Simultaneous | + |
| IV | 12 | Left S6, Sq | Left S10, small | Subcarinal, Sq | Surgery | Simultaneous | − |
| V | 24 | Right lower, acinar Ad | Left lower, solid Ad | Left lobar, solid Ad | Surgery | Simultaneous | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, R.; Nakagomi, T.; Goto, T.; Hirotsu, Y.; Shikata, D.; Yokoyama, Y.; Otake, S.; Amemiya, K.; Oyama, T.; Mochizuki, H.; et al. Identification of Clonality through Genomic Profile Analysis in Multiple Lung Cancers. J. Clin. Med. 2020, 9, 573. https://doi.org/10.3390/jcm9020573
Higuchi R, Nakagomi T, Goto T, Hirotsu Y, Shikata D, Yokoyama Y, Otake S, Amemiya K, Oyama T, Mochizuki H, et al. Identification of Clonality through Genomic Profile Analysis in Multiple Lung Cancers. Journal of Clinical Medicine. 2020; 9(2):573. https://doi.org/10.3390/jcm9020573
Chicago/Turabian StyleHiguchi, Rumi, Takahiro Nakagomi, Taichiro Goto, Yosuke Hirotsu, Daichi Shikata, Yujiro Yokoyama, Sotaro Otake, Kenji Amemiya, Toshio Oyama, Hitoshi Mochizuki, and et al. 2020. "Identification of Clonality through Genomic Profile Analysis in Multiple Lung Cancers" Journal of Clinical Medicine 9, no. 2: 573. https://doi.org/10.3390/jcm9020573
APA StyleHiguchi, R., Nakagomi, T., Goto, T., Hirotsu, Y., Shikata, D., Yokoyama, Y., Otake, S., Amemiya, K., Oyama, T., Mochizuki, H., & Omata, M. (2020). Identification of Clonality through Genomic Profile Analysis in Multiple Lung Cancers. Journal of Clinical Medicine, 9(2), 573. https://doi.org/10.3390/jcm9020573





