Prognostic Significance of Glucose Metabolism as GLUT1 in Patients with Pulmonary Pleomorphic Carcinoma
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
2.2. Immunohistochemical Staining
2.3. Statistical Analysis
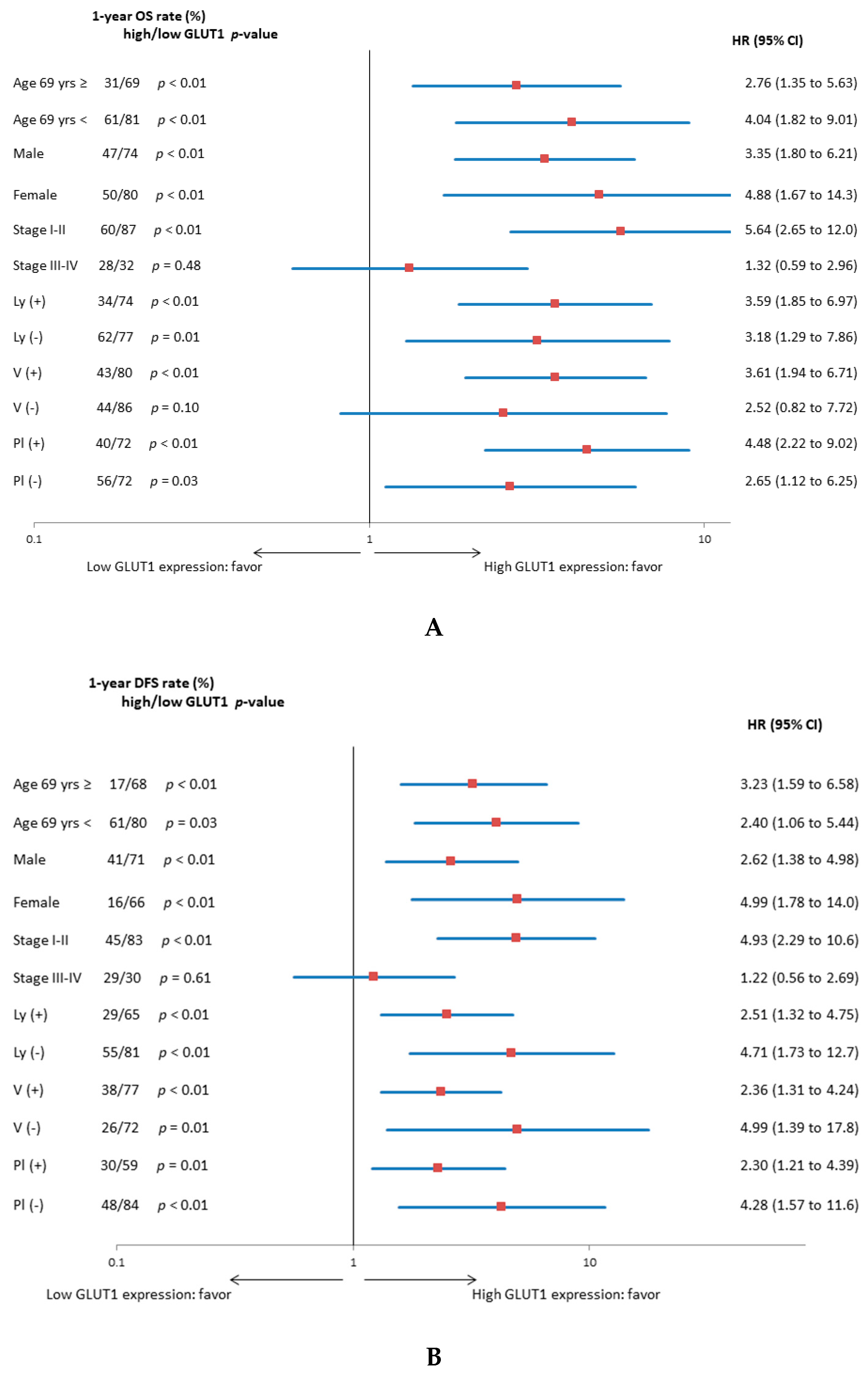
3. Results
3.1. Patient Demographics and Immunohistochemistry
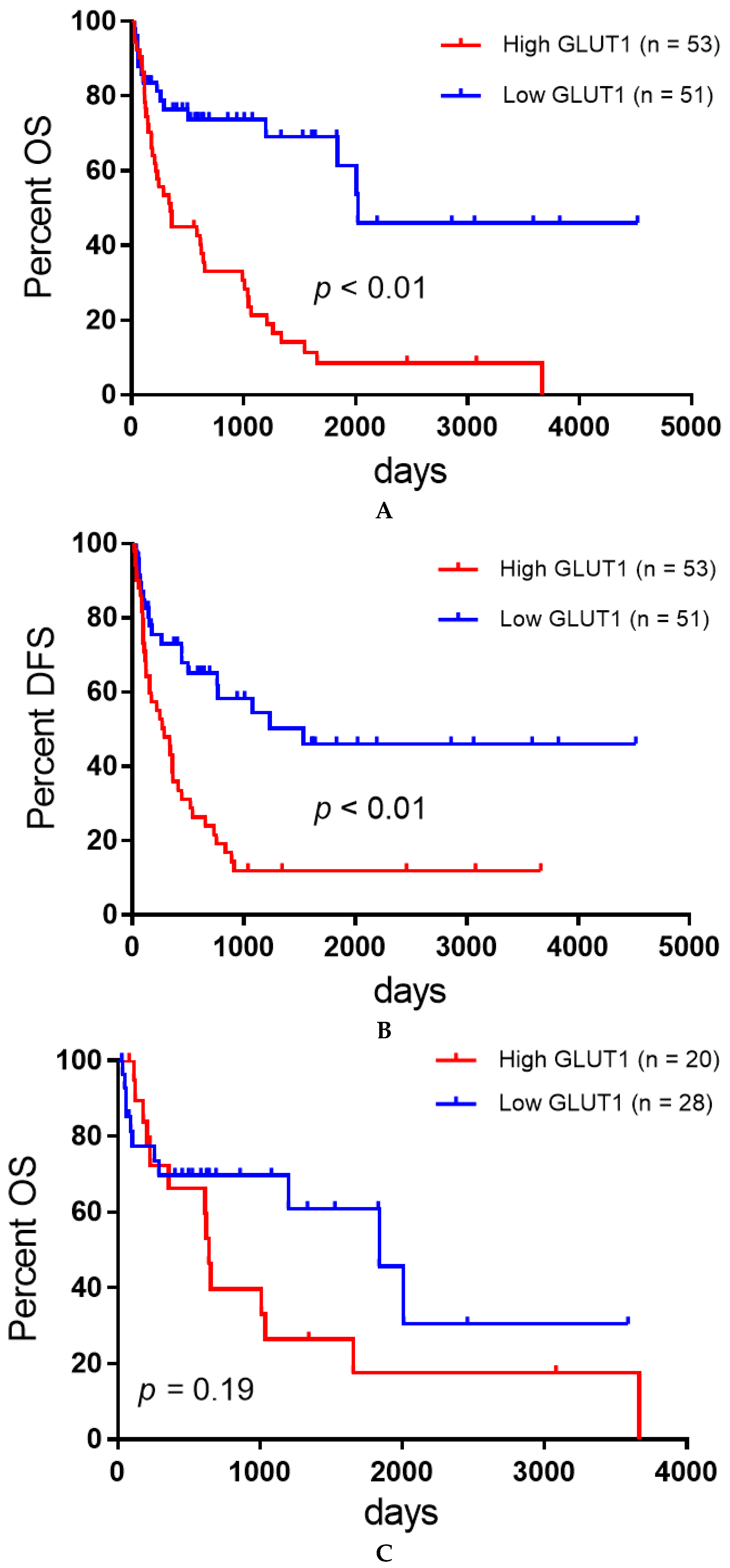
3.2. Univariate and Multivariate Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, Y.L.; Lee, Y.C.; Shih, J.Y.; Wu, C.T. Pulmonary pleomorphic (spindle) cell carcinoma: Peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer 2001, 34, 91–97. [Google Scholar] [CrossRef]
- Kerr, K.M.; Pelosi, G.; Austin, J.H.M. Pleomorphic, spindle cell, and giant cell carcinoma. In WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart; Travis, W.D., Brambilla, E., Burke, A.P., Marx, A., Nicholson, A.G., Eds.; IARC Press: Lyon, France, 2015; Volume 7, pp. 88–90. [Google Scholar]
- Fishback, N.F.; Travis, W.D.; Moran, C.A.; Guinee, D.G., Jr.; McCarthy, W.F.; Koss, M.N. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 1994, 73, 2936–2945. [Google Scholar] [CrossRef]
- Kaira, K.; Endo, M.; Abe, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Takahashi, T.; Murakami, H.; Tsuya, A.; Nakamura, Y.; et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J. Clin. Oncol. 2010, 28, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, Z.; Li, B. The clinicopathologic impacts and prognostic significance of GLUT1 expression in patients with lung cancer: A meta-analysis. Gene 2019, 689, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Endo, M.; Abe, M.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Takahashi, T.; Murakami, H.; Tsuya, A.; Nakamura, Y.; et al. Biologic correlates of ¹⁸F-FDG uptake on PET in pulmonary pleomorphic carcinoma. Lung Cancer 2011, 71, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Kawashima, O.; Endoh, H.; Imaizumi, K.; Goto, Y.; Kamiyoshihara, M.; Sugano, M.; Yamamoto, R.; Osaki, T.; Tanaka, S.; et al. Expression of amino acid transporter (LAT1 and 4F2hc) in pulmonary pleomorphic carcinoma. Hum. Pathol. 2019, 84, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Kamiyoshihara, M.; Kawashima, O.; Endoh, H.; Imaizumi, K.; Sugano, M.; Tanaka, S.; Fujita, A.; Kogure, Y.; Shimizu, A.; et al. Prognostic impact of β2 adrenergic receptor expression in surgically resected pulmonary pleomorphic carcinoma. Anticancer Res. 2019, 39, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Shimizu, K.; Kawashima, O.; Endoh, H.; Imaizumi, K.; Goto, Y.; Kamiyoshihara, M.; Sugano, M.; Yamamoto, R.; Tanaka, S.; et al. Clinical significance of various drug-sensitivity markers in patients with surgically resected pulmonary pleomorphic carcinoma. Cancers 2019, 11, 1636. [Google Scholar] [CrossRef]
- Kaira, K.; Shimizu, K.; Kitahara, S.; Yajima, T.; Atsumi, J.; Kosaka, T.; Ohtaki, Y.; Higuchi, T.; Oyama, T.; Asao, T.; et al. 2-Deoxy-2-[fluorine-18] fluoro-d-glucose uptake on positron emission tomography is associated with programmed death ligand-1 expression in patients with pulmonary adenocarcinoma. Eur. J. Cancer 2018, 101, 181–190. [Google Scholar] [CrossRef] [PubMed]
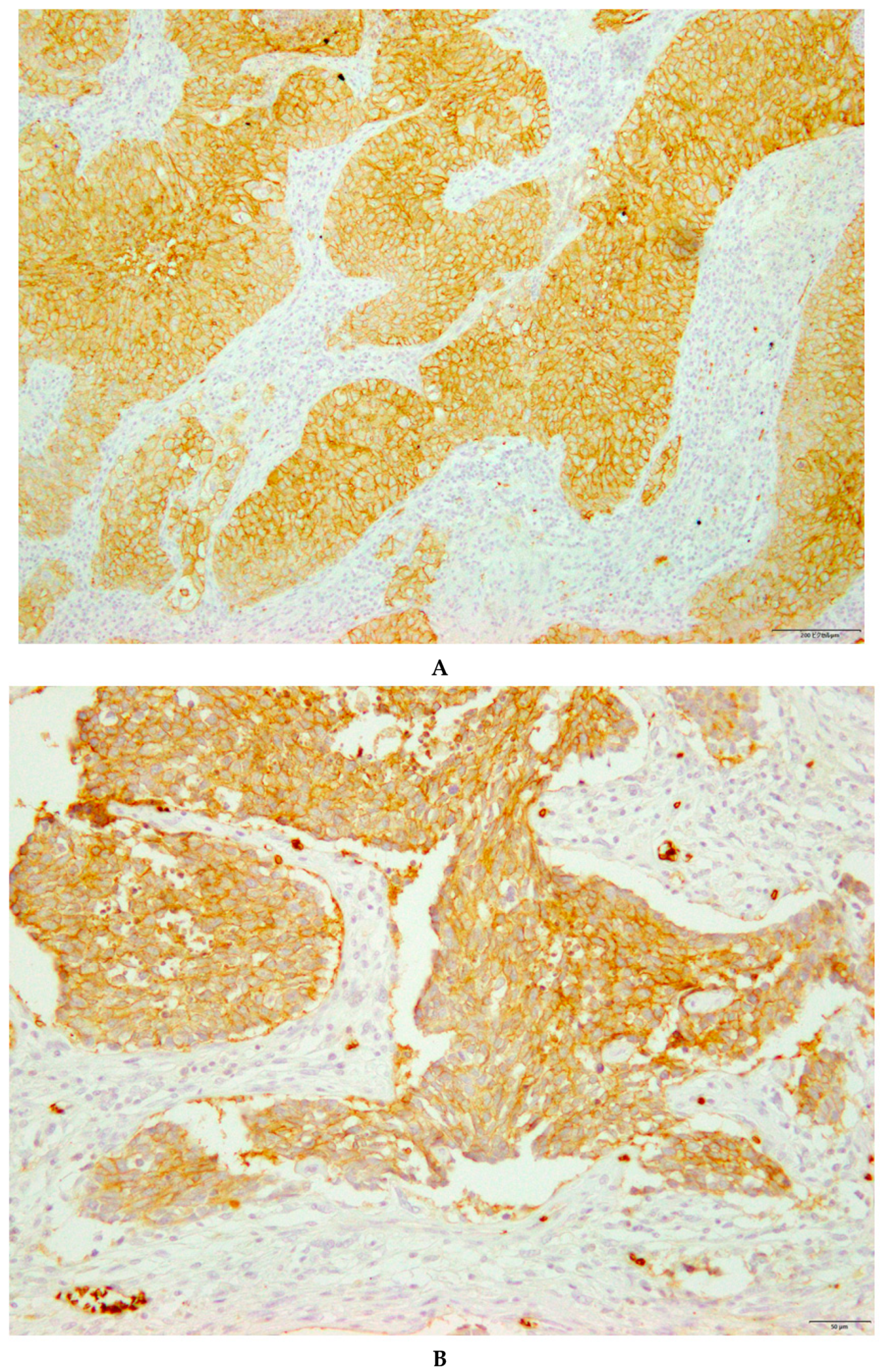
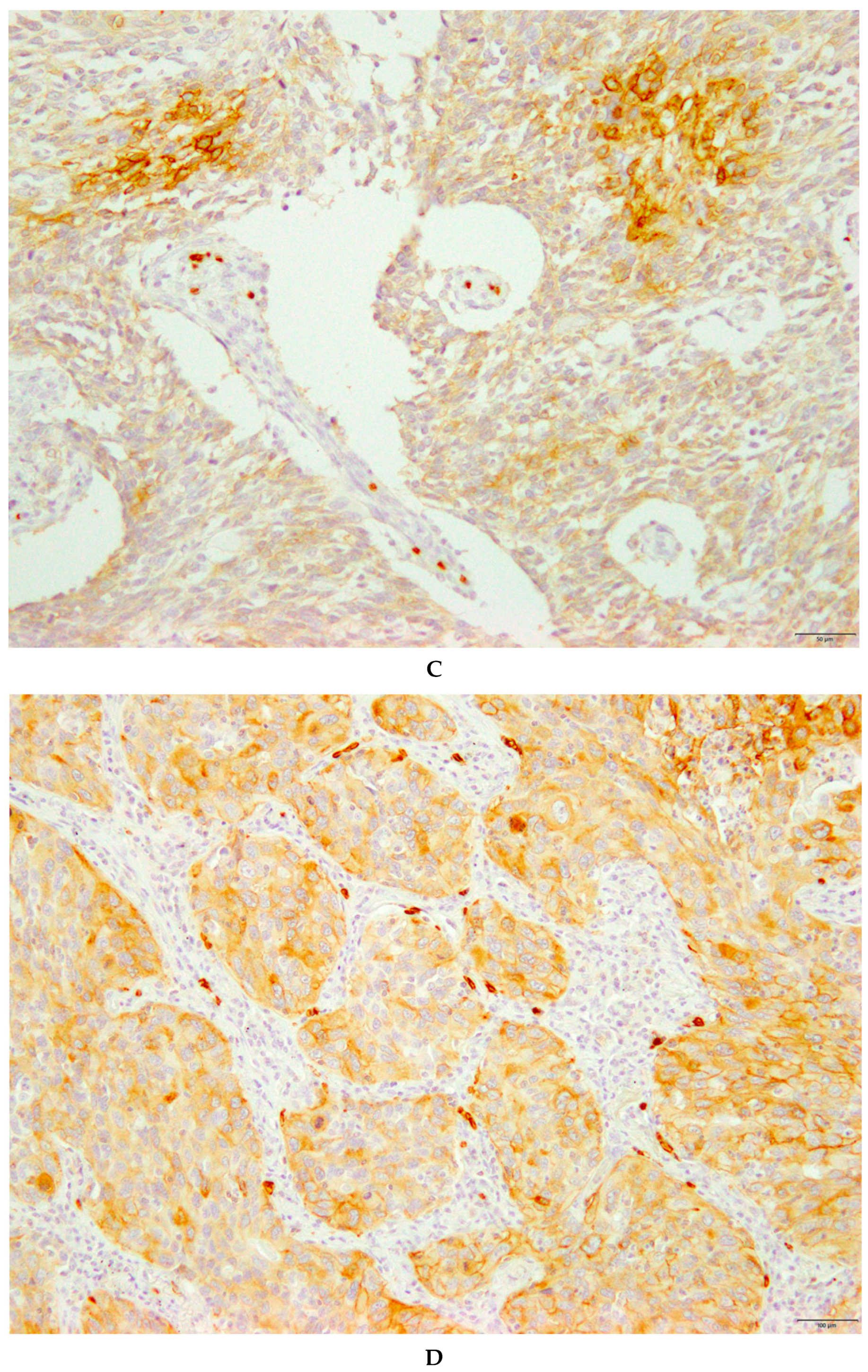

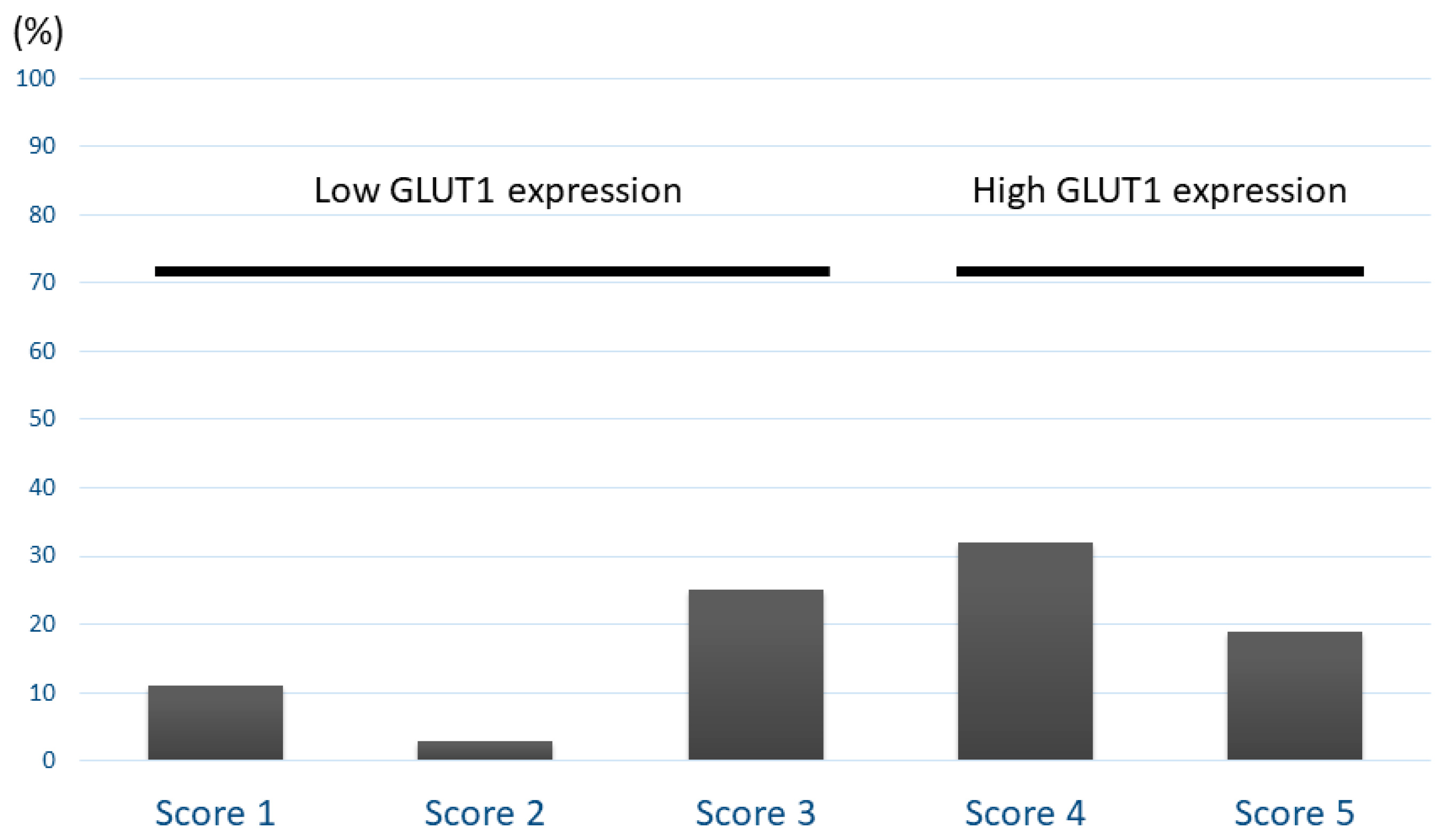

| Variables | GLUT1 Expression in All Patients | |||
|---|---|---|---|---|
| Total (n = 104) | High (n = 50) | Low (n = 54) | p-Value | |
| Age | ||||
| <69 years/≥69 years | 54/50 | 30/20 | 24/30 | 0.12 |
| Gender | ||||
| Male/Female | 79/25 | 35/15 | 44/10 | 0.25 |
| Smoking | ||||
| Yes/No | 84/20 | 40/10 | 44/10 | >0.99 |
| T factor | ||||
| T1-2/T3-4 | 65/39 | 25/25 | 40/14 | 0.11 |
| N factor | ||||
| Absent/Present | 72/32 | 33/17 | 39/15 | 0.53 |
| Stage | ||||
| I-II/III-IV | 69/35 | 28/22 | 41/13 | 0.03* |
| Lymphatic permeation | ||||
| Absent/Present | 41/63 | 17/33 | 24/30 | 0.31 |
| Vascular invasion | ||||
| Absent/Present | 31/73 | 9/41 | 22/32 | 0.02* |
| Pleural invasion | ||||
| Absent/Present | 48/56 | 17/33 | 31/13 | <0.01* |
| Adjuvant chemotherapy | ||||
| Absent/Present | 77/27 | 35/15 | 42/12 | 0.38 |
| Ki-67 labeling index | ||||
| High/Low | 50/54 | 31/19 | 19/35 | <0.01* |
| * <0.05 | ||||
| Variables | Overall survival (OS) in Total Patients | ||||
|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||
| 1-Year Rate (%) | p-Value | HR | 95% CI | p-Value | |
| Age (<69/≥69) | 48/73 | 0.41 | |||
| Gender (female/male) | 60/58 | 0.66 | |||
| p-stage (I-II/III-IV) | 75/29 | <0.01* | 1.53 | 1.21–2.12 | <0.01* |
| Ly (present/absent) | 52/71 | 0.21 | |||
| v (present/absent) | 59/61 | 0.23 | |||
| Pl (present/absent) | 53/66 | 0.07 | |||
| Adjuvant CTx (present/absent) | 66/57 | 0.18 | |||
| GLUT1 expression (high/low) | 43/75 | <0.01* | 1.72 | 1.29–2.34 | <0.01* |
| Ki-67 labeling index (high/low) | 60/61 | 0.77 | |||
| Disease-Free Survival (DFS) in Total Patients | |||||
| Age (<69/≥69) | 41/69 | 0.12 | |||
| Gender (female/male) | 58/46 | 0.17 | |||
| p-stage (I-II/III-IV) | 67/31 | <0.01* | 1.58 | 1.19–2.09 | <0.01* |
| ly (present/absent) | 47/68 | 0.04 | |||
| v (present/absent) | 56/62 | 0.08 | |||
| pl (present/absent) | 43/71 | <0.01* | 1.15 | 0.57–1.02 | 0.07 |
| Adjuvant CTx (present/absent) | 57/52 | 0.97 | |||
| GLUT1 expression (high/low) | 36/72 | <0.01* | 1.44 | 1.08–1.95 | 0.01* |
| Ki-67 labeling index (high/low) | 51/59 | 0.64 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, H.; Kaira, K.; Endoh, H.; Imaizumi, K.; Goto, Y.; Kamiyoshihara, M.; Kosaka, T.; Yajima, T.; Ohtaki, Y.; Osaki, T.; et al. Prognostic Significance of Glucose Metabolism as GLUT1 in Patients with Pulmonary Pleomorphic Carcinoma. J. Clin. Med. 2020, 9, 413. https://doi.org/10.3390/jcm9020413
Imai H, Kaira K, Endoh H, Imaizumi K, Goto Y, Kamiyoshihara M, Kosaka T, Yajima T, Ohtaki Y, Osaki T, et al. Prognostic Significance of Glucose Metabolism as GLUT1 in Patients with Pulmonary Pleomorphic Carcinoma. Journal of Clinical Medicine. 2020; 9(2):413. https://doi.org/10.3390/jcm9020413
Chicago/Turabian StyleImai, Hisao, Kyoichi Kaira, Hideki Endoh, Kazuyoshi Imaizumi, Yasuhiro Goto, Mitsuhiro Kamiyoshihara, Takayuki Kosaka, Toshiki Yajima, Yoichi Ohtaki, Takashi Osaki, and et al. 2020. "Prognostic Significance of Glucose Metabolism as GLUT1 in Patients with Pulmonary Pleomorphic Carcinoma" Journal of Clinical Medicine 9, no. 2: 413. https://doi.org/10.3390/jcm9020413
APA StyleImai, H., Kaira, K., Endoh, H., Imaizumi, K., Goto, Y., Kamiyoshihara, M., Kosaka, T., Yajima, T., Ohtaki, Y., Osaki, T., Kogure, Y., Tanaka, S., Fujita, A., Oyama, T., Minato, K., Asao, T., & Shirabe, K. (2020). Prognostic Significance of Glucose Metabolism as GLUT1 in Patients with Pulmonary Pleomorphic Carcinoma. Journal of Clinical Medicine, 9(2), 413. https://doi.org/10.3390/jcm9020413






