Intraoperative Oxygen Delivery and Acute Kidney Injury after Liver Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anesthesia and Surgical Technique
2.3. Data Collection and Study Outcomes
2.4. Statistical Analysis
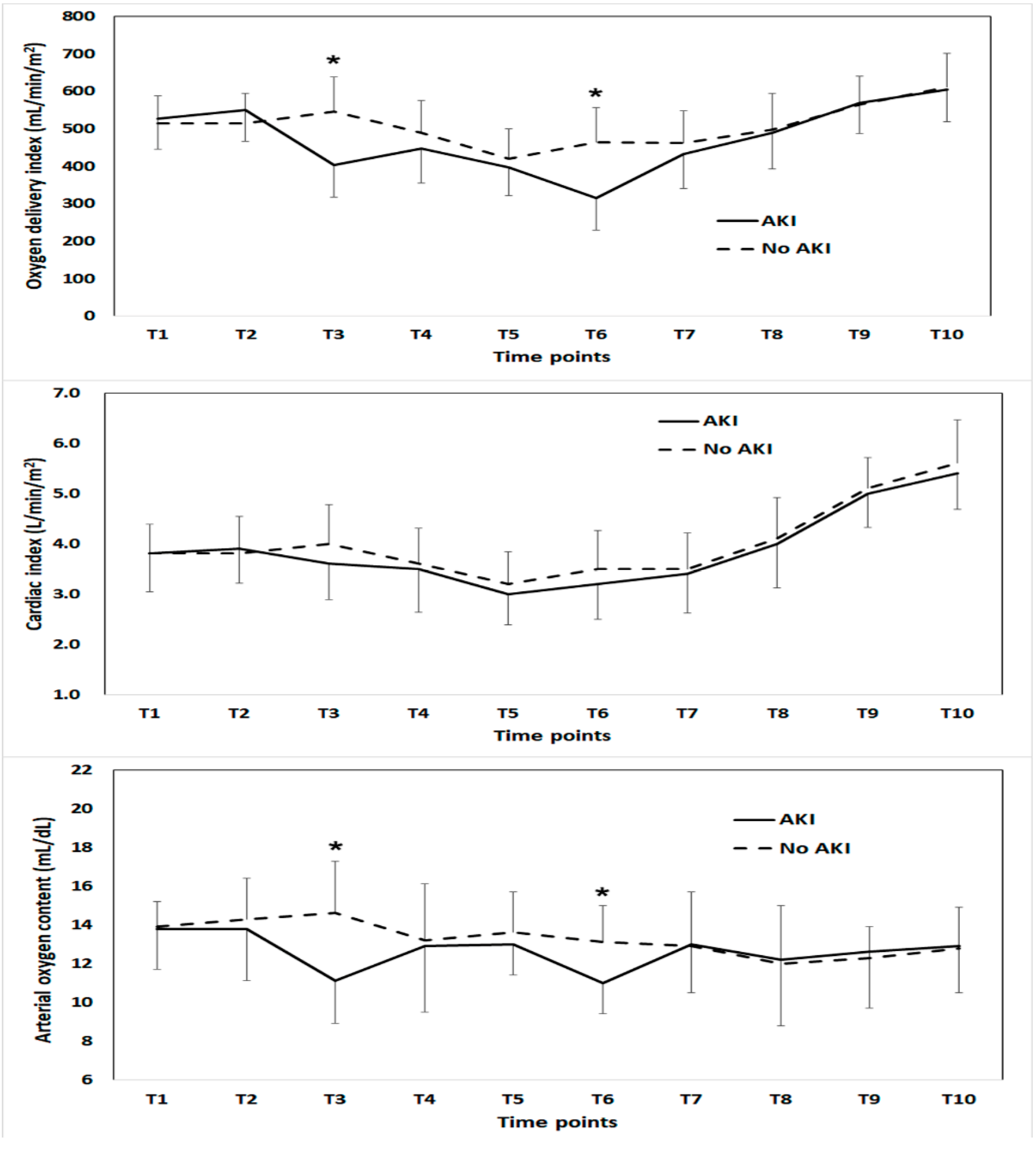
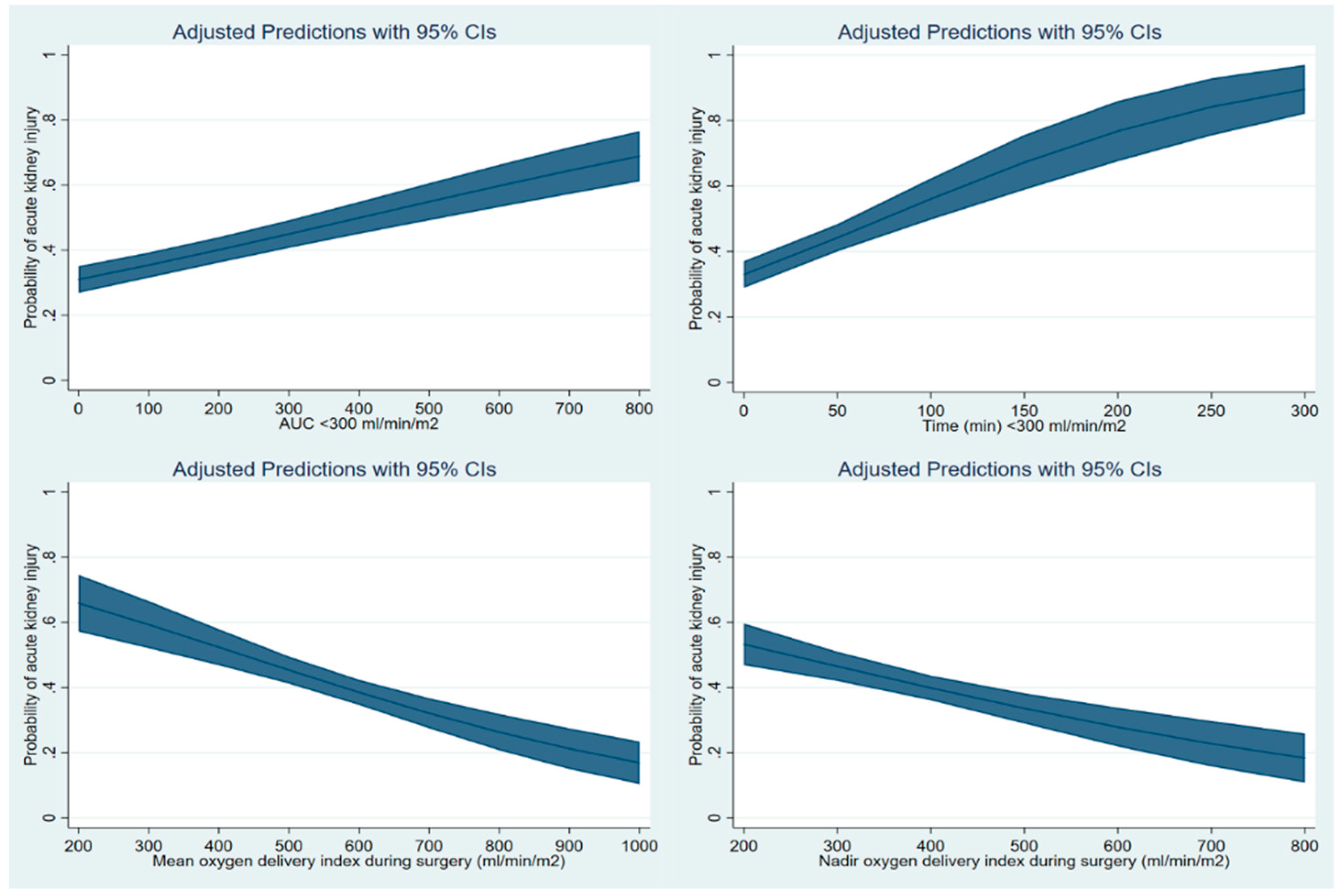
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Utsumi, M.; Umeda, Y.; Sadamori, H.; Nagasaka, T.; Takaki, A.; Matsuda, H.; Shinoura, S.; Yoshida, R.; Nobuoka, D.; Satoh, D.; et al. Risk factors for acute renal injury in living donor liver transplantation: Evaluation of the RIFLE criteria. Transpl. Int. 2013, 26, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Lebron Gallardo, M.; Herrera Gutierrez, M.E.; Seller Perez, G.; Curiel Balsera, E.; Fernandez Ortega, J.F.; Quesada Garcia, G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. 2004, 10, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Barri, Y.M.; Sanchez, E.Q.; Jennings, L.W.; Melton, L.B.; Hays, S.; Levy, M.F.; Klintmalm, G.B. Acute kidney injury following liver transplantation: Definition and outcome. Liver Trasnpl. 2009, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.M.; Arons, R.R. The burden of acute renal failure in nonrenal solid organ transplantation. Transplantation 2004, 78, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Paramesh, A.S.; Roayaie, S.; Doan, Y.; Schwartz, M.E.; Emre, S.; Fishbein, T.; Florman, S.; Gondolesi, G.E.; Krieger, N.; Ames, S.; et al. Post-liver transplant acute renal failure: Factors predicting development of end-stage renal disease. Clin. Transplant. 2004, 18, 94–99. [Google Scholar] [CrossRef]
- Trinh, E.; Alam, A.; Tchervenkov, J.; Cantarovich, M. Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin. Transplant. 2017, 31, e12863. [Google Scholar] [CrossRef]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Planinsic, R.; Boucek, C.; Sakai, T.; Chang, C.C.; Kellum, J.A. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 2015, 114, 919–926. [Google Scholar] [CrossRef] [Green Version]
- Park, M.H.; Shim, H.S.; Kim, W.H.; Kim, H.J.; Kim, D.J.; Lee, S.H.; Kim, C.S.; Gwak, M.S.; Kim, G.S. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS ONE 2015, 10, e0136230. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Soyama, A.; Takatsuki, M.; Hidaka, M.; Muraoka, I.; Kanematsu, T.; Eguchi, S. Acute kidney injury following living donor liver transplantation. Clin. Transplant. 2012, 26, E530–E535. [Google Scholar] [CrossRef]
- Chen, J.; Singhapricha, T.; Hu, K.Q.; Hong, J.C.; Steadman, R.H.; Busuttil, R.W.; Xia, V.W. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: A matched study. Transplantation 2011, 91, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Sakai, T.; Donaldson, J.; Winger, D.G.; Kellum, J.A. Acute kidney injury after orthotopic liver transplantation using living donor versus deceased donor grafts: A propensity score-matched analysis. Liver Transpl. 2015, 21, 1179–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.H.; Oh, H.W.; Yang, S.M.; Yu, J.H.; Lee, H.C.; Jung, C.W.; Suh, K.S.; Lee, K.H. Intraoperative Hemodynamic Parameters and Acute Kidney Injury After Living Donor Liver Transplantation. Transplantation 2019, 103, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Lee, H.C.; Lim, L.; Ryu, H.G.; Jung, C.W. Intraoperative Oliguria with Decreased SvO(2) Predicts Acute Kidney Injury after Living Donor Liver Transplantation. J. Clin. Med. 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLellan, S.; Walsh, T. Oxygen delivery and haemoglobin. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 123–126. [Google Scholar] [CrossRef]
- Mukaida, H.; Matsushita, S.; Kuwaki, K.; Inotani, T.; Minami, Y.; Saigusa, A.; Amano, A. Time-dose response of oxygen delivery during cardiopulmonary bypass predicts acute kidney injury. J. Thorac. Cardiovasc. Surg. 2019, 158, 492–499. [Google Scholar] [CrossRef]
- Ranucci, M.; Johnson, I.; Willcox, T.; Baker, R.A.; Boer, C.; Baumann, A.; Justison, G.A.; de Somer, F.; Exton, P.; Agarwal, S.; et al. Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J. Thorac. Cardiovasc. Surg. 2018, 156, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Rudnick, M.R.; Marchi, L.D.; Plotkin, J.S. Hemodynamic monitoring during liver transplantation: A state of the art review. World J. Hepatol. 2015, 7, 1302–1311. [Google Scholar] [CrossRef]
- Vallet, B.; Futier, E. Perioperative oxygen therapy and oxygen utilization. Curr. Opin. Crit. Care 2010, 16, 359–364. [Google Scholar] [CrossRef]
- Lees, N.; Hamilton, M.; Rhodes, A. Clinical review: Goal-directed therapy in high risk surgical patients. Crit. Care 2009, 13, 231. [Google Scholar] [CrossRef] [Green Version]
- Durand, F.; Francoz, C.; Asrani, S.K.; Khemichian, S.; Pham, T.A.; Sung, R.S.; Genyk, Y.S.; Nadim, M.K. Acute Kidney Injury after Liver Transplantation. Transplantation 2018, 102, 1636–1649. [Google Scholar] [CrossRef]
- Kalisvaart, M.; Schlegel, A.; Umbro, I.; de Haan, J.E.; Scalera, I.; Polak, W.G.; JNM, I.J.; Mirza, D.F.; Perera, M.; Isaac, J.I.; et al. The Impact of Combined Warm Ischemia Time on Development of Acute Kidney Injury in Donation after Circulatory Death Liver Transplantation: Stay within the Golden Hour. Transplantation 2018, 102, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yoon, S.B.; Yang, S.M.; Kim, W.H.; Ryu, H.G.; Jung, C.W.; Suh, K.S.; Lee, K.H. Prediction of Acute Kidney Injury after Liver Transplantation: Machine Learning Approaches vs. Logistic Regression Model. J. Clin. Med. 2018, 7, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paugam-Burtz, C.; Kavafyan, J.; Merckx, P.; Dahmani, S.; Sommacale, D.; Ramsay, M.; Belghiti, J.; Mantz, J. Postreperfusion syndrome during liver transplantation for cirrhosis: Outcome and predictors. Liver Transpl. 2009, 15, 522–529. [Google Scholar] [CrossRef]
- Vives, M.; Callejas, R.; Duque, P.; Echarri, G.; Wijeysundera, D.N.; Hernandez, A.; Sabate, A.; Bes-Rastrollo, M.; Monedero, P. Modern hydroxyethyl starch and acute kidney injury after cardiac surgery: A prospective multicentre cohort. Br. J. Anaesth. 2016, 117, 458–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selzner, M.; Kashfi, A.; Cattral, M.S.; Selzner, N.; McGilvray, I.D.; Greig, P.D.; Levy, G.A.; Renner, E.L.; Grant, D.R. Live donor liver transplantation in high MELD score recipients. Ann. Surg. 2010, 251, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010, 16, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef]
- Group, K.W. KDIGO 2012 clinical practice guidelines for evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Mayer, K.; Trzeciak, S.; Puri, N.K. Assessment of the adequacy of oxygen delivery. Curr. Opin. Crit. Care 2016, 22, 437–443. [Google Scholar] [CrossRef]
- Vernooij, L.M.; van Klei, W.A.; Machina, M.; Pasma, W.; Beattie, W.S.; Peelen, L.M. Different methods of modelling intraoperative hypotension and their association with postoperative complications in patients undergoing non-cardiac surgery. Br. J. Anaesth. 2018, 120, 1080–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, J.N.; Altman, D.G.; Campbell, M.J.; Royston, P. Analysis of serial measurements in medical research. BMJ 1990, 300, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimundo, M.; Crichton, S.; Syed, Y.; Martin, J.R.; Beale, R.; Treacher, D.; Ostermann, M. Low Systemic Oxygen Delivery and BP and Risk of Progression of Early AKI. Clin. J. Am. Soc. Nephrol. 2015, 10, 1340–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umbro, I.; Tinti, F.; Scalera, I.; Evison, F.; Gunson, B.; Sharif, A.; Ferguson, J.; Muiesan, P.; Mitterhofer, A.P. Acute kidney injury and post-reperfusion syndrome in liver transplantation. World J. Gastroenterol. 2016, 22, 9314–9323. [Google Scholar] [CrossRef] [PubMed]
- Saner, F.H.; Cicinnati, V.R.; Sotiropoulos, G.; Beckebaum, S. Strategies to prevent or reduce acute and chronic kidney injury in liver transplantation. Liver Int. 2012, 32, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U.; Bernhardt, W.M.; Weidemann, A.; Warnecke, C.; Rosenberger, C.; Wiesener, M.S.; Willam, C. Role of hypoxia in the pathogenesis of renal disease. Kidney Int. Suppl. 2005, 68, S46–S51. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.G.; Goddard, D.; Eppel, G.A.; O’Connor, P.M. Factors that render the kidney susceptible to tissue hypoxia in hypoxemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R931–R940. [Google Scholar] [CrossRef] [Green Version]
- Chae, M.S.; Lee, N.; Da Hye Park, J.L.; Jung, H.S.; Park, C.S.; Lee, J.; Choi, J.H.; Hong, S.H. Influence of oxygen content immediately after graft reperfusion on occurrence of postoperative acute kidney injury in living donor liver transplantation. Medicine 2017, 96, e7626. [Google Scholar] [CrossRef]
- Skytte Larsson, J.; Bragadottir, G.; Redfors, B.; Ricksten, S.E. Renal function and oxygenation are impaired early after liver transplantation despite hyperdynamic systemic circulation. Crit. Care 2017, 21, 87. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.Z.L.; Martin, A.; Cochrane, A.D.; Smith, J.A.; Thrift, A.G.; Harrop, G.K.; Ngo, J.P.; Evans, R.G. Urinary hypoxia: An intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrol. Dial. Transplant. 2018, 33, 2191–2201. [Google Scholar] [CrossRef] [Green Version]
- Lankadeva, Y.R.; Cochrane, A.D.; Marino, B.; Iguchi, N.; Hood, S.G.; Bellomo, R.; May, C.N.; Evans, R.G. Strategies that improve renal medullary oxygenation during experimental cardiopulmonary bypass may mitigate postoperative acute kidney injury. Kidney Int. 2019, 95, 1338–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fede, G.; Privitera, G.; Tomaselli, T.; Spadaro, L.; Purrello, F. Cardiovascular dysfunction in patients with liver cirrhosis. Ann. Gastroenterol. 2015, 28, 31–40. [Google Scholar]
- Boyd, O.; Grounds, R.M.; Bennett, E.D. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 1993, 270, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.A.; Timmins, A.C.; Yau, E.H.; Palazzo, M.; Hinds, C.J.; Watson, D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N. Engl. J. Med. 1994, 330, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.S.; Fish, D.N.; Teitelbaum, I. Assessing renal function in cirrhotic patients: Problems and pitfalls. Am. J. Kidney Dis. 2003, 41, 269–278. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Mean DO2I ≥ 500 mL/min/m2 | Mean DO2I < 500 mL/min/m2 | p-Value |
|---|---|---|---|
| Sample size | 444 (65.7) | 232 (34.3) | |
| Demographic data | |||
| Age, years | 53 (47–58) | 58 (52–63) | <0.001 |
| Female, n | 107 (24.1) | 81 (34.9) | 0.003 |
| Body-mass index, kg/m2 | 23.2 (21.2–25.3) | 23.0 (21.0–25.6) | 0.711 |
| Etiology of liver disease | |||
| Alcoholic liver cirrhosis, n | 58 (13.1) | 28 (12.1) | 0.713 |
| Hepatitis B viral hepatitis, n | 213 (48.0) | 95 (40.9) | 0.082 |
| Hepatitis C viral hepatitis, n | 42 (9.5) | 30 (12.9) | 0.165 |
| Hepatocellular carcinoma, n | 217 (48.9) | 87 (37.5) | 0.005 |
| Cholestatic disease, n | 11 (2.5) | 7 (3.0) | 0.679 |
| Non-alcoholic steatohepatitis, n | 23 (5.2) | 8 (3.4) | 0.340 |
| Baseline medical status | |||
| Hypertension, n | 46 (10.4) | 49 (21.1) | 0.001 |
| Diabetes mellitus, n | 66 (14.9) | 44 (19.0) | 0.170 |
| Preoperative hemoglobin, g/dL | 11.4 (9.7–13.1) | 10.0 (8.7–11.7) | <0.001 |
| Preoperative serum albumin level, mg/dL | 3.0 (2.6–3.6) | 2.9 (2.5–3.3) | 0.001 |
| Model for end-stage liver disease score | 13 (9–20) | 17 (11–26) | <0.001 |
| Preoperative serum creatinine, mg/dL | 0.84 (0.70–1.00) | 0.90 (0.74–1.30) | <0.001 |
| Preoperative prothrombin time, INR | 1.5 (1.2–1.9) | 1.6 (1.3–2.2) | 0.016 |
| Preoperative total bilirubin, mg/dL | 3.0 (1.6–7.4) | 3.5 (1.8–11.2) | 0.061 |
| Child-Turcotte-Pugh score | 8 (6–10) | 8 (7–10) | 0.005 |
| Child class, n (A/B/C) | 153 (34.5)/180 (40.5)/111 (25.0) | 54 (23.3)/102 (44.0)/76 (32.8) | 0.002 |
| Preoperative LVEF, % | 65 (62–68) | 65 (63–69) | 0.596 |
| Preoperative beta-blocker, n | 17 (3.8) | 27 (11.6) | <0.001 |
| Preoperative diuretics, n | 12 (2.7) | 18 (7.8) | 0.005 |
| Previous abdominal surgery, n | 8 (1.8) | 9 (3.9) | 0.122 |
| Donor/graft factors | |||
| Living/deceased donor, n | 352 (79.3)/92 (20.7) | 129 (55.6)/103 (44.4) | <0.001 |
| Age, years | 30 (22–40) | 31 (25–41) | 0.118 |
| Estimated GRWR | 1.20 (1.03–1.43) | 1.22 (1.11–1.48) | 0.035 |
| Operation and anesthesia details | |||
| Operation time, h | 6.8 (5.9–7.8) | 6.2 (5.3–7.7) | 0.003 |
| Cold ischemic time, min | 77 (65–118) | 118 (72–240) | <0.001 |
| Warm ischemic time, min | 30 (27–38) | 30 (27–33) | 0.048 |
| Intraoperative dose of epinephrine bolus, mcg | 0 (0–20) | 10 (0–40) | <0.001 |
| Intraoperative mean blood glucose, mg/dL | 165 (145–180) | 156 (140–179) | 0.014 |
| Crystalloid administration, mL | 3700 (2500–5000) | 3700 (2500–6000) | 0.096 |
| Colloid administration, mL | 0 (0–900) | 0 (0–600) | 0.526 |
| Bleeding and transfusion amount | |||
| pRBC transfusion, units | 5 (0–10) | 7 (3–14) | <0.001 |
| FFP transfusion, units | 4 (0–10) | 5 (0–14) | 0.030 |
| Variable | Multivariable-Adjusted OR | 95% CI | p-Value |
|---|---|---|---|
| Body-mass index, kg/m2 | 1.23 | 1.06–1.20 | <0.001 |
| Child class B or C vs. A | 1.99 | 1.16–3.40 | 0.012 |
| Baseline right ventricular end-diastolic volume, mL | 1.02 | 1.01–1.02 | <0.001 |
| Area under curve below DO2I of 300 mL/min/m2 | 1.10 | 1.06–1.13 | <0.001 |
| Or cumulative time (per 10 min) below DO2I of 300 mL/min/m2 | 1.10 | 1.08–1.14 | <0.001 |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Characteristic | Mean DO2I ≥ 500 mL/min/m2 (n = 444) | Mean DO2I < 500 mL/min/m2 (n = 232) | p-Value | Mean DO2I ≥ 500 mL/min/m2 (n = 192) | Mean DO2I < 500 mL/min/m2 (n = 192) | p-Value |
| Length of hospital stay, days | 16 (14–24) | 21 (16–31) | <0.001 | 17 (14–26) | 21 (16–31) | 0.008 |
| Length of ICU stay, days | 5 (4–6) | 5 (4–8) | <0.001 | 5 (4–7) | 5 (4–8) | <0.001 |
| Acute kidney injury, n | 142 (32.0) | 133 (57.3) | <0.001 | 64 (33.3) | 106 (55.2) | <0.001 |
| Stage 1, n | 117 (26.4) | 90 (38.8) | 0.001 | 52 (27.1) | 76 (39.6) | 0.009 |
| Stage 2 or 3, n | 25 (5.6) | 43 (18.5) | <0.001 | 12 (6.3) | 30 (15.6) | 0.003 |
| Early allograft dysfunction, n | 6 (1.4) | 7 (3.6) | 0.074 | 3 (1.6) | 7 (4.3) | 0.196 |
| In-hospital mortality, n | 12 (2.7) | 12 (5.2) | 0.125 | 4 (2.1) | 10 (5.2) | 0.172 |
| Chronic hemodialysis, n | 5 (1.1) | 10 (4.3) | 0.012 | 2 (1.0) | 9 (4.7) | 0.032 |
| Chronic kidney disease, n | 99 (22.3) | 61 (26.3) | 0.005 | 38 (19.8) | 55 (28.6) | 0.043 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.H.; Lee, H.-J.; Yoon, H.-C.; Lee, K.H.; Suh, K.-S. Intraoperative Oxygen Delivery and Acute Kidney Injury after Liver Transplantation. J. Clin. Med. 2020, 9, 564. https://doi.org/10.3390/jcm9020564
Kim WH, Lee H-J, Yoon H-C, Lee KH, Suh K-S. Intraoperative Oxygen Delivery and Acute Kidney Injury after Liver Transplantation. Journal of Clinical Medicine. 2020; 9(2):564. https://doi.org/10.3390/jcm9020564
Chicago/Turabian StyleKim, Won Ho, Ho-Jin Lee, Hee-Chul Yoon, Kook Hyun Lee, and Kyung-Suk Suh. 2020. "Intraoperative Oxygen Delivery and Acute Kidney Injury after Liver Transplantation" Journal of Clinical Medicine 9, no. 2: 564. https://doi.org/10.3390/jcm9020564
APA StyleKim, W. H., Lee, H.-J., Yoon, H.-C., Lee, K. H., & Suh, K.-S. (2020). Intraoperative Oxygen Delivery and Acute Kidney Injury after Liver Transplantation. Journal of Clinical Medicine, 9(2), 564. https://doi.org/10.3390/jcm9020564




