Childhood Leukemia Survivors and Metabolic Response to Exercise: A Pilot Controlled Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Physical Activity Assessment
2.3. Experimental Procedure
2.4. Measurements
2.5. Data Analysis
2.6. Statistical Considerations
3. Results
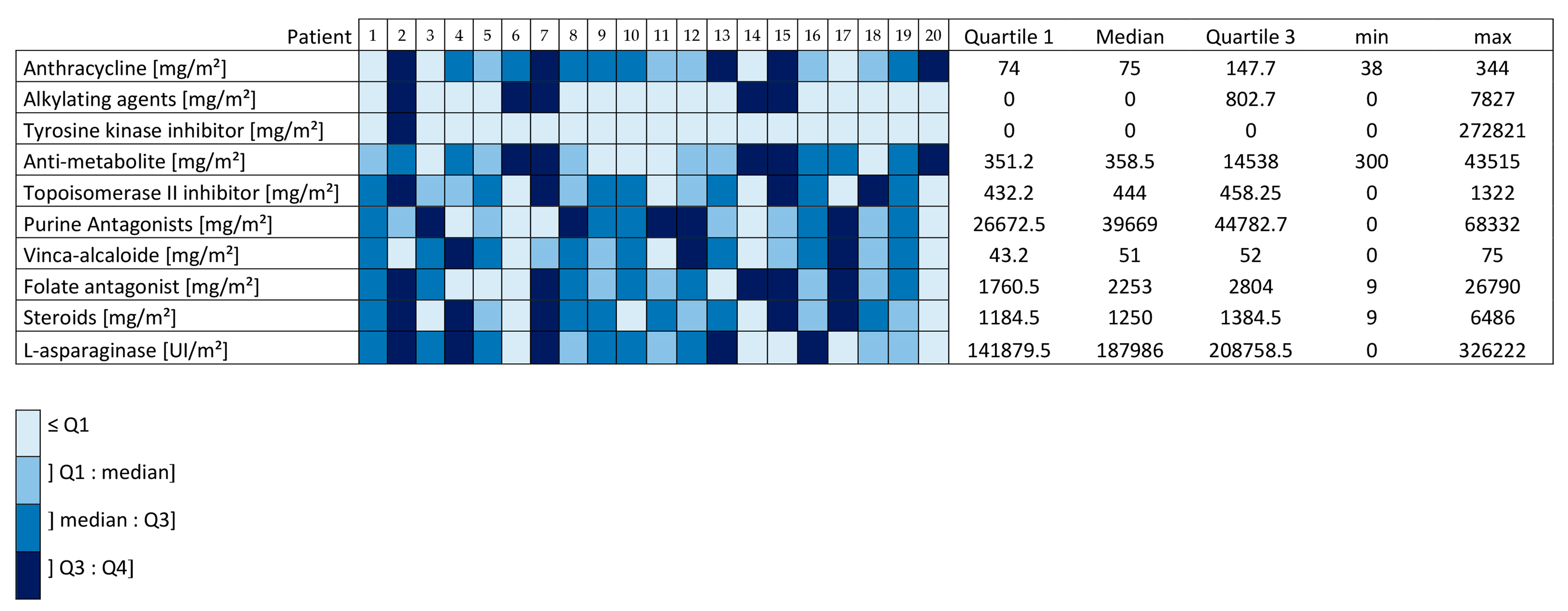
3.1. Patients
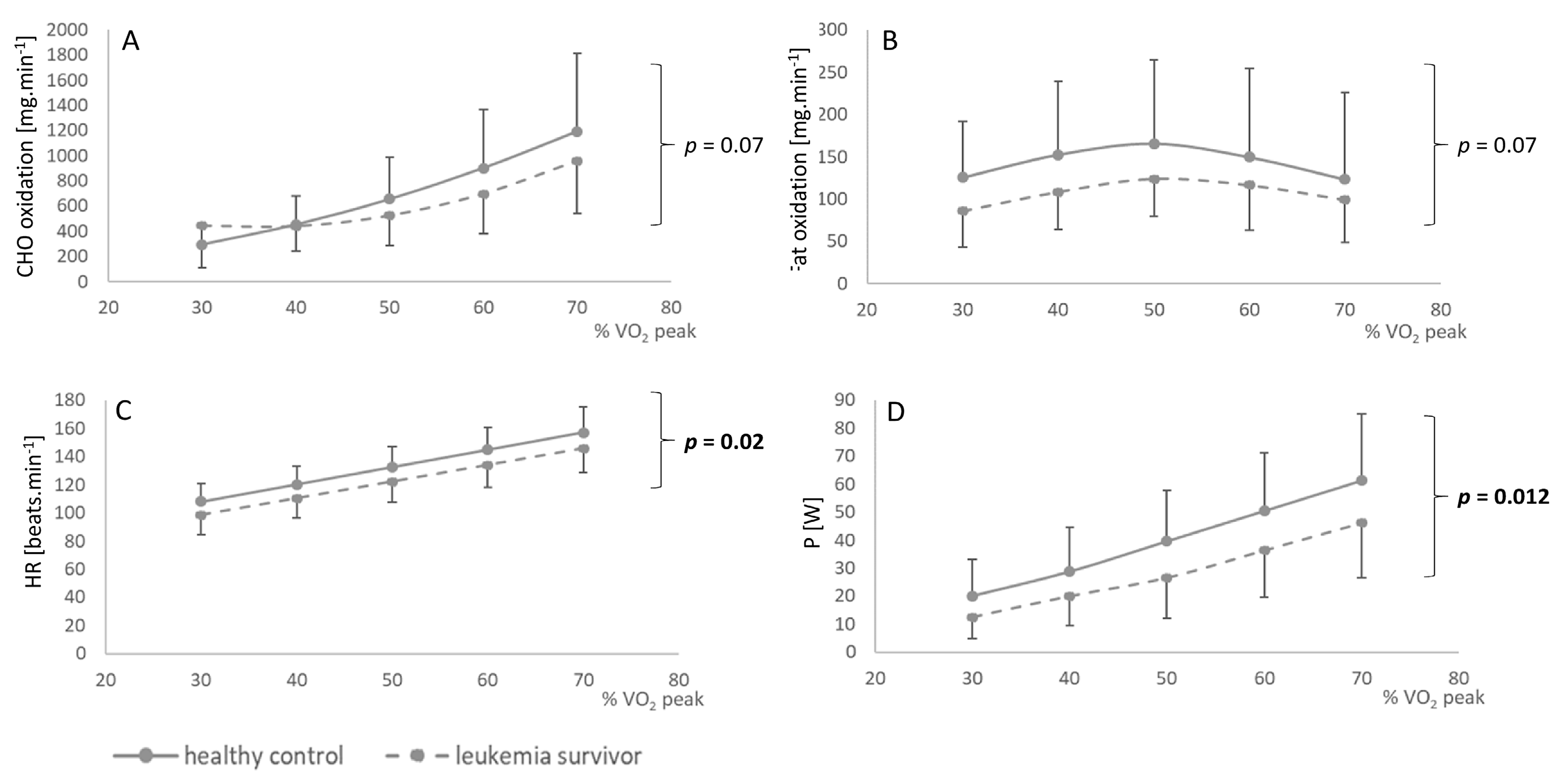
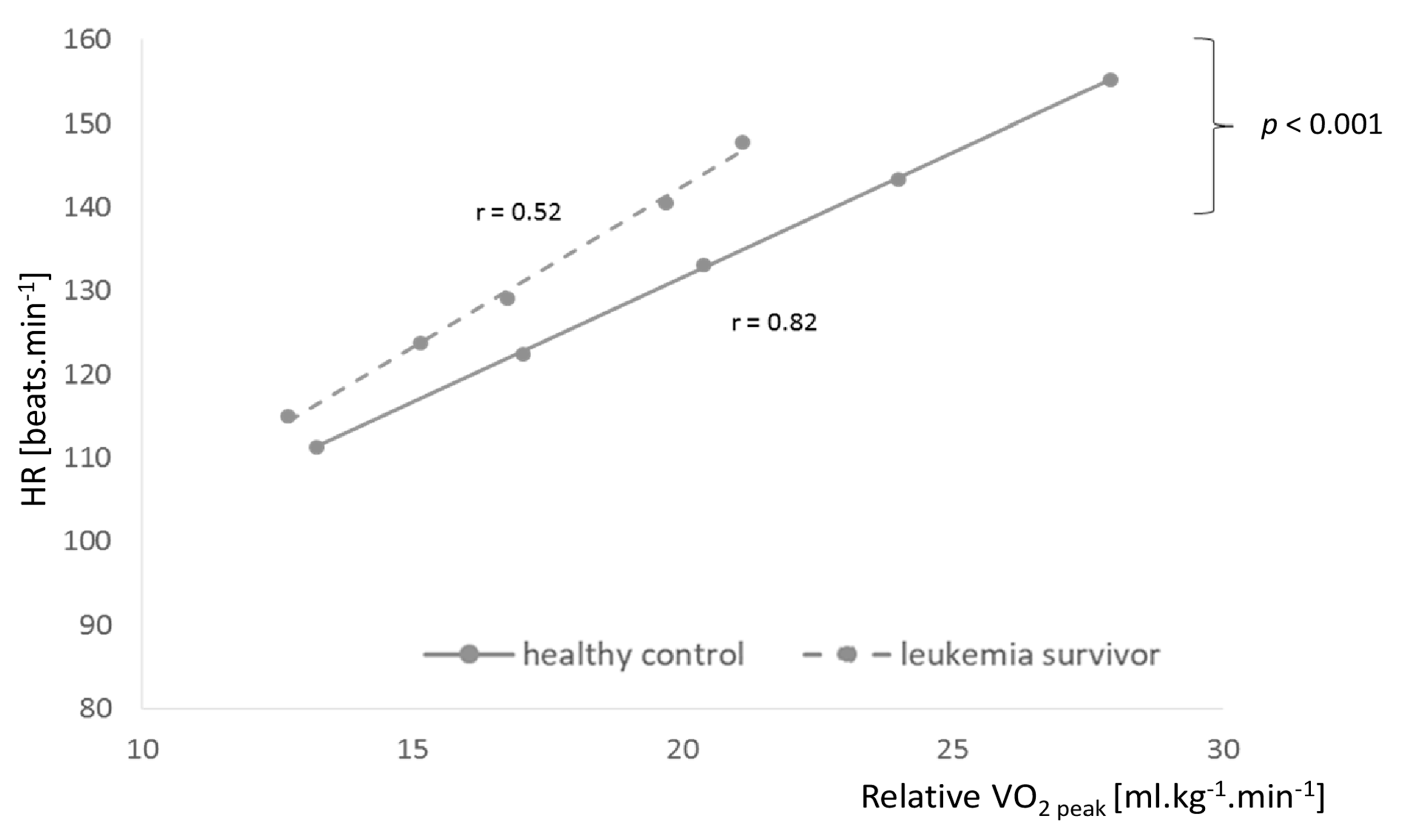
3.2. Oxidation of Metabolites
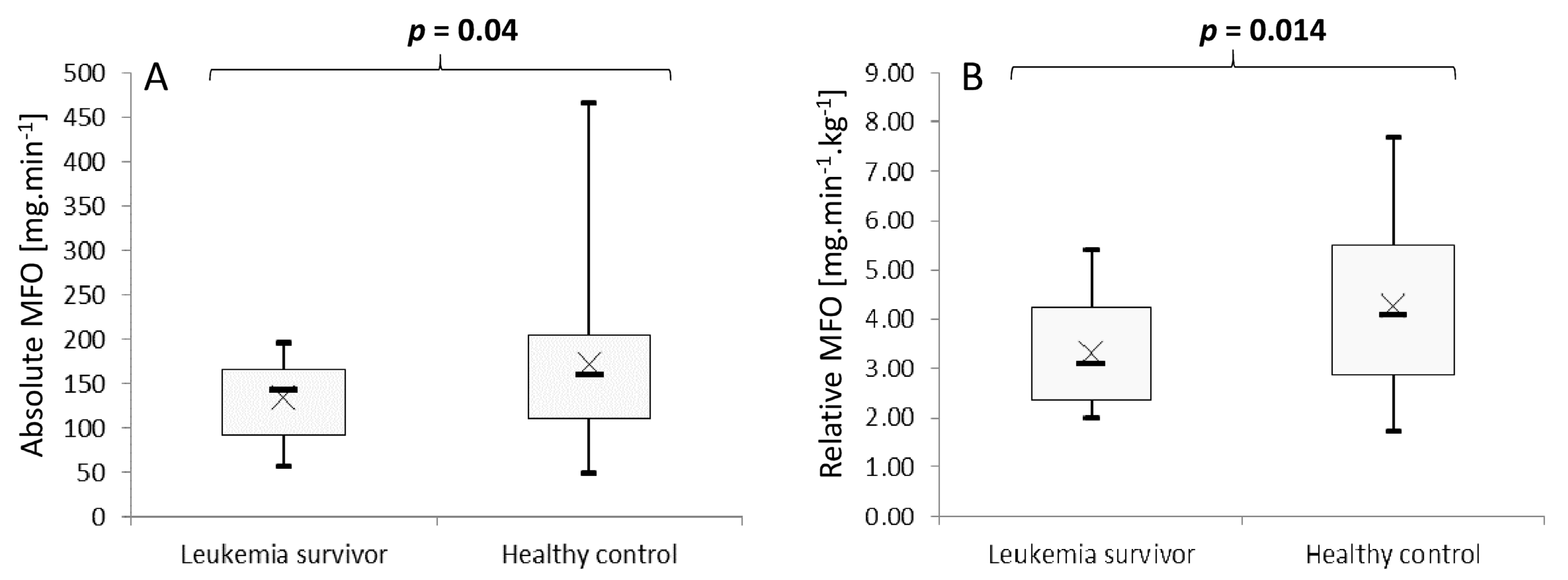
3.3. Maximal Fat Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | acute lymphoblastic leukemia |
| AML | acute myeloblastic leukemia |
| BMI | body mass index |
| CHO | carbohydrates |
| CPP | Comité de Protection des Personnes |
| HR | heart rate |
| IPAQ-Q | International Physical Activity Questionnaire for Adolescents |
| MET | metabolic equivalent of task |
| MFO | maximal fat oxidation |
| O2 | oxygen |
| PA | physical activity |
| PAL | physical activity level |
| RER | respiratory exchange ratio |
| SD | standard derivation |
| VCO2 | carbon dioxide volume |
| VO2 | oxygen volume |
| W | watt |
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Gatta, G.; Zigon, G.; Capocaccia, R.; Coebergh, J.W.; Desandes, E.; Kaatsch, P.; Pastore, G.; Peris-Bonet, R.; Stiller, C.A. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur. J. Cancer 2009, 45, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Zwaan, C.M.; Kolb, E.A.; Reinhardt, D.; Abrahamsson, J.; Adachi, S.; Aplenc, R.; De Bont, E.S.; De Moerloose, B.; Dworzak, M.; Gibson, B.E.; et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 2949–2962. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.; Li, S.; Dover, D.C.; Sallan, S.; Leisenring, W.; Oeffinger, K.C.; Yasui, Y.; Robison, L.L.; Neglia, J. Twenty-five–year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood 2008, 111, 5515–5523. [Google Scholar] [CrossRef]
- Gandemer, V.; Bonneau, J.; Oudin, C.; Berbis, J.; Bertrand, Y.; Tabone, M.-D.; Ducassou, S.; Chastagner, P.; Brethon, B.; Dalle, J.-H.; et al. Late effects in survivors of infantile acute leukemia: A study of the L.E.A program. Blood Cancer J. 2017, 7, e518. [Google Scholar] [CrossRef]
- Leung, W.; Hudson, M.; Zhu, Y.; Rivera, G.K.; Ribeiro, R.C.; Sandlund, J.T.; Bowman, L.C.; Evans, W.E.; Kun, L.; Pui, C.H. Late effects in survivors of infant leukemia. Leukemia 2000, 14, 1185–1190. [Google Scholar] [CrossRef][Green Version]
- Veringa, S.J.; van Dulmen-den Broeder, E.; Kaspers, G.J.; Veening, M.A. Blood pressure and body composition in long-term survivors of childhood acute lymphoblastic leukemia. Pediatric Blood Cancer 2012, 58, 278–282. [Google Scholar] [CrossRef]
- Leahy, J.; Spahis, S.; Bonneil, E.; Garofalo, C.; Grimard, G.; Morel, S.; Laverdiere, C.; Krajinovic, M.; Drouin, S.; Delvin, E.; et al. Insight from mitochondrial functions and proteomics to understand cardiometabolic disorders in survivors of acute lymphoblastic leukemia. Metabolism 2018, 85, 151–160. [Google Scholar] [CrossRef]
- Mertens, A.C.; Yasui, Y.; Liu, Y.; Stovall, M.; Hutchinson, R.; Ginsberg, J.; Sklar, C.; Robison, L.L. Pulmonary complications in survivors of childhood and adolescent cancer. Cancer 2002, 95, 2431–2441. [Google Scholar] [CrossRef]
- Harila-Saari, A.H.; Huuskonen, U.E.; Tolonen, U.; Vainionpää, L.K.; Lanning, B.M. Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: A study with motor evoked potentials*. Med. Pediatr. Oncol. 2001, 36, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E. Exposure to Anthracyclines During Childhood Causes Cardiac Injury. Semin. Oncol. 2006, 33, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Samoilenko, M.; Morel, S.; England, J.; Amre, D.; Bertout-Affret, L.; Drouin, S.; Laverdière, C.; Krajinovic, M.; Sinnett, D.; et al. Cardiometabolic Risk Factors in Childhood, Adolescent and Young Adult Survivors of Acute Lymphoblastic Leukemia – A Petale Cohort. Sci. Rep. 2017, 7, 17684. [Google Scholar] [CrossRef] [PubMed]
- Talvensaari, K.K.; Lanning, M.; Tapanainen, P.; Knip, M. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 3051–3055. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.S.; Chow, E.J.; Goodman, P.J.; Leisenring, W.M.; Dietz, A.C.; Perkins, J.L.; Chow, L.; Sinaiko, A.; Moran, A.; Petryk, A.; et al. Impact of treatment exposures on cardiovascular risk and insulin resistance in childhood cancer survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, C.; Ramírez, C.; Goedeke, L.; Suárez, Y. MicroRNAs in metabolic disease. Arter. Thromb. Vasc. Boil. 2013, 33, 178–185. [Google Scholar] [CrossRef]
- Brun, J.-F.; Varlet-Marie, E.; Jrme, A.; Mercier, J. Measurement and Physiological Relevance of the Maximal Lipid Oxidation Rate During Exercise (LIPOXmax). In An International Perspective on Topics in Sports Medicine and Sports Injury; Zaslav, K.R., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Zakrzewski, J.K.; Tolfrey, K. Comparison of fat oxidation over a range of intensities during treadmill and cycling exercise in children. Eur. J. Appl. Physiol. 2012, 112, 163–171. [Google Scholar] [CrossRef]
- Hocking, M.C.; Schwartz, L.A.; Hobbie, W.L.; DeRosa, B.W.; Ittenbach, R.F.; Mao, J.J.; Ginsberg, J.P.; Kazak, A.E. Prospectively examining physical activity in young adult survivors of childhood cancer and healthy controls. Pediatric Blood Cancer 2013, 60, 309–315. [Google Scholar] [CrossRef]
- Howell, C.R.; Wilson, C.L.; Ehrhardt, M.J.; Partin, R.E.; Kaste, S.C.; Lanctot, J.Q.; Pui, C.-H.; Robison, L.L.; Hudson, M.M.; Ness, K.K. Clinical impact of sedentary behaviors in adult survivors of acute lymphoblastic leukemia: A report from the St. Jude Lifetime Cohort Study. Cancer 2018, 124, 1036–1043. [Google Scholar] [CrossRef]
- Lucia, A.; Earnest, C.; Pérez, M. Cancer–related fatigue: Can exercise physiology assist oncologists? Lancet Oncol. 2003, 4, 616–625. [Google Scholar] [CrossRef]
- Jansen, H.; Postma, A.; Stolk, R.; Kamps, W.A. Acute lymphoblastic leukemia and obesity: Increased energy intake or decreased physical activity? Support. Care Cancer 2008, 17, 103–106. [Google Scholar] [CrossRef]
- Hartman, A.; Hop, W.; Takken, T.; Pieters, R.; van den Heuvel-Eibrink, M. Motor performance and functional exercise capacity in survivors of pediatric acute lymphoblastic leukemia. Pediatric Blood Cancer. 2013, 60, 494–499. [Google Scholar] [CrossRef]
- Lemay, V.; Caru, M.; Samoilenko, M.; Drouin, S.; Alos, N.; Lefebvre, G.; Levy, E.; Lippé, S.; Marcil, V.; Sultan, S.; et al. Prevention of Long-term Adverse Health Outcomes with Cardiorespiratory Fitness and Physical Activity in Childhood Acute Lymphoblastic Leukemia Survivors. J. Pediatric Hematol. 2019, 41, e450–e458, Publish Ahead of Print. [Google Scholar] [CrossRef]
- Warner, J.; Bell, W.; Webb, D.; Gregory, J. Relationship between cardiopulmonary response to exercise and adiposity in survivors of childhood malignancy. Arch. Dis. Child. 1997, 76, 298–303. [Google Scholar] [CrossRef]
- Huang, T.-T.; Ness, K.K. Exercise Interventions in Children with Cancer: A Review. Int. J. Pediatr. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Ottevaere, C.; Huybrechts, I.; De Bourdeaudhuij, I.; Sjöström, M.; Ruiz, J.R.; Ortega, F.B.; Hagströmer, M.; Widhalm, K.; Molnar, D.; Moreno, L.; et al. Comparison of the IPAQ-A and Actigraph in relation to VO2max among European adolescents: The HELENA study. J. Sci. Med. Sport 2011, 14, 317–324. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S516. [Google Scholar] [CrossRef]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best Practice Methods to Apply to Measurement of Resting Metabolic Rate in Adults: A Systematic Review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef]
- Riddell, M.C.; Jamnik, V.K.; Iscoe, K.E.; Timmons, B.W.; Gledhill, N. Fat oxidation rate and the exercise intensity that elicits maximal fat oxidation decreases with pubertal status in young male subjects. J. Appl. Physiol. 2008, 105, 742–748. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Dafoe, W. Principles of Exercise Testing and Interpretation. Can. J. Cardiol. 2007, 23, 274. [Google Scholar]
- Péronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 355, i5239. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, N.J., Ed.; L. Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Christiansen, J.R.; Kanellopoulos, A.; Lund, M.B.; Massey, R.; Dalen, H.; Kiserud, C.E.; Ruud, E.; Aakhus, S. Impaired exercise capacity and left ventricular function in long-term adult survivors of childhood acute lymphoblastic leukemia. Pediatric Blood Cancer 2015, 62, 1437–1443. [Google Scholar] [CrossRef]
- Filomena, D.; Versacci, P.; Cimino, S.; Mattiucci, C.; Maestrini, V.; Cantisani, D.; Petronilli, V.; Agati, L.; Schiavetti, A. Echocardiographic long-term follow-up of adult survivors of pediatric cancer treated with Dexrazoxane-Anthracyclines association. Int. J. Cardiol. 2020, 299, 271–275. [Google Scholar] [CrossRef]
- Alias, H.; Nazi, N.A.M.; Chong, D.L.S. Participation in Physical Activity and Physical Education in School Among Children with Acute Lymphoblastic Leukemia After Intensive Chemotherapy. Front. Pediatric 2019, 7, 73. [Google Scholar] [CrossRef]
- Hung, S.H.; Rankin, A.; Virji-Babul, N.; Pritchard, S.; Fryer, C.; Campbell, K.L. Associating Physical Activity Levels with Motor Performance and Physical Function in Childhood Survivors of Acute Lymphoblastic Leukemia. Physiother. Can. 2017, 69, 57–64. [Google Scholar] [CrossRef]
- Venables, M.; Achten, J.; Jeukendrup, A.E. Determinants of fat oxidation during exercise in healthy men and women: A cross-sectional study. J. Appl. Physiol. 2005, 98, 160–167. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wust, R.C.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Myrdal, O.H.; Kanellopoulos, A.; Christensen, J.R.; Ruud, E.; Edvardsen, E.; Kongerud, J.; Sikkeland, L.I.; Lund, M.B. Risk factors for impaired pulmonary function and cardiorespiratory fitness in very long-term adult survivors of childhood acute lymphoblastic leukemia after treatment with chemotherapy only. Acta Oncol. 2018, 57, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Stratton, K.; Leisenring, W.L.; Oeffinger, K.C.; Nathan, P.C.; Wasilewski-Masker, K.; Hudson, M.M.; Castellino, S.M.; Stovall, M.; Armstrong, G.T.; et al. Decline in physical activity level in the Childhood Cancer Survivor Study cohort. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.K.; Baker, K.S.; Dengel, D.R.; Youngren, N.; Sibley, S.; Mertens, A.C.; Gurney, J.G. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatric Blood Cancer 2007, 49, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Lanfranconi, F.; Pollastri, L.; Ferri, A.; Fraschini, D.; Masera, G.; Miserocchi, G. Near Infrared Spectroscopy (NIRS) as a New Non-Invasive Tool to Detect Oxidative Skeletal Muscle Impairment in Children Survived to Acute Lymphoblastic Leukaemia. PLoS ONE 2014, 9, e99282. [Google Scholar] [CrossRef] [PubMed]
- Van Brussel, M.; Takken, T.; Lucia, A.; van der Net, J.; Helders, P.J. Is physical fitness decreased in survivors of childhood leukemia? A systematic review. Leukemia 2005, 19, 13. [Google Scholar] [CrossRef]
| AL Survivors | Healthy Controls | p | Hedge’s g | |
|---|---|---|---|---|
| n | 20 | 20 | ||
| Sex (male/female) | 10/10 | 10/10 | ||
| Age (years) mean ± SD | 12.2 ± 3.3 | 12.3 ± 3.4 | ns | |
| Tanner stage (I–II/III–V) | 13/7 | 13/7 | ||
| Body mass index for age percentile, mean ± SD | 50.5 ± 28.9 | 42.3 ± 27.3 | ns | −0.29 (−0.91, 0.34) |
| BMI (kg/m²) mean ± SD | 19.0 ± 3.7 | 17.8 ± 2.9 | ns | |
| Absolute VO2 peak (mL/min) mean ± SD | 1245.4 ± 413.3 | 1603.2 ± 621.9 | 0.003 | |
| Relative VO2 peak (mL/kg/min) mean ± SD | 29.4 ± 6.9 | 40.6 ± 11.3 | <0.001 | |
| Rest metabolism (kcal/day) mean ± SD | 1577.9 ± 579.4 | 1553.5 ± 517.3 | ns | −0.04 (−0.66, 0.58) |
| Exercise maximum heart rate (bpm) mean ± SD | 155.4 ± 17.0 | 168.1.7 ± 19.3 | 0.016 | |
| Level of physical activity (MET-minutes/week) mean ± SD | 1883.5 ± 1042.9 | 2527.7 ± 1496.2 | ns | 0.49 (−0.14, 1.12) |
| Age at diagnosis (years), mean ± SD | 4.8 ± 3.4 | NA | ||
| Time since end of treatment (years) mean ± SD | 4.9 ± 3.5 | NA | ||
| Type of acute leukemia (n) | 20 | NA | ||
| AL lymphoblastic | 18 | |||
| Leukocytosis < 50,000/mm3 + no MI | 15 | |||
| Leukocytosis > 50,000/mm3 + no MI | 1 | |||
| Leukocytosis > 50,000/mm3 + MI | 2 | |||
| AL myeloblastic * | 2 | |||
| LVEF (%) mean ± SD | 69.2 ± 2.7 | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pegon, C.; Rochette, E.; Rouel, N.; Pereira, B.; Doré, E.; Isfan, F.; Grèze, V.; Merlin, E.; Kanold, J.; Duché, P., on behalf of HERCCULE Group. Childhood Leukemia Survivors and Metabolic Response to Exercise: A Pilot Controlled Study. J. Clin. Med. 2020, 9, 562. https://doi.org/10.3390/jcm9020562
Pegon C, Rochette E, Rouel N, Pereira B, Doré E, Isfan F, Grèze V, Merlin E, Kanold J, Duché P on behalf of HERCCULE Group. Childhood Leukemia Survivors and Metabolic Response to Exercise: A Pilot Controlled Study. Journal of Clinical Medicine. 2020; 9(2):562. https://doi.org/10.3390/jcm9020562
Chicago/Turabian StylePegon, Charline, Emmanuelle Rochette, Nadège Rouel, Bruno Pereira, Eric Doré, Florentina Isfan, Victoria Grèze, Etienne Merlin, Justyna Kanold, and Pascale Duché on behalf of HERCCULE Group. 2020. "Childhood Leukemia Survivors and Metabolic Response to Exercise: A Pilot Controlled Study" Journal of Clinical Medicine 9, no. 2: 562. https://doi.org/10.3390/jcm9020562
APA StylePegon, C., Rochette, E., Rouel, N., Pereira, B., Doré, E., Isfan, F., Grèze, V., Merlin, E., Kanold, J., & Duché, P., on behalf of HERCCULE Group. (2020). Childhood Leukemia Survivors and Metabolic Response to Exercise: A Pilot Controlled Study. Journal of Clinical Medicine, 9(2), 562. https://doi.org/10.3390/jcm9020562






