Recurrent Campylobacter Enteritis in Patients with Hypogammaglobulinemia: Review of the Literature
Abstract
1. Introduction
2. Methods
3. Results
3.1. Investigations in Local Microbiology Laboratories
3.2. Literature Review
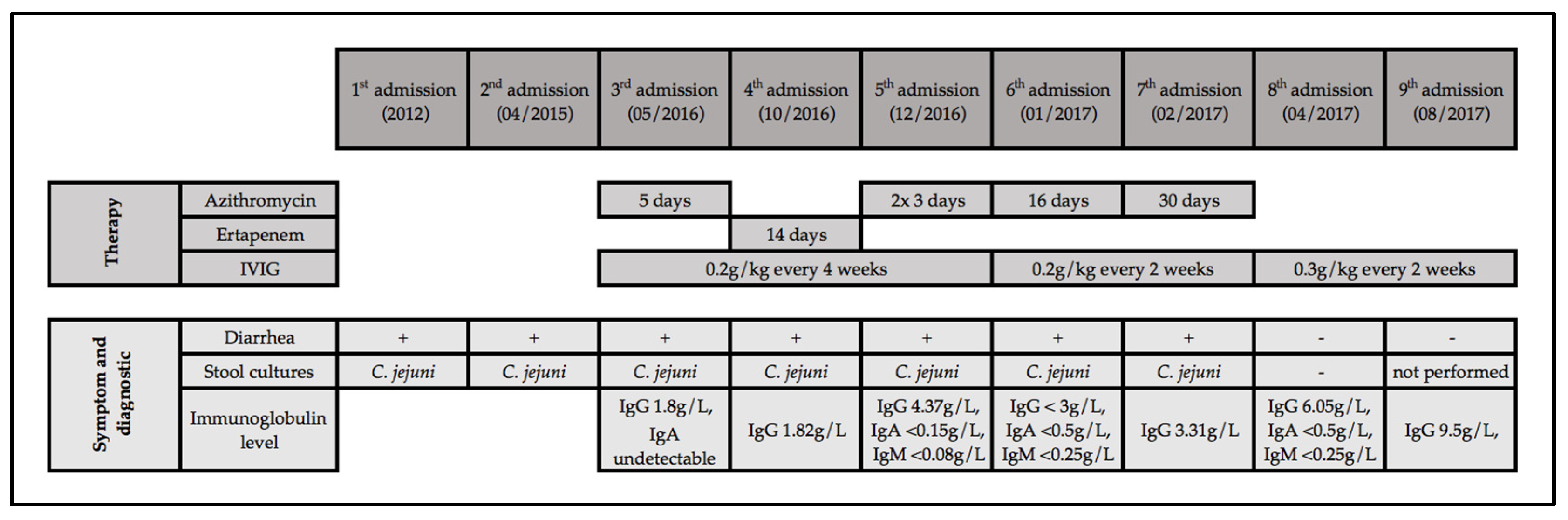
4. Illustrative Case Report
5. Discussion
5.1. Recurrent Campylobacteriosis
5.2. Recurrent Campylobacter Enteritis in Immunodeficient Persons
5.3. Infections Following Rituximab
5.4. Infections and Hypogammaglobulinemia Following Rituximab
5.5. IVIG Replacement for Rituximab-Associated Hypogammaglobulinemia
6. Conclusions
- Physicians should be aware of the association of recurrent campylobacteriosis and immunodeficiency, especially humoral immunodeficiency.
- Patients with recurrent enteritis (or with a first episode of Campylobacter bacteremia) should be evaluated for humoral immunodeficiency by measuring serum immune globulin levels and circulating B-cells [2].
- This should routinely be done also before administering rituximab, in order to identify patients with undiagnosed preexisting hypogammaglobulinemia, typically those with chronic lymphatic leukemia or lymphoma, and occasionally patients with undiagnosed CVID. In these patients, “subclinical immunodeficiency” might be unmasked by rituximab, and they may be at an increased risk of infection after rituximab is given [51,72].
- In patients who develop recurrent infections after rituximab, new or worsened hypogammaglobulinemia should again be looked for, and specialist referral and IVIG replacement therapy should be considered.
Funding
Acknowledgments
Conflicts of Interest
References
- Oksenhendler, E.; Gérard, L.; Fieschi, C.; Malphettes, M.; Mouillot, G.; Jaussaud, R.; Viallard, J.F.; Gardembas, M.; Galicier, L.; Schleinitz, N.; et al. Infections in 252 patients with common variable immunodeficiency. Clin. Infect. Dis. 2008, 46, 1547–1554. [Google Scholar] [CrossRef]
- Fernández-Cruz, A.; Muñoz, P.; Mohedano, R.; Valerio, M.; Marín, M.; Alcalá, L.; Rodriguez-Créixems, M.; Cercenado, E.; Bouza, E. Campylobacter bacteremia: Clinical characteristics, incidence, and outcome over 23 years. Medicine (Baltimore) 2010, 89, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimol, S.; Carmi, A.; Greenberg, D. Demographic and clinical characteristics of Campylobacter bacteremia in children with and without predisposing factors. Pediatr. Infect. Dis. J. 2013, 32, e414–e418. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.; Ravel, A.; Michel, P.; Berke, O.; Gosselin, P. Do patients with recurrent episodes of campylobacteriosis differ from those with a single disease event? BMC Public Health 2011, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.E.; Notarangelo, L.D.; Etzioni, A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin. Immunol. 1999, 93, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Kerstens, P.J.; Endtz, H.P.; Meis, J.F.; Oyen, W.J.; Koopman, R.J.; van den Broek, P.J.; van der Meer, J.W. Erysipelas-like skin lesions associated with Campylobacter jejuni septicemia in patients with hypogammaglobulinemia. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 842–847. [Google Scholar] [CrossRef]
- Borleffs, J.C.; Schellekens, J.F.; Brouwer, E.; Rozenberg-Arska, M. Use of an immunoglobulin M containing preparation for treatment of two hypogammaglobulinemic patients with persistent Campylobacter jejuni infection. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 772–775. [Google Scholar] [CrossRef]
- Spelman, D.W.; Davidson, N.; Buckmaster, N.D.; Spicer, W.J.; Ryan, P. Campylobacter bacteraemia: A report of 10 cases. Med. J. Aust. 1986, 145, 503–505. [Google Scholar] [CrossRef]
- Hopkins, S.; Abuzakouk, M.; Brannigan, E.; Bergin, C.; Feighery, C. Campylobacter jejuni cellulitis in a patient with pan-hypogammaglobulinaemia. BMJ Case Rep. 2011. [Google Scholar] [CrossRef]
- Ariganello, P.; Angelino, G.; Scarselli, A.; Salfa, I.; Corte Della, M.; De Matteis, A.; D’Argenio, P.; Livadiotti, S.; Manno, E.C.; Russo, C.; et al. Relapsing Campylobacter jejuni Systemic Infections in a Child with X-Linked Agammaglobulinemia. Case Rep. Pediatr. 2013, 2013, 735108. [Google Scholar] [CrossRef]
- Hagiya, H.; Kimura, K.; Nishi, I.; Yoshida, H.; Yamamoto, N.; Akeda, Y.; Tomono, K. Emergence of Carbapenem Non-susceptible Campylobacter coli after Long-term Treatment against Recurrent Bacteremia in a Patient with X-linked Agammaglobulinemia. Intern Med. 2018, 57, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, K.; Nishi, J.; Miyanohara, H.; Sarantuya, J.; Iwashita, M.; Kamenosono, A.; Hizukuri, K.; Wakimoto, N.; Yoshinaga, M. Relapsing cellulitis associated with Campylobacter coli bacteremia in an agammaglobulinemic patient. Pediatr. Infect. Dis. J. 2004, 23, 577–579. [Google Scholar] [CrossRef]
- Arai, A.; Kitano, A.; Sawabe, E.; Kanegane, H.; Miyawaki, T.; Miura, O. Relapsing Campylobacter coli bacteremia with reactive arthritis in a patient with X-linked agammaglobulinemia. Intern. Med. 2007, 46, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, I.B.; Schuster, V.; Ewald, J.; Harmsen, D.; Kreth, H.W. An unusual case of refractory Campylobacter jejuni infection in a patient with X-linked agammaglobulinemia: Successful combined therapy with maternal plasma and ciprofloxacin. Clin. Infect. Dis. 1996, 23, 526–531. [Google Scholar] [CrossRef]
- Rafi, A.; Matz, J. An unusual case of Campylobacter jejuni pericarditis in a patient with X-linked agammaglobulinemia. Ann. Allergy Asthma Immunol. 2002, 89, 362–367. [Google Scholar] [CrossRef]
- Langereis, J.D.; Henriet, S.S.; Kuipers, S.; Weemaes, C.M.R.; van der Burg, M.; de Jonge, M.I.; van der Flier, M. IgM Augments Complement Bactericidal Activity with Serum from a Patient with a Novel CD79a Mutation. J. Clin. Immunol. 2018, 38, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.H.; Markusse, H.M. Campylobacter jejuni arthritis in secondary amyloidosis. Clin. Rheumatol. 1995, 14, 214–216. [Google Scholar] [CrossRef]
- Pönkä, A.; Tilvis, R.; Kosunen, T.U. Prolonged campylobacter gastroenteritis in a patient with hypogammaglobulinaemia. Acta Med. Scand. 1983, 213, 159–160. [Google Scholar] [CrossRef]
- Melamed, I.; Bujanover, Y.; Igra, Y.S.; Schwartz, D.; Zakuth, V.; Spirer, Z. Campylobacter enteritis in normal and immunodeficient children. Am. J. Dis. Child. 1983, 137, 752–753. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nolan, C.; Wang, S.P.; Shelton, W.R.; Blaser, M.J. Persistent Campylobacter jejuni infection in an immunocompromised patient. Ann. Intern. Med. 1984, 100, 832–834. [Google Scholar] [CrossRef]
- Perlman, D.M.; Ampel, N.M.; Schifman, R.B.; Cohn, D.L.; Patton, C.M.; Aguirre, M.L.; Wang, W.L.; Blaser, M.J. Persistent Campylobacter jejuni infections in patients infected with the human immunodeficiency virus (HIV). Ann. Intern. Med. 1988, 108, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.T.; Klein, K.; Borthistle, B.K. Jejunal infection with Campylobacter. Arch. Intern. Med. 1984, 144, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Ahnen, D.J.; Brown, W.R. Campylobacter enteritis in immune-deficient patients. Ann. Intern. Med. 1982, 96, 187–188. [Google Scholar] [CrossRef]
- Aguilar-Company, J.; Los-Arcos, I.; Pigrau, C.; Rodríguez-Pardo, D.; Larrosa, M.N.; Rodríguez-Garrido, V.; Sihuay-Diburga, D.; Almirante, B. Potential Use of Fosfomycin-Tromethamine for Treatment of Recurrent Campylobacter Species Enteritis. Antimicrob. Agents Chemother. 2016, 60, 4398–4400. [Google Scholar] [CrossRef] [PubMed]
- Gharamti, A.A.; Nour, M.; Ali, T.; Kanafani, Z.A. Recurrent Campylobacter Bacteremia as the First Manifestation of Hypogammaglobulinemia: A Case Report and Literature Review. J. Infect. Chemother. 2019, 51, e41. [Google Scholar] [CrossRef]
- van den Bruele, T.; Mourad-Baars, P.E.C.; Claas, E.C.J.; van der Plas, R.N.; Kuijper, E.J.; Bredius, R.G.M. Campylobacter jejuni bacteremia and Helicobacter pylori in a patient with X-linked agammaglobulinemia. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1315–1319. [Google Scholar] [CrossRef]
- LeBar, W.D.; Menard, R.R.; Check, F.E. Hypogammaglobulinemia and recurrent Campylobacter jejuni infection. J. Infect. Dis. 1985, 152, 1099–1100. [Google Scholar] [CrossRef]
- van der Meer, J.W.; Mouton, R.P.; Daha, M.R.; Schuurman, R.K. Campylobacter jejuni bacteraemia as a cause of recurrent fever in a patient with hypogammaglobulinaemia. J. Infect. 1986, 12, 235–239. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, J.A.; Han, S.B.; Cho, B.; Jeong, D.C.; Kang, J.H. Recurrent Campylobacter jejuni bacteremia in a patient with hypogammaglobulinemia: A case report. Medicine (Baltimore) 2017, 96, e7238. [Google Scholar] [CrossRef]
- Tarr, P.E.; Sneller, M.C.; Mechanic, L.J.; Economides, A.; Eger, C.M.; Strober, W.; Cunningham-Rundles, C.; Lucey, D.R. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltimore) 2001, 80, 123–133. [Google Scholar] [CrossRef]
- Moore, J.; Curran, M.; Wareing, D.; Fox, A.; Boyd, N.; Glynn, G.; Millar, B.; Daly, G.; Murphy, P. Investigation of infection with Campylobacter jejuni in a man with hypogammaglobulinaemia using PCR-single-stranded conformational polymorphism (PCR-SSCP) typing. Int. J. Med. Microbiol. 2001, 291, 21–25. [Google Scholar] [CrossRef]
- Lever, A.M.; Dolby, J.M.; Webster, A.D.; Price, A.B. Chronic campylobacter colitis and uveitis in patient with hypogammaglobulinaemia. Br. Med. J. (Clin. Res. Ed.) 1984, 288, 531. [Google Scholar] [CrossRef] [PubMed]
- Makatsori, M.; Kiani-Alikhan, S.; Manson, A.L.; Verma, N.; Leandro, M.; Gurugama, N.P.; Longhurst, H.J.; Grigoriadou, S.; Buckland, M.; Kanfer, E.; et al. Hypogammaglobulinaemia after rituximab treatment-incidence and outcomes. QJM 2014, 107, 821–828. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO. The Global View of CAMPYLOBACTERIOSIS; World Health Organization WHO: Geneva, Switzerland, 2012; pp. 1–69. Available online: www.who.int/iris/bitstream/10665/80751/1/9789241564601_eng.pdf (accessed on 15 December 2019).
- Thomas, M.K.; Majowicz, S.E.; Sockett, P.N.; Fazil, A.; Pollari, F.; Doré, K.; Flint, J.A.; Edge, V.L. Estimated Numbers of Community Cases of Illness Due to Salmonella, Campylobacter and Verotoxigenic Escherichia Coli: Pathogen-specific Community Rates. Can. J. Infect. Dis. Med. Microbiol. 2006, 17, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Dionisi, A.M.; Milito, C.; Martini, H.; Pesce, A.M.; Mitrevski, M.; Granata, G.; Lucarelli, C.; Parisi, A.; Luzzi, I.; Quinti, I. High prevalence of intestinal carriage of Campylobacter coli in patients with primary antibody deficiencies: A silent infection that could shift to a life-threatening condition. J. Clin. Gastroenterol. 2011, 45, 474–475. [Google Scholar] [CrossRef]
- Freeman, A.F.; Holland, S.M. Persistent bacterial infections and primary immune disorders. Curr. Opin. Microbiol. 2007, 10, 70–75. [Google Scholar] [CrossRef]
- Dingle, K.E.; Colles, F.M.; Wareing, D.R.; Ure, R.; Fox, A.J.; Bolton, F.E.; Bootsma, H.J.; Willems, R.J.; Urwin, R.; Maiden, M.C. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 14–23. [Google Scholar] [CrossRef]
- Strid, M.A.; Engberg, J.; Larsen, L.B.; Begtrup, K.; Mølbak, K.; Krogfelt, K.A. Antibody responses to Campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin. Diagn. Lab. Immunol. 2001, 8, 314–319. [Google Scholar] [CrossRef]
- Janssen, R.; Krogfelt, K.A.; Cawthraw, S.A.; van Pelt, W.; Wagenaar, J.A.; Owen, R.J. Host-pathogen interactions in Campylobacter infections: The host perspective. Clin. Microbiol. Rev. 2008, 21, 505–518. [Google Scholar] [CrossRef]
- Wassenaar, T.M.; Blaser, M.J. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1999, 1, 1023–1033. [Google Scholar] [CrossRef]
- Pazzaglia, G.; Bourgeois, A.L.; Diwany El, K.; Nour, N.; Badran, N.; Hablas, R. Campylobacter diarrhoea and an association of recent disease with asymptomatic shedding in Egyptian children. Epidemiol. Infect. 1991, 106, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, T.A.; Nielsen, P.N.; Petersen, J.; Kofoed, T.; Crawford, J.S.; Morsczeck, C.; Boysen, A.; Schrotz-King, P. Novel surface polypeptides of Campylobacter jejuni as traveller’s diarrhoea vaccine candidates discovered by proteomics. Vaccine 2006, 24, 6446–6455. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, B.; Wormser, G.P.; Abdoo, R.A.; Cabello, F.; Aguero, M.E.; Sivak, S.L. Persistence of multiply antibiotic-resistant Campylobacter jejuni in a patient with the acquired immune deficiency syndrome. Am. J. Med. 1986, 80, 965–970. [Google Scholar] [CrossRef]
- Ruiz-Palacios, G.M.; Calva, J.J.; Pickering, L.K.; Lopez-Vidal, Y.; Volkow, P.; Pezzarossi, H.; West, M.S. Protection of breast-fed infants against Campylobacter diarrhea by antibodies in human milk. J. Pediatr. 1990, 116, 707–713. [Google Scholar] [CrossRef]
- Okada, H.; Kitazawa, T.; Harada, S.; Itoyama, S.; Hatakeyama, S.; Ota, Y.; Koike, K. Combined Treatment with Oral Kanamycin and Parenteral Antibiotics for a Case of Persistent Bacteremia and Intestinal Carriage with Campylobacter coli. Intern. Med. Jpn. Soc. Intern. Med. 2008, 47, 1363–1366. [Google Scholar] [CrossRef]
- Edwards, J.C.W.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [Google Scholar] [CrossRef]
- Di Bisceglie, A.M.; Lok, A.S.; Martin, P.; Terrault, N.; Perrillo, R.P.; Hoofnagle, J.H. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: Just the tip of the iceberg? Hepatology 2015, 61, 703–711. [Google Scholar] [CrossRef]
- Perrillo, R.P.; Martin, P.; Lok, A.S. Preventing hepatitis B reactivation due to immunosuppressive drug treatments. JAMA 2015, 313, 1617–1618. [Google Scholar] [CrossRef]
- Paul, S.; Saxena, A.; Terrin, N.; Viveiros, K.; Balk, E.M.; Wong, J.B. Hepatitis B Virus Reactivation and Prophylaxis During Solid Tumor Chemotherapy: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 30–40. [Google Scholar] [CrossRef]
- Buch, M.H.; Smolen, J.S.; Betteridge, N.; Breedveld, F.C.; Burmester, G.; Dörner, T.; Ferraccioli, G.; Gottenberg, J.-E.; Isaacs, J.; Kvien, T.K.; et al. Rituximab Consensus Expert Committee Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 909–920. [Google Scholar] [CrossRef]
- Casulo, C.; Maragulia, J.; Zelenetz, A.D. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin. Lymphoma Myeloma Leuk. 2013, 13, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Keystone, E.; Fleischmann, R.; Emery, P.; Furst, D.E.; van Vollenhoven, R.; Bathon, J.; Dougados, M.; Baldassare, A.; Ferraccioli, G.; Chubick, A.; et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: An open-label extension analysis. Arthritis Rheum. 2007, 56, 3896–3908. [Google Scholar] [CrossRef] [PubMed]
- van Vollenhoven, R.F.; Emery, P.; Bingham, C.O.; Keystone, E.C.; Fleischmann, R.M.; Furst, D.E.; Tyson, N.; Collinson, N.; Lehane, P.B. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann. Rheum. Dis. 2013, 72, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/product-information/mabthera-epar-product-information_en.pdf. (accessed on 31 January 2019).
- Tudesq, J.-J.; Cartron, G.; Rivière, S.; Morquin, D.; Iordache, L.; Mahr, A.; Pourcher, V.; Klouche, K.; Cerutti, D.; Le Quellec, A.; et al. Clinical and microbiological characteristics of the infections in patients treated with rituximab for autoimmune and/or malignant hematological disorders. Autoimmun. Rev. 2018, 17, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Gea-Banacloche, J.C. Rituximab-associated infections. Semin. Hematol. 2010, 47, 187–198. [Google Scholar] [CrossRef]
- Brah, S.; Chiche, L.; Brun, M.; Schleinitz, N.; Harle, J.-R.; Durand, J.-M. Campylobacter fetus Bacteremia Revealed by Cellulitis without Gastrointestinal Symptoms in the Context of Acquired Hypogammaglobulinemia: A Report of Three Cases. Case Rep. Gastrointest. Med. 2011, 2011, 628902–628903. [Google Scholar] [CrossRef]
- Meyer, A.; Theulin, A.; Chatelus, E.; Argemi, X.; Sordet, C.; Javier, R.M.; Hansmann, Y.; Sibilia, J.; Gottenberg, J.-E. Campylobacter fetus infection in three rheumatoid arthritis patients treated with rituximab. Ann. Rheum. Dis. 2012, 71, 1094–1095. [Google Scholar] [CrossRef]
- Gupta, A.; Tse, L. Successful conservative management of campylobacter cholecystitis occurring post chemotherapy and rituximab: A rare disease entity. N. Z. Med. J. 2015, 128, 110–112. [Google Scholar]
- Nakazawa, H.; Nishina, S.; Sakai, H.; Ito, T.; Ishida, F.; Kitano, K. Successful Empiric Therapy for Postsplenectomy Sepsis with Campylobacter fetus in an Abattoir Worker with Follicular Lymphoma. Intern. Med. 2018, 57, 3329–3332. [Google Scholar] [CrossRef]
- Filanovsky, K.; Shvidel, L.; Shtalrid, M.; Haran, M.; Duek, A.; Berrebi, A. Predictive Factors to Hypogammaglobulinemia and Non-Neutropenic Infection Complications after Rituximab/Chemotherapy Treatment. Blood 2007, 110, 1288. [Google Scholar] [CrossRef]
- McLaughlin, P.; Grillo-López, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, F.; Liboy, I.; Pavia, O.; Rivera, E. High incidence of non-neutropenic infections induced by rituximab plus fludarabine and associated with hypogammaglobulinemia: A frequently unrecognized and easily treatable complication. Ann. Oncol. 2006, 17, 1424–1427. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Endo, T.; Fujimoto, K.; Yamamoto, S.; Obara, M.; Yamaguchi, K.; Takeda, Y.; Goto, H.; Kasahara, I.; Sato, N.; et al. FCGR3A-158V/F polymorphism may correlate with the levels of immunoglobulin in patients with non-Hodgkin’s lymphoma after rituximab treatment as an adjuvant to autologous stem cell transplantation. Eur. J. Haematol. 2009, 82, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Christou, E.A.A.; Giardino, G.; Worth, A.; Ladomenou, F. Risk factors predisposing to the development of hypogammaglobulinemia and infections post-Rituximab. Int. Rev. Immunol. 2017, 36, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Barmettler, S.; Price, C. Continuing IgG replacement therapy for hypogammaglobulinemia after rituximab--for how long? J. Allergy Clin. Immunol. 2015, 136, 1407–1409. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Emery, P.; Bingham, C.O.; Keystone, E.C.; Fleischmann, R.; Furst, D.E.; Macey, K.; Sweetser, M.; Kelman, A.; Rao, R. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J. Rheumatol. 2010, 37, 558–567. [Google Scholar] [CrossRef]
- Diwakar, L.; Gorrie, S.; Richter, A.; Chapman, O.; Dhillon, P.; Al-Ghanmi, F.; Noorani, S.; Krishna, M.T.; Huissoon, A. Does rituximab aggravate pre-existing hypogammaglobulinaemia? J. Clin. Pathol. 2010, 63, 275–277. [Google Scholar] [CrossRef]
- Mogensen, T.H.; Bernth-Jensen, J.M.; Petersen, C.C.; Petersen, M.S.; Nyvold, C.; Gadegaard, K.H.; Hokland, M.; Hokland, P.; Larsen, C.S. Common variable immunodeficiency unmasked by treatment of immune thrombocytopenic purpura with Rituximab. BMC Blood Disord. 2013, 13, 1. [Google Scholar] [CrossRef]
- Kaplan, B.; Kopyltsova, Y.; Khokhar, A.; Lam, F.; Bonagura, V. Rituximab and immune deficiency: Case series and review of the literature. J. Allergy Clin. Immunol. Pract. 2014, 2, 594–600. [Google Scholar] [CrossRef]
- Barmettler, S.; Ong, M.-S.; Farmer, J.R.; Choi, H.; Walter, J. Association of Immunoglobulin Levels, Infectious Risk, and Mortality With Rituximab and Hypogammaglobulinemia. JAMA Netw. Open 2018, 1, e184169. [Google Scholar] [CrossRef]
- Cunningham-Rundles, C.; Carol, B. Common Variable Immunodeficiency: Clinical and Immunological Features of 248 Patients. Clin. Immunol. 1999, 92, 1–15. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Age/Sex (yrs.) | Immuno-Deficiency | Clinical Features | Campylobacter Species * | Bacteremia (Number of Episodes) | Intravenous Immune Globulin Treatment (IVIG) | Outcome |
|---|---|---|---|---|---|---|---|
| [22] | 42 M | CVID | Weight loss Diarrhea | C. jejuni (2 separate isolates, one in small bowel) | - | - | Diarrhea improved and repeat stool cultures remained negative 14 months after discontinuing antibiotics |
| [23] | 63 M | CVID | Diarrhea | C. jejuni (3 separate isolates) | - | - | Diarrhea improved: stool cultures remained negative after discontinuing antibiotics |
| [23] | 64 W | CVID | Diarrhea | C. jejuni (2 separate isolates) | - | - | Diarrhea improved |
| [24] | 64 W | CVID | Diarrhea, hypo-volemic shock | C. jejuni (1 isolate), C. coli (1 isolate) | - | IVIG every 21 days | Diarrhea resolved and stool cultures remained negative 12 months after discontinuing antibiotics |
| [27] | 39 W | CVID | Cellulitis, nausea, vomiting, rash, diarrhea | C. jejuni (2 separate isolates) | 2 | - | Symptoms resolved |
| [24] | 83 W | Good Syndrome | Diarrhea | Campylobacter sp (1 isolate), C. coli (1 isolate) | - | IVIG every 14 days | Diarrhea resolved and stool cultures remained negative 2 and 6 months after discontinuing antibiotics |
| [26] | 15 M | XLA | Loss of appetite, weight loss, fever | C. jejuni (2 separate isolates, one in gastric Antrum) | 1 | IVIG every 28 days | Weight gain, stool cultures remained negative 6 month after discontinuing antibiotics |
| [27] | 24 M | XLA | Fever, nausea, cramping, vomiting | C. jejuni (2 separate isolates) | 2 | - | Died of Sepsis complicated by DIC and multiple organ failure |
| [28] | 24 M | XLA | Fever, Diarrhea | C. jejuni (4 separate isolates) | 4 | - | Fever bouts recurred after second course of antimicrobial treatment |
| [30] | 54 M | Good Syndrome | Diarrhea | C. jejuni (2 separate isolates) | 2 | IVIG every 28 days | Diarrhea resolved |
| [29] | 18 M | Probable XLA | Fever, diarrhea and cellulitis | C. jejuni (4 separate isolates) | 4 | IVIG every 21 days | Diarrhea improved |
| [32] | 34 W | Hypogamma-globulinemia, probable CVID | Diarrhea | C. jejuni (2 separate isolates) | - | IVIG every 14 days | Diarrhea resolved and follow up stool cultures were negative |
| [31] | M | Hypogamma-globulinemia, probably primary | Diarrhea, sepsis | C. jejuni (4 separate isolates) | 4 | - | not reported |
| [25] | 30 F | Probable CVID, corticosteroids and rituximab for autoimmune hemolytic anemia | Fever, diarrhea, dyspnea, myalgia, arthralgia | C. spp (5 separate isolates in blood and stool cultures) | 4 | - | Diarrhea resolved, no recurrence during five years follow-up |
| Present case | 73 M | Secondary hypogamma-globulinemia | Diarrhea | C. jejuni (6 separate isolates) | - | IVIG every 21 days Then IVIG 14 days | Persistent mild diarrhea, stool cultures remained negative 6 months after discontinuing antibiotics |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najjar, I.; Paluca, F.; Loukidis, K.; Tarr, P.E. Recurrent Campylobacter Enteritis in Patients with Hypogammaglobulinemia: Review of the Literature. J. Clin. Med. 2020, 9, 553. https://doi.org/10.3390/jcm9020553
Najjar I, Paluca F, Loukidis K, Tarr PE. Recurrent Campylobacter Enteritis in Patients with Hypogammaglobulinemia: Review of the Literature. Journal of Clinical Medicine. 2020; 9(2):553. https://doi.org/10.3390/jcm9020553
Chicago/Turabian StyleNajjar, Iris, Florina Paluca, Konstantinos Loukidis, and Philip E. Tarr. 2020. "Recurrent Campylobacter Enteritis in Patients with Hypogammaglobulinemia: Review of the Literature" Journal of Clinical Medicine 9, no. 2: 553. https://doi.org/10.3390/jcm9020553
APA StyleNajjar, I., Paluca, F., Loukidis, K., & Tarr, P. E. (2020). Recurrent Campylobacter Enteritis in Patients with Hypogammaglobulinemia: Review of the Literature. Journal of Clinical Medicine, 9(2), 553. https://doi.org/10.3390/jcm9020553






