Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Collection
2.3. Saliva Collection
2.4. Dental Examination
2.5. Biochemical Determination
2.6. Antioxidant Enzymes
2.7. Non-Enzymatic Antioxidants
2.8. Redox Status
2.9. Products of Oxidative Damage to Proteins and Lipids
2.10. Statistical Analysis
3. Results
3.1. General Characteristics
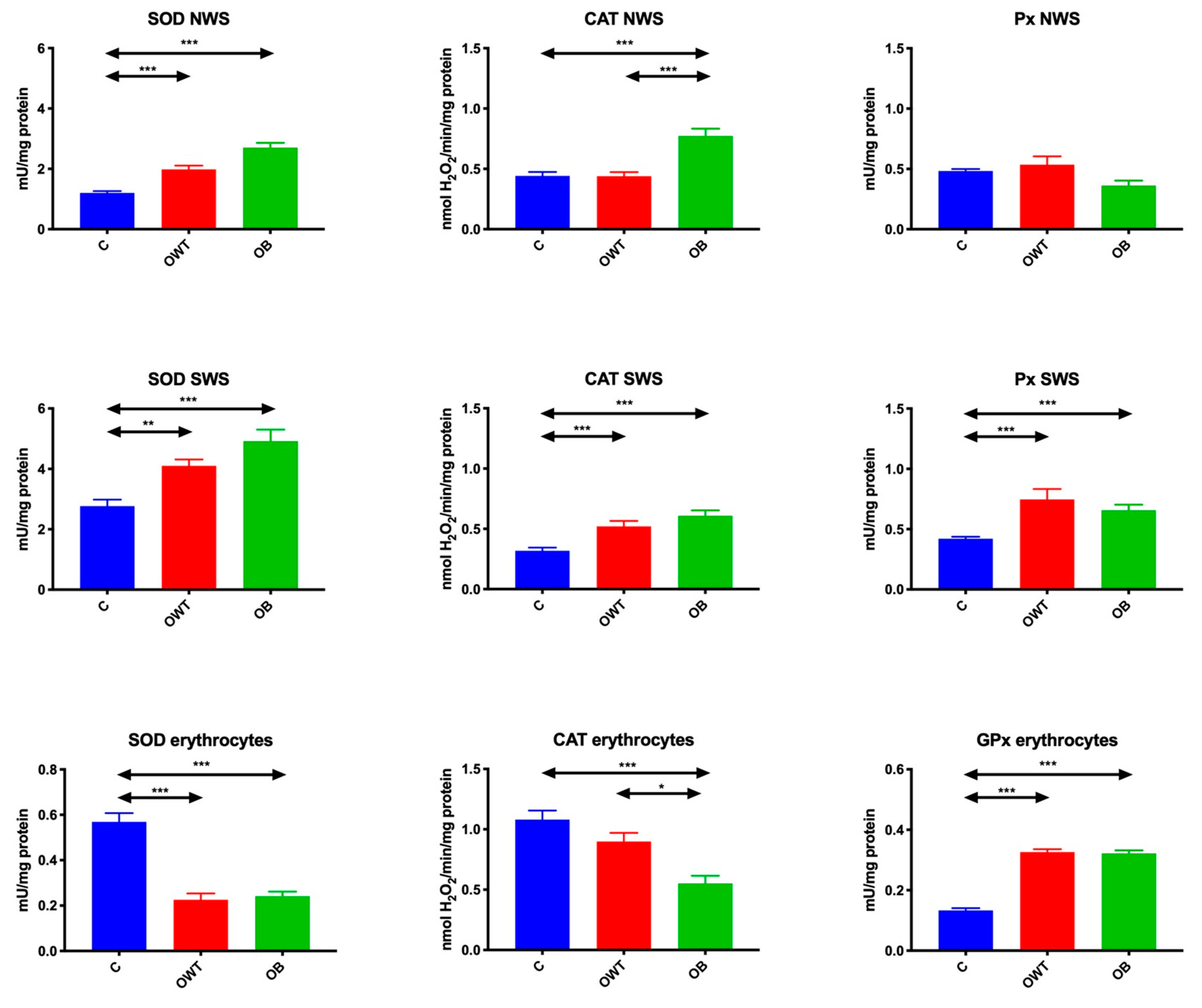
3.2. Enzymatic Antioxidants
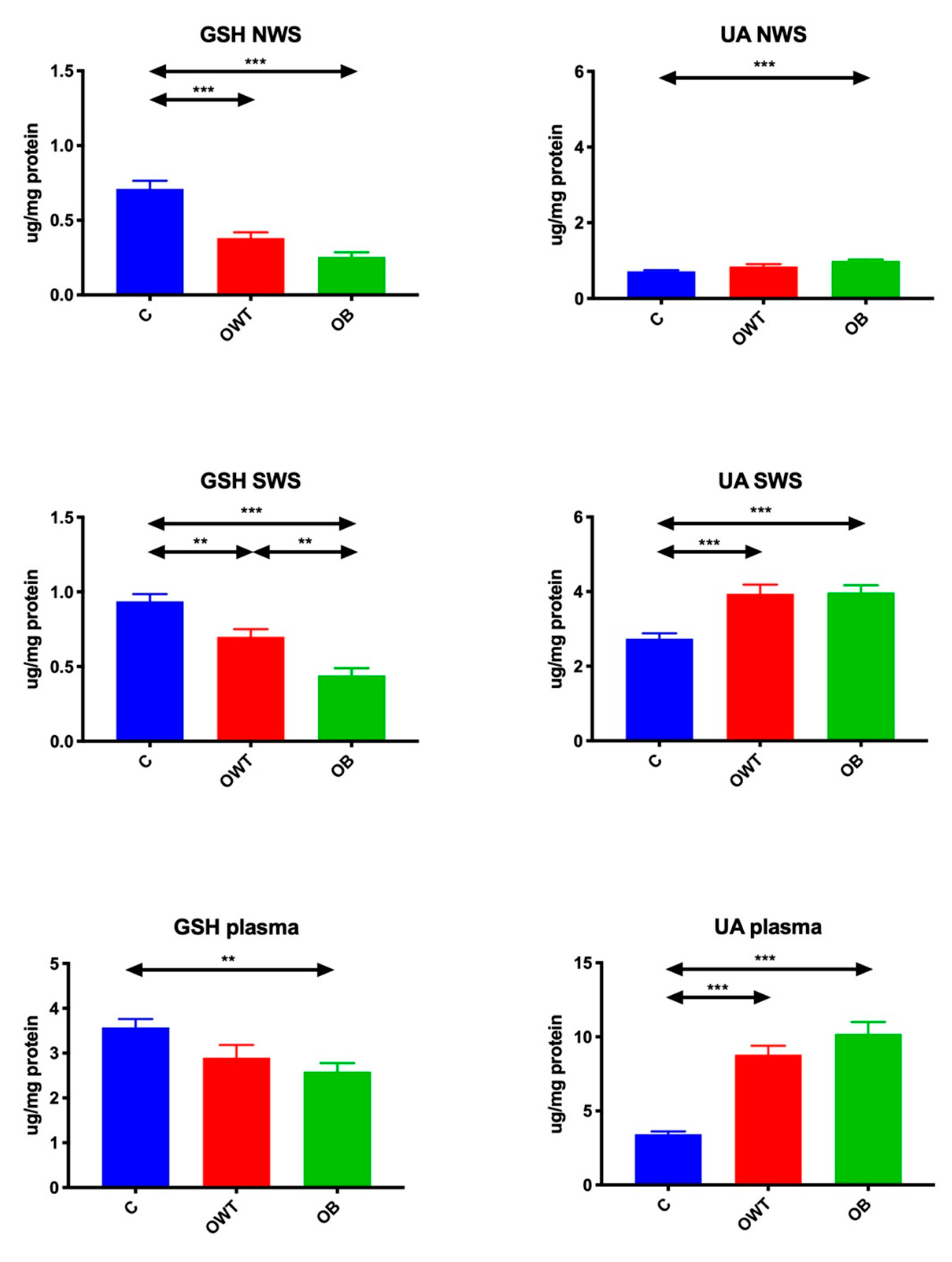
3.3. Non-Enzymatic Antioxidants
3.4. Redox Status
3.5. Oxidation Products
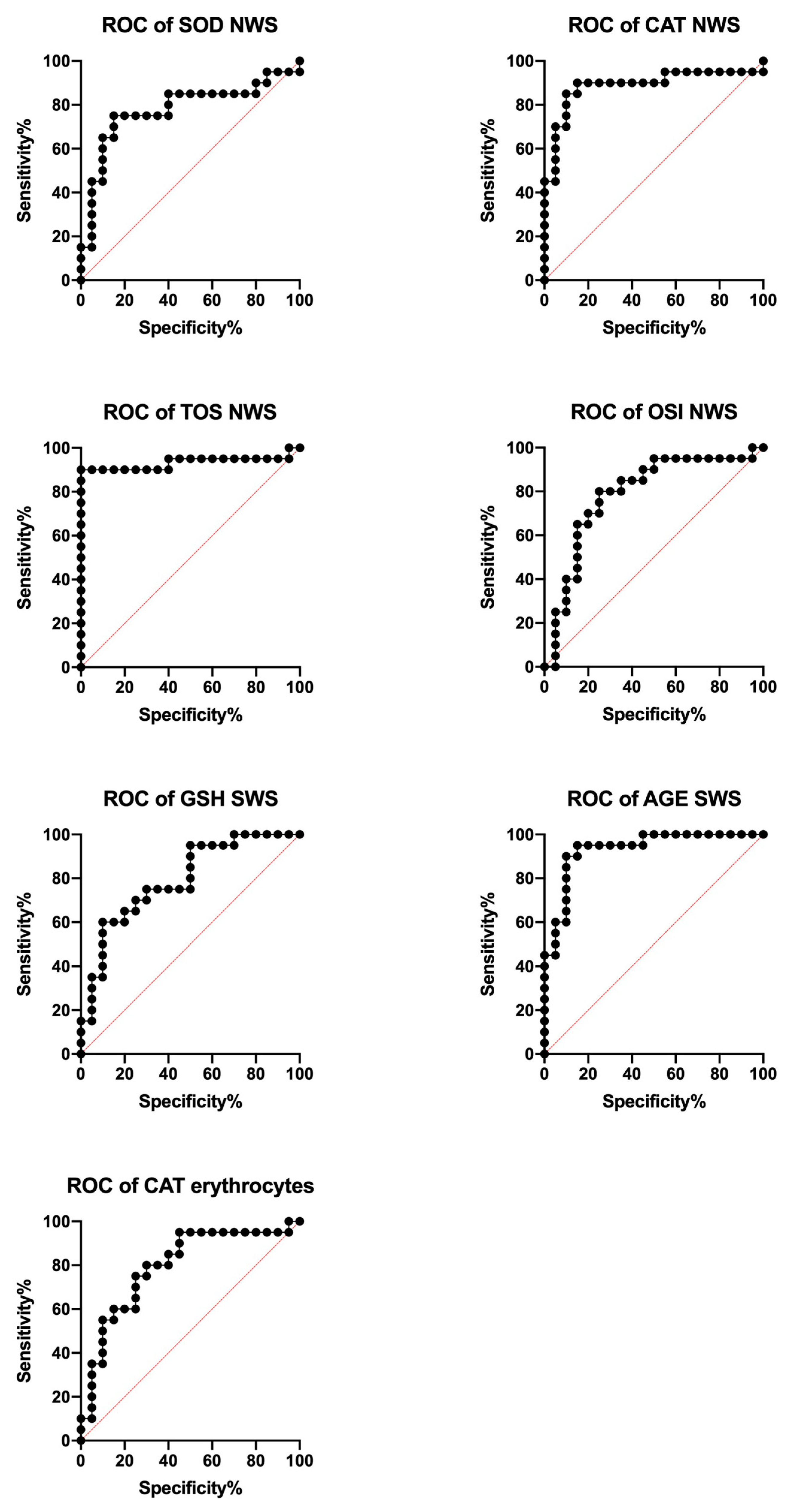
3.6. ROC Analysis
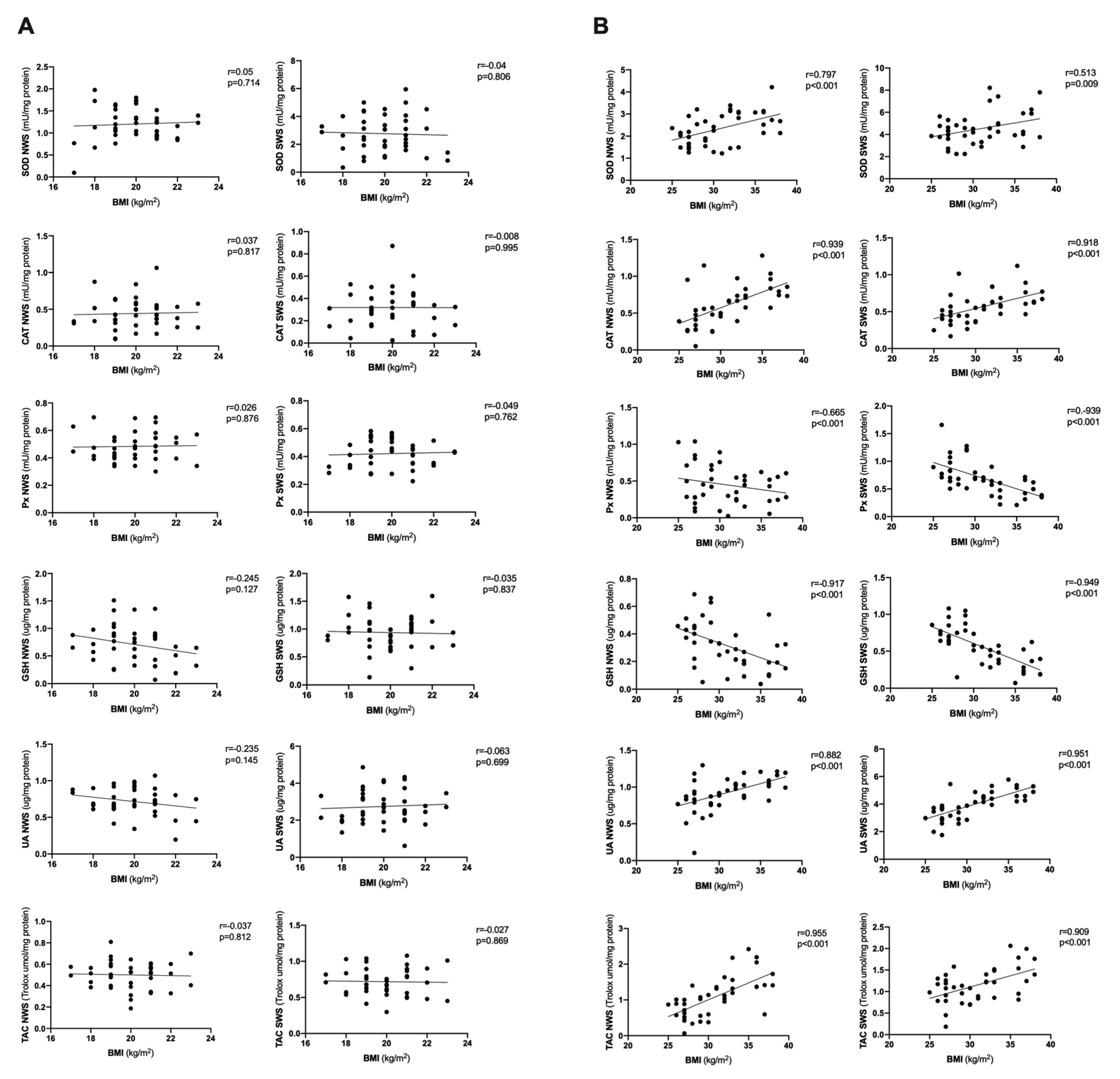
3.7. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Rowicka, G.; Dylag, H.; Ambroszkiewicz, J.; Riahi, A.; Weker, H.; Chelchowska, M. Total Oxidant and Antioxidant Status in Prepubertal Children with Obesity. Oxid. Med. Cell. Longev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Matczuk, J.; Zalewska, A.; Łukaszuk, B.; Knaś, M.; Maciejczyk, M.; Grabowska, M.; Ziembicka, D.M.; Waszkiel, D.; Chabowski, A.; Żendzian-Piotrowska, M.; et al. Insulin resistance and obesity affect lipid profile in the salivary glands. J. Diabetes Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Database on Body Mass Index. Available online: http://www.who.int/bmi (accessed on 16 January 2020).
- Freedman, D.S.; Khan, L.K.; Serdula, M.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. The relation of childhood BMI to adult adiposity: The Bogalusa Heart Study. Pediatrics 2005, 115, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Knaś, M.; Maciejczyk, M.; Waszkiel, D.; Zalewska, A. Oxidative stress and salivary antioxidants. Dent. Med. Probl. 2013, 50, 461–466. [Google Scholar]
- Holven, K.B.; Ulven, S.M.; Bogsrud, M.P. Hyperlipidemia and cardiovascular disease with focus on familial hypercholesterolemia. Curr. Opin. Lipidol. 2017, 28, 445–447. [Google Scholar] [CrossRef]
- Kurek, K.; Miklosz, A.; Lukaszuk, B.; Chabowski, A.; Gorski, J.; Zendzian-Piotrowska, M. Inhibition of Ceramide De Novo Synthesis Ameliorates Diet Induced Skeletal Muscles Insulin Resistance. J. Diabetes Res. 2015. [Google Scholar] [CrossRef]
- Outon, S.; Galceran, I.; Pascual, J.; Oliveras, A. Central blood pressure in morbid obesity and after bariatric surgery. Nefrologia 2019. [Google Scholar] [CrossRef]
- Greig, F.; Hyman, S.; Wallach, E.; Hildebrandt, T.; Rapaport, R. Which obese youth are at increased risk for type 2 diabetes? Latent class analysis and comparison with diabetic youth. Pediatr. Diabetes 2012, 13, 181–188. [Google Scholar] [CrossRef]
- Sanchez, A.; Furberg, H.; Kuo, F.; Vuong, L.; Ged, Y.; Patil, S.; Ostrovnaya, I.; Petruzella, S.; Reising, A.; Patel, P.; et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: A cohort study. Lancet Oncol. 2019. [Google Scholar] [CrossRef]
- Gerdin, E.W.; Angbratt, M.; Aronsson, K.; Eriksson, E.; Johansson, I. Dental caries and body mass index by socio-economic status in Swedish children. Commun. Dent. Oral. Epidemiol. 2008, 36, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Willerhausen, B.; Blettner, M.; Kasaj, A.; Hohenfellner, K. Association between body mass index and dental health in 1,290 children of elementary schools in a German city. Clin. Oral. Invesitg. 2007, 11, 195–200. [Google Scholar] [CrossRef]
- Zeigler, C.C.; Persson, G.R.; Wondimu, B.; Marcus, C.; Sobko, T.; Modèer, T. Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity 2012, 20, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Chielle, E.O.; Casarin, J.N. Evaluation of salivary oxidative parameters in overweight and obese young adults. Arch. Epidemiol. Metab. 2017, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Knaś, M.; Maciejczyk, M.; Sawicka, K.; Hady Razak, H.; Niczyporuk, M.; Ładny, J.R.; Matczuk, J.; Waszkiel, D.; Żendzian-Piotrowska, M.; Zalewska, A. Impact of morbid obesity and bariatric surgery on antioxidant/oxidant balance of the unstimulated and stimulated saliva. J. Oral. Pathol. Med. 2016, 45, 455–464. [Google Scholar] [CrossRef]
- Fejfer, K.; Buczko, P.; Niczyporuk, M.; Ładny, J.R.; Hady, R.H.; Knaś, M.; Waszkiel, D.; Klimiuk, A.; Zalewska, A.; Maciejczyk, M. Oxidative Modicication of biomolecules in the nonstiumulated and stimulated saliva of patients with morbid obesity treated with bariatric surgery. BioMed Res. Int. 2017. [Google Scholar] [CrossRef]
- Narotzki, B.; Reznick, A.Z.; Mitki, T.; Aizenbud, D.; Levy, Y. Enhanced cardiovascular risk and altered oxidative status in elders with moderate excessive body fat. Rejuvenation Res. 2014, 17, 334–340. [Google Scholar] [CrossRef]
- Cole, T.J.; Green, P.J. Smoothing reference centile curves: The LMS method and penalized likelihood. Stat. Med. 1992, 11, 1305–1319. [Google Scholar] [CrossRef]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-Forage: Methods and Development; WHO: Genewa, Switzerland, 2006. [Google Scholar]
- De Onis, M.; Lobstein, T. Defining obesity risk status in the general childhood population: Which cut-offs should we use? Int. J. Pediatr. Obese. 2010, 5, 458–460. [Google Scholar] [CrossRef]
- Mansson-Rahemtulla, B.; Baldone, D.C.; Pruitt, K.M.; Rahemtulla, F. Specific assays for peroxidases in human saliva. Arch. Oral. Biol. 1986, 31, 661–668. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 227–285. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Kołodziej, U.; Maciejczyk, M.; Niklińska, W.; Waszkiel, D.; Żendzian-Piotrowska, M.; Żukowski, P.; Zalewska, A. Chronic high-protein diet induces oxidative stress and alters the salivary gland function in rats. Arch. Oral. Biol. 2017, 84, 6–12. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. Oxidative stress index (OSI) as a new tool to assess redox status in dairy cattle during transition period. Animal 2013, 7, 1374–1378. [Google Scholar] [CrossRef]
- Kalousová, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar]
- Maciejczyk, M.; Zalewska, A.; Ladny, J.R. Salivary Antioxidant Barrier, Redox Status, and Oxidative Damage to Proteins and Lipids in Healthy Children, Adults, and the Elderly. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Hajiluian, G.; Nameni, G.; Shahabi, P. Adipose Tissue Inflammation and Oxidative Stress: The Ameliorative Effects of Vitamin D. Inflammation 2017, 40, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Alcala, M.; Calderon-Dominguez, M.; Bustos, E.; Ramos, P.; Casals, N.; Serra, D.; Viana, M.; Herrero, L. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, E.; Silverio Amancio, O.M.; de Souza Vitalle, M.S.; Nesadal de Souza, D.; Medeiros Mendes, F.; Nicolau, J. Analysis of the stimulated whole saliva in overweight and obese children. Rev. Assoc. Med. Bras. 2010, 56, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jin, C.; Li, L.; Wang, Y. Increased Expression of TNF-alpha Occurs Before the Development of Periodontitis Among Obese Chinese Children: A Potential Marker for Prediction and Prevention of Periodontitis. Oral. Health Prev. Dent. 2016, 14, 71–75. [Google Scholar] [CrossRef]
- Scorzetti, L.; Marcattili, D.; Pasini, M.; Mattei, A.; Marchetti, E.; Marzo, G. Association between obesity and periodontal disease in children. Eur. J. Pediatr. Dent. 2013, 14, 181–184. [Google Scholar]
- Modeer, T.; Blomberg, C.C.; Wondimu, B.; Julihn, A.; Marcus, C. Association between obesity, flow rate of whole saliva, and dental caries in adolescents. Obesity 2010, 18, 2367–2373. [Google Scholar] [CrossRef]
- Fadel, H.T.; Pliaki, A.; Gronowitz, E.; Mårild, S.; Ramberg, P.; Dahlèn, G.; Yucel-Lindberg, T.; Heijl, L.; Birkhed, D. Clinical and biological indicators of dental caries and periodontal disease in adolescents with or without obesity. Clin. Oral. Investig. 2014, 18, 359–368. [Google Scholar] [CrossRef]
- Patrascu, V.; Giurca, C.; Ciurea, R.N.; Georgescu, C.C.; Ciurea, M.E. Ulcerated necrobiosis lipoidica to a teenager with diabetes mellitus and obesity. Rom. J. Morphol. Embryol. 2014, 55, 171–176. [Google Scholar]
- Dursun, E.; Akalin, F.A.; Genc, T.; Cinar, N.; Erel, O.; Yildiz, B.O. Oxidative stress and periodontal disease in obesity. Medicine 2016, 95, e3136. [Google Scholar] [CrossRef]
- Brown, L.A.; Kerr, C.J.; Whiting, P.; Finer, N.; McEneny, J.; Ashton, T. Oxidant stress in healthy normal-weight, overweight, and obese individuals. Obesity 2009, 17, 460–466. [Google Scholar] [CrossRef]
- Goth, L.; Lenkey, A.; Bigler, W.N. Blood catalase deficiency and diabetes in Hungary. Diabetes Care 2001, 24, 1839–1840. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Knaś, M.; Waszkiewicz, N.; Klimiuk, A.; Litwin, K.; Sierakowski, S.; Waszkiel, D. Salivary antioxidants in patients with systemic sclerosis. J. Oral. Pathol. Med. 2014, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Knaś, M.; Żendzian-Piotrowska, M.; Waszkiewicz, N.; Szulimowska, J.; Prokopiuk, S.; Waszkiel, D.; Car, H. Antioxidant profile of salivary glands in high fat diet- induced insulin resistance rats. Oral. Dis. 2014, 20, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, N.H. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diabetes Vasc. Dis. Res. 2011, 8, 22–28. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, X.; Shi, X.; Tian, R.; Zhao, Z. Association of serum uric acid with different levels of glucose and related factors. Chin. Med. J. 2011, 124, 1443–1448. [Google Scholar] [PubMed]
- Wang, Z.N.; Li, P.; Jiang, R.H.; Li, L.; Li, X.; Li, L.; Liu, C.; Tian, C.L. The association between serum uric acid and metabolic syndrome among adolescents in northeast China. Int. J. Clin. Exp. Med. 2015, 8, 21122–21129. [Google Scholar]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Lushchak, V.I. Classification of oxidative stress based on its intensity. Exp. Clin. Sci. 2014, 13, 922–937. [Google Scholar]
- Adamczyk-Sowa, M.; Bieszczad-Bedrejczuk, E.; Galiniak, S.; Rozmilowska, I.; Czyzewski, D.; Bartosz, G.; Sadowska-Bartosz, I. Oxidative modifications of blood serum proteins in myasthenia gravis. J. Neuroimmunol. 2017, 305, 145–153. [Google Scholar] [CrossRef]
- Gębicki, J.M.; Bartosz, G. Rola białek jako przekaźników uszkodzeń indukowanych przez reaktywne formy tlenu in vivo. Postepy Biochem. 2010, 56, 115–123. [Google Scholar]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int. J. Mol. Sci. 2017, 18, 2205. [Google Scholar] [CrossRef] [PubMed]
- Bozzato, A.; Burger, P.; Zenk, J.; Ulter, W.; Iro, H. Salivary gland biometry in female patients with eating disorders. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265, 1095–1102. [Google Scholar] [CrossRef]
- Heo, M.S.; Lee, S.C.; Lee, S.S.; Choi, H.M.; Choi, S.C.; Park, T.W. Quantitative analysis of normal major salivary glands using computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, M.; Iwasaki, T.; Kitano, M.; Kuno, H.; Hashimoto, N.; Kawahito, Y.; Azuma, M.; Hla, T.; Sano, H. Role of sphingosine 1-phosphate in the pathogenesis of Sjögren’s syndrome. J. Immunol. 2008, 180, 1921–1928. [Google Scholar] [CrossRef]
- Uemura, T.; Suzuki, T.; Saiki, R.; Dohmae, N.; Ito, S.; Takahashi, H.; Toida, T.; Kashiwagi, K.; Igarashi, K. Activation of MMP-9 activity by acrolein in saliva from patients with primary Sjögren’s syndrome and its mechanism. Int. J. Biochem. Cell Biol. 2017, 88, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Norheim, K.B.; Jonsson, G.; Harboe, E.; Hanasand, M.; Goransson, L.; Omdal, R. Oxidative stress, as measured by protein oxidation, is increased in primary Sjögren’s syndrome. Free Radic. Res. 2012, 46, 141–146. [Google Scholar] [CrossRef] [PubMed]




| C n = 40 | OWT n = 20 | OB n = 20 | |
|---|---|---|---|
| Age (years) | 16 ± 2.0 | 16 ± 1.9 | 15.8 ± 2.2 |
| Sex (male/female) n | 20/20 | 10/10 | 10/10 |
| Weight (kg) | 55 ± 3.1 | 65 ± 10 * | 90 ± 21 * |
| Height (cm) | 167 ± 4.5 | 163 ± 12 | 162 ± 14 |
| BMI (kg/m2) | 20 ± 1.5 | 28 ± 1.5 * | 34 ± 2.7 * |
| cc BMI | 50 ± 2.5 | 97 ± 1.2 | 99 ± 0.83 |
| SDS BMI | 0.5 ± 0.2 | 2.5 ± 0.28 | 3.9 ± 0.81 |
| Systolic BP (mmHg) | 109 ± 1.0 | 110 ± 1.2 | 111 ± 1.0 |
| Diastolic BP (mmHg) | 58 ± 4.2 | 73 ± 10 | 76 ± 9.8 |
| WBC (thousand/μL) | 5.8 ± 1.3 | 6.7 ± 2.2 | 6.7 ± 1.1 |
| Hgb (g/dL) | 13.9 ± 1.1 | 14 ± 1.2 | 14 ± 1.0 |
| Hct (%) | 42.0 ± 2.3 | 41 ± 3.3 | 42 ± 3.2 |
| PLT (thousand/μL) | 278 ± 46 | 294 ± 71 | 263 ± 48 |
| sCre (mg/dL) | 0.63 ± 0.1 | 0.64 ± 0.19 | 0.79 ± 0.56 |
| Urea (mg/dL) | 15.4 ± 3.6 | 24 ± 9.7 * | 27 ± 13 * |
| HDL (mg/dL) | 47 ± 2.2 | 52 ± 1.4 | 43 ± 8.1 |
| LDL (mg/dL) | 70 ± 5.2 | 87 ± 24 | 106 ± 21 * |
| Total cholesterol (mg/dL) | 120 ± 9 | 132 ± 30 | 161 ± 39 * |
| TG (mg/dL) | 68 ± 8 | 102 ± 75 * | 139 ± 75 * |
| Glucose (mg/dL) | 86 ± 5.3 | 86 ± 9.9 | 91 ± 5.4 |
| eGFR (mL/min/1.73 m2) | 132 ± 10 | 120 ± 42 | 122 ± 43 |
| IL-6 (pg/mL) | 1.7 ± 0.71 | 2.3 ± 0.74 | 3 ± 1.13 |
| C n = 40 | O n = 20 | OB n = 20 | |
|---|---|---|---|
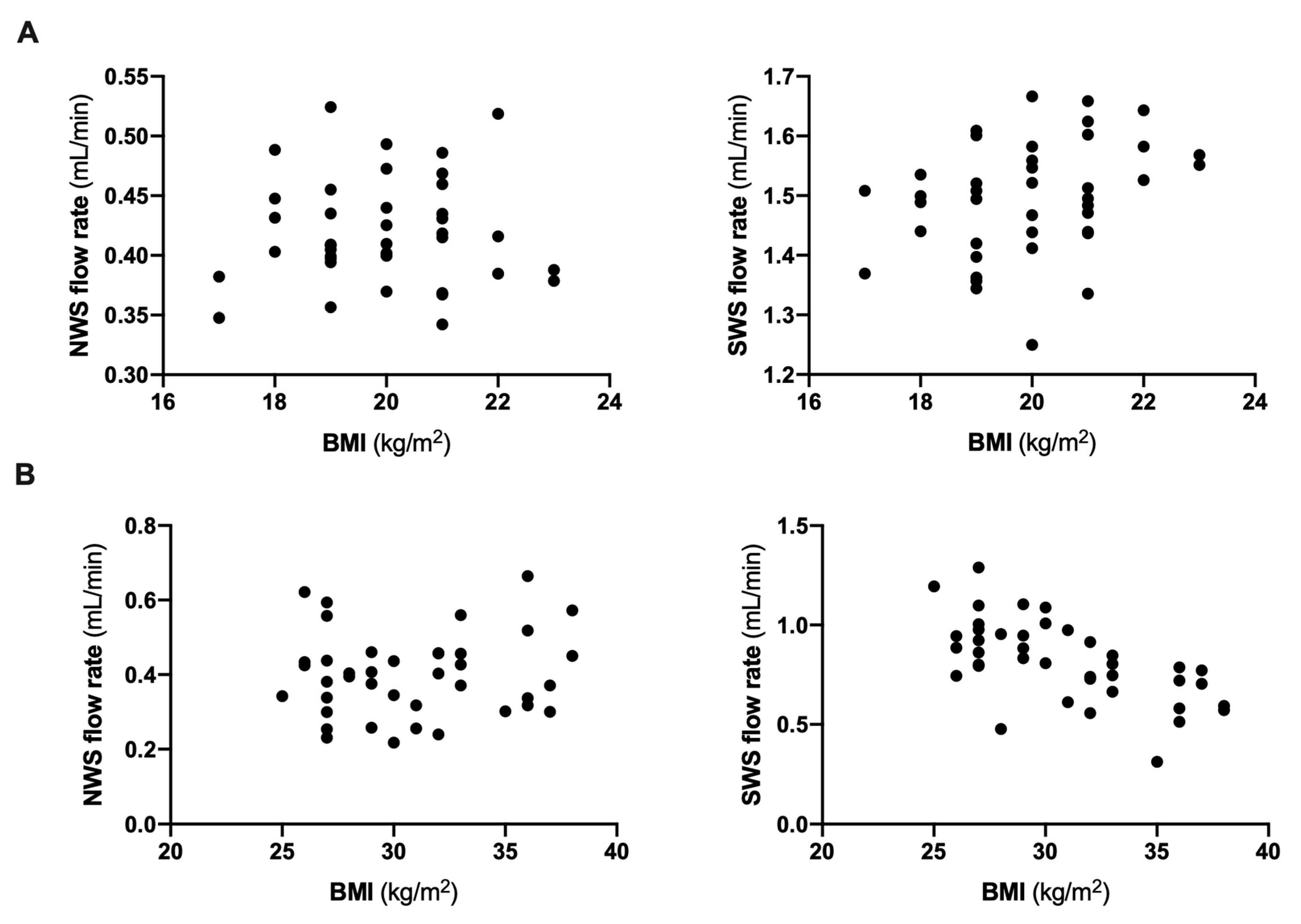
| NWS (mL/min) | 0.42 ± 0.05 | 0.39 ± 0.11 | 0.41 ± 0.11 |
| SWS (mL/min) | 1.5 ± 0.1 | 0.9 ± 0.2 * | 0.74 ± 0.18 *# |
| TP NWS (μg/mL) | 1291 ± 227 | 1167 ± 299 | 1139 ± 245 |
| TP SWS (μg/mL) | 986 ± 327 | 658 ± 208 * | 598 ± 197 * |
| DMFT | 6 ± 2 | 8 ± 3 | 8 ± 2 |
| GI | 0.1 ± 0.1 | 0.1 ± 0.15 | 0.1 ± 0.12 |
| NWS | SWS | Plasma/Erythrocytes | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | AUC | 95% Confidence Interval | p Value | Cut-Off | Sensitivity (%) | Specificity (%) | AUC | 95% Confidence Interval | p Value | Cut-Off | Sensitivity (%) | Specificity (%) | AUC | 95% Confidence Interval | p Value | Cut-Off | Sensitivity (%) | Specificity (%) |
| Antioxidants | ||||||||||||||||||
| SOD (mU/mg protein) | 0.7775 | 0.6222–0.9328 | 0.0027 | >2.469 | 53.13 | 63.96 | 0.615 | 0.4360–0.7940 | 0.2134 | >4.536 | 29.93 | 43.29 | 0.5325 | 0.3490–0.7160 | 0.7251 | >0.2348 | 38.66 | 38.66 |
| CAT (nmol H2O2/min/mg protein) | 0.8875 | 0.7711–1.000 | <0.0001 | >0.5747 | 63.96 | 69.90 | 0.6675 | 0.4954–0.8396 | 0.0699 | >0.5692 | 43.29 | 48.10 | 0.795 | 0.6515–0.9385 | 0.0014 | <0.6559 | 53.13 | 53.13 |
| Px/GPx (mU/mg protein) | 0.6575 | 0.4818–0.8332 | 0.0884 | <0.4494 | 43.29 | 38.66 | 0.5525 | 0.3675–0.7375 | 0.57 | <0.6815 | 38.66 | 34.21 | 0.5 | 0.3167–0.6833 | >0.9999 | >0.3117 | 34.21 | 29.93 |
| GSH (μg/mg protein) | 0.7 | 0.5359–0.8641 | 0.0305 | <0.3304 | 53.1 | 43.29 | 0.7925 | 0.6531–0.9319 | 0.0016 | <0.5908 | 53.13 | 48.10 | 0.5875 | 0.4058–0.7692 | 0.3438 | <2.823 | 43.29 | 34.21 |
| UA (ng/mg protein) | 0.67 | 0.5018–0.8382 | 0.0659 | >0.8843 | 48.10 | 34.21 | 0.5075 | 0.3247–0.6903 | 0.9353 | >3.912 | 29.93 | 29.93 | 0.6125 | 0.4345–0.7905 | 0.2235 | >9.354 | 38.66 | 43.29 |
| Redox Status | ||||||||||||||||||
| TAC (Trolox μmol/mg protein) | 0.52 | 0.3370–0.7030 | 0.8287 | >1.018 | 34.21 | 29.93 | 0.5175 | 0.3348–0.7002 | 0.8498 | >1.202 | 29.93 | 38.66 | 0.5425 | 0.3511–0.7339 | 0.6456 | >1.143 | 34.21 | 38.66 |
| TOS (nmol H2O2 Equiv./mg protein) | 0.9325 | 0.8344–1.000 | <0.0001 | >20.77 | 90 | 100 | 0.7425 | 0.5847–0.9003 | 0.0087 | >45.71 | 70 | 80 | 0.55 | 0.3685–0.7315 | 0.5885 | <14.48 | 34.21 | 29.93 |
| OSI (TOS/TAC ratio) | 0.7875 | 0.6377–0.9373 | 0.0019 | >20.12 | 80 | 75 | 0.655 | 0.4836–0.8264 | 0.0935 | >38.18 | 65 | 60 | 0.5525 | 0.3664–0.7386 | 0.57 | <12.63 | 43.29 | 34.21 |
| Oxidative Damage | ||||||||||||||||||
| AGE (AFU/mg protein) | 0.5825 | 0.3937–0.7713 | 0.372 | >6.116 | 38.66 | 48.10 | 0.9325 | 0.8547–1.000 | <0.0001 | >10.24 | 69.90 | 69.90 | 0.6175 | 0.4395–0.7955 | 0.2036 | >6.360 | 38.66 | 38.66 |
| MDA (μmol/mg protein) | 0.5325 | 0.3480–0.7170 | 0.7251 | >127.4 | 38.66 | 38.66 | 0.565 | 0.3828–0.7472 | 0.4819 | >98.43 | 43.29 | 34.21 | 0.6625 | 0.4817–0.8433 | 0.0787 | >143.0 | 53.13 | 48.10 |
| 4-HNE (ng/mg protein) | 0.7625 | 0.6138–0.9112 | 0.0045 | >1.787 | 65 | 70 | 0.705 | 0.5400–0.8700 | 0.0265 | >1.268 | 70 | 70 | 0.81 | 0.6730–0.9470 | 0.0008 | >1.386 | 75 | 80 |
| 8-OHdG (pg/mg protein) | 0.8625 | 0.7452–0.9798 | <0.0001 | <3.440 | 80 | 80 | 0.795 | 0.6501–0.9399 | 0.0014 | <2.226 | 75 | 80 | 0.8075 | 0.6761–0.9389 | 0.0009 | >2.690 | 65 | 70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewska, A.; Kossakowska, A.; Taranta-Janusz, K.; Zięba, S.; Fejfer, K.; Salamonowicz, M.; Kostecka-Sochoń, P.; Wasilewska, A.; Maciejczyk, M. Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents. J. Clin. Med. 2020, 9, 548. https://doi.org/10.3390/jcm9020548
Zalewska A, Kossakowska A, Taranta-Janusz K, Zięba S, Fejfer K, Salamonowicz M, Kostecka-Sochoń P, Wasilewska A, Maciejczyk M. Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents. Journal of Clinical Medicine. 2020; 9(2):548. https://doi.org/10.3390/jcm9020548
Chicago/Turabian StyleZalewska, Anna, Agnieszka Kossakowska, Katarzyna Taranta-Janusz, Sara Zięba, Katarzyna Fejfer, Małgorzata Salamonowicz, Paula Kostecka-Sochoń, Anna Wasilewska, and Mateusz Maciejczyk. 2020. "Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents" Journal of Clinical Medicine 9, no. 2: 548. https://doi.org/10.3390/jcm9020548
APA StyleZalewska, A., Kossakowska, A., Taranta-Janusz, K., Zięba, S., Fejfer, K., Salamonowicz, M., Kostecka-Sochoń, P., Wasilewska, A., & Maciejczyk, M. (2020). Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents. Journal of Clinical Medicine, 9(2), 548. https://doi.org/10.3390/jcm9020548







