TLR2 Expression on Select Lymphocyte Subsets as a New Marker in Glomerulonephritis
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Patients and Controls
2.2. Comparisons of Protein Concentrations, Complement Components, Renal Function Parameters, and Blood Cell Counts between Patients with PGN, NPGN, and Controls
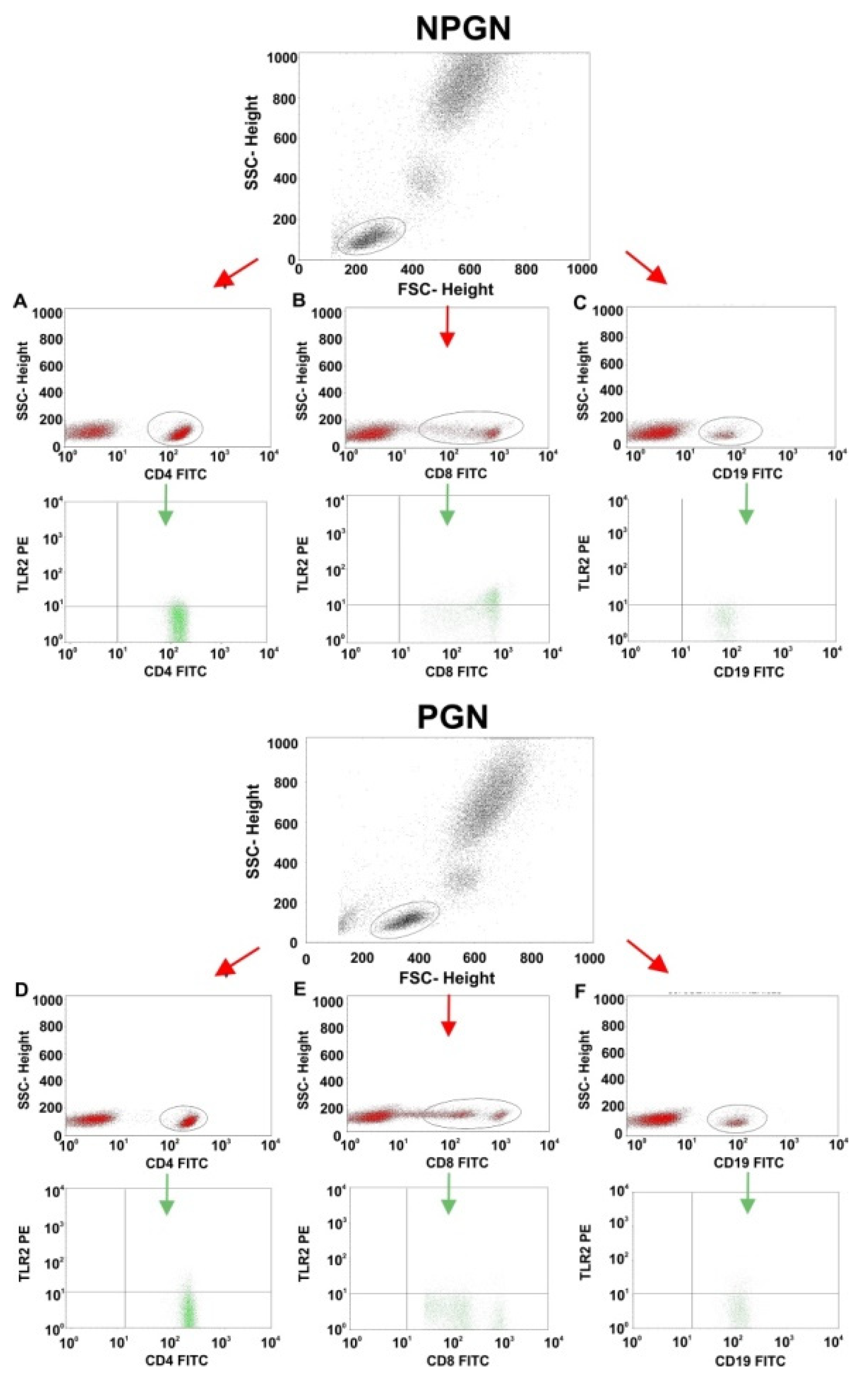
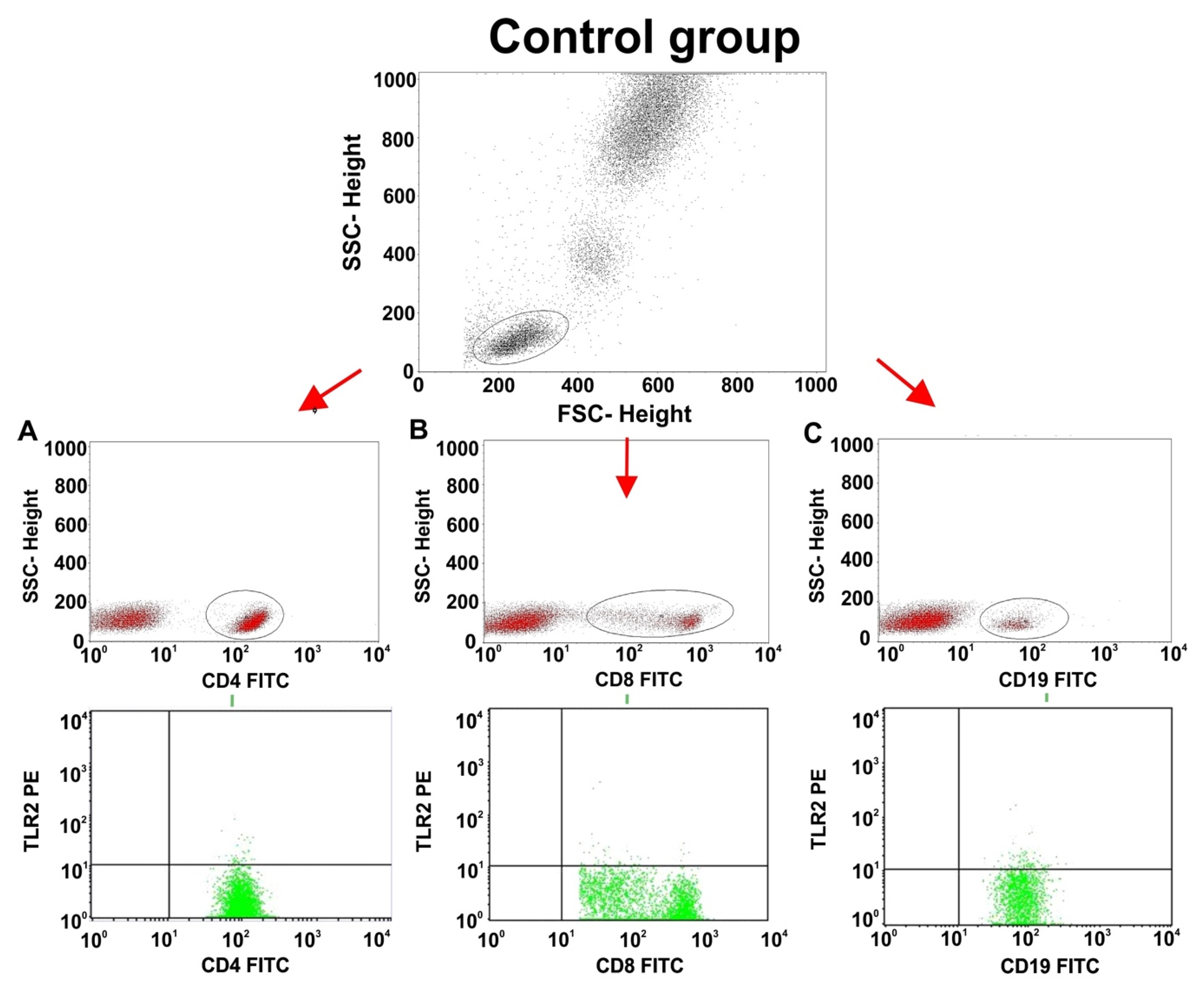
2.3. Comparisons of TLR2 Expression on T and B Lymphocyte Subsets between Patients with PGN, NPGN, and Controls
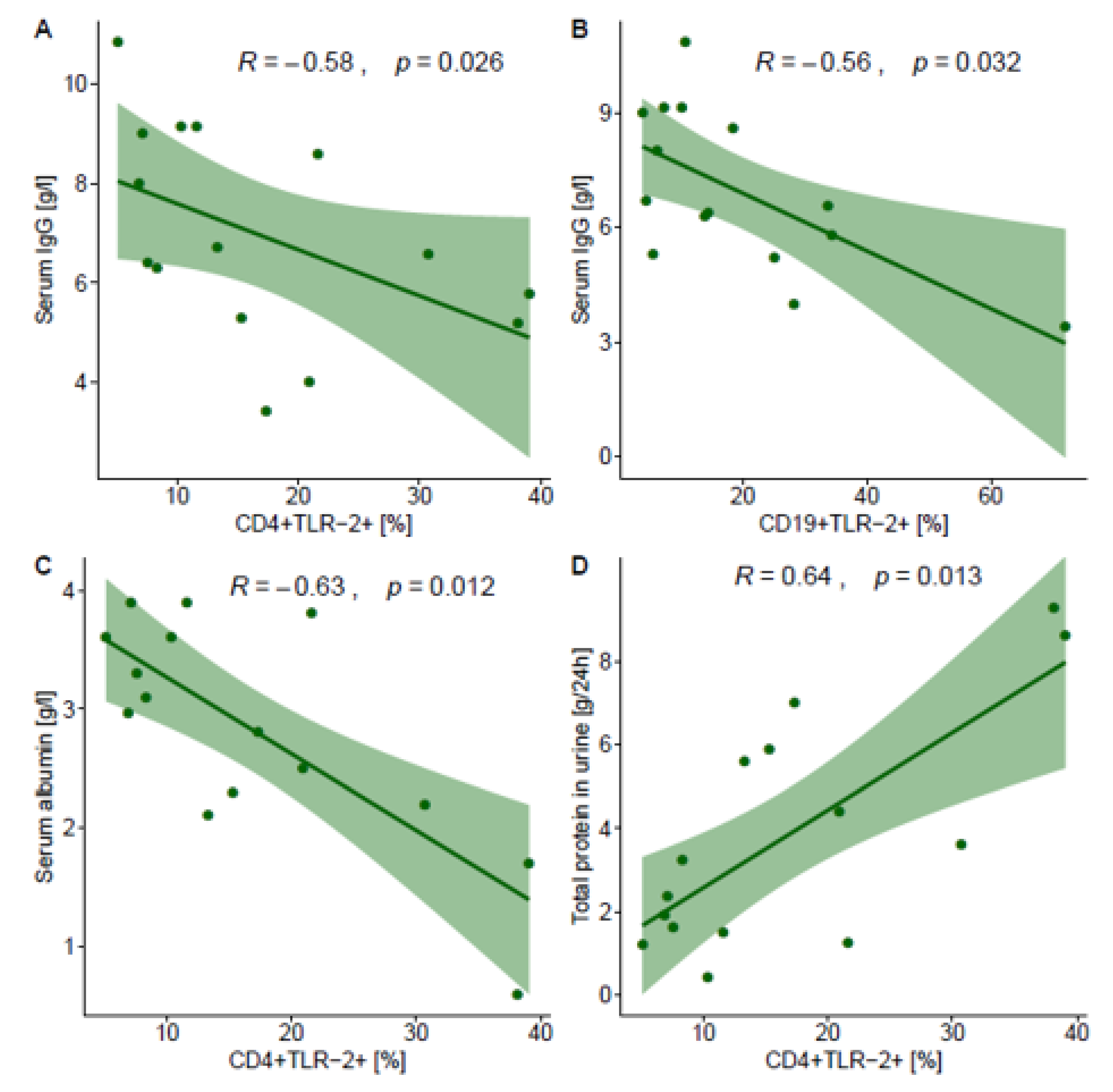
2.4. Correlations between TLR2 Expression on T and B Lymphocyte Subsets and Selected Laboratory Parameters in NPGN and PGN Patients
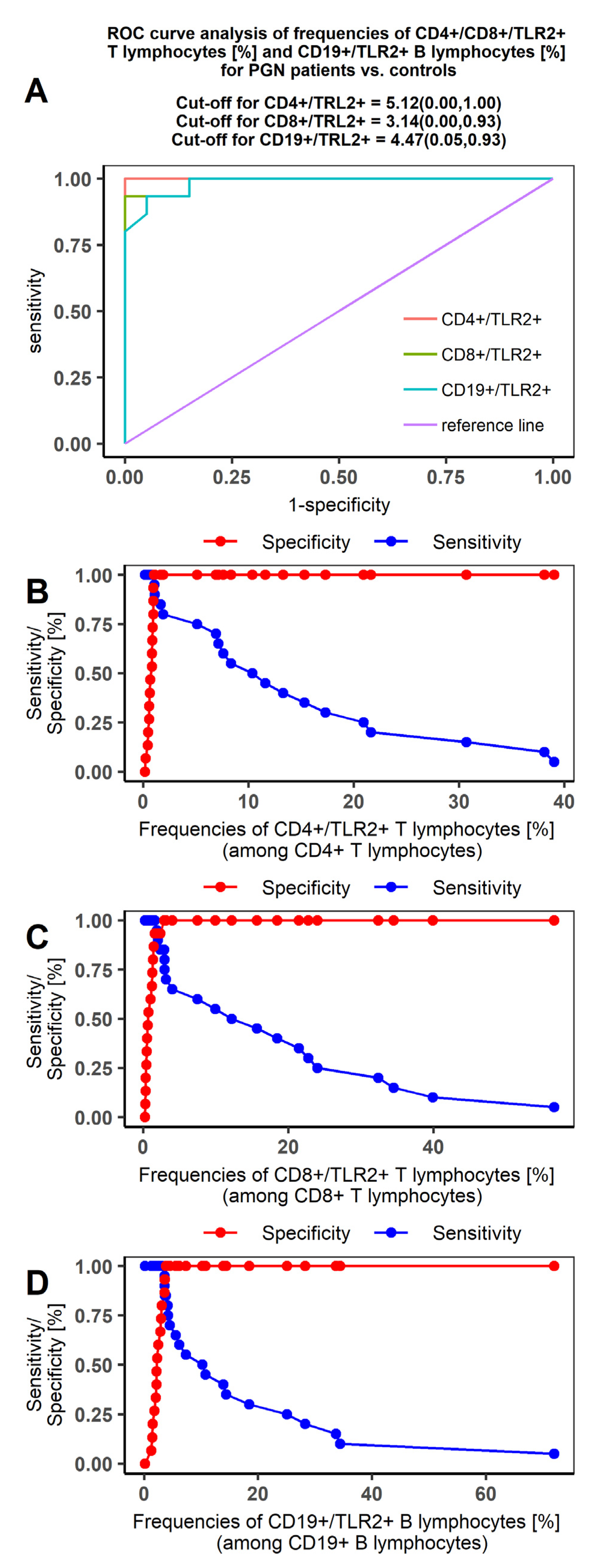
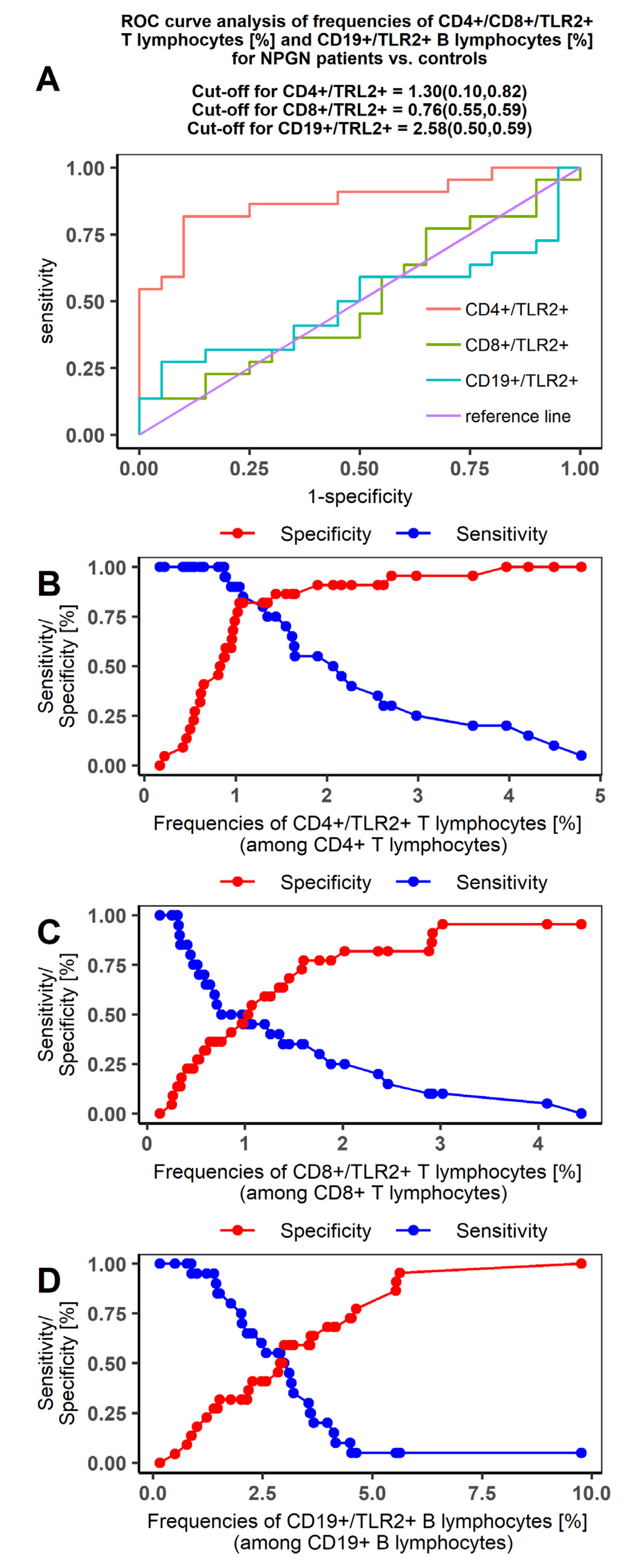
2.5. Receiver Operating Characteristic (ROC) Curve Analysis to Determine The Diagnostic Accuracy of TLR2 Expression on T and B Lymphocytes in Patients with PGN vs NPGN and in Patients vs Controls
3. Discussion
4. Materials and Methods
4.1. Peripheral Blood Collection
4.2. Flow Cytometry and Sample Preparation
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ig | immunoglobulin |
| NPGN | non-proliferative glomerulonephritis |
| PGN | proliferative glomerulonephritis |
| ROC | receiver operating characteristic |
| TLR | Toll-like receptor |
Appendix A

References
- Ferri, F.F. Ferri’s Clinical Advisor 2018 E-Book: 5 Books in 1; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; p. 889. ISBN 9780323529570. [Google Scholar]
- Couser, W.G. Pathogenesis and treatment of glomerulonephritis-an update. Brazilian J. Nephrol. 2016, 38, 107–122. [Google Scholar] [CrossRef]
- Mühlig, A.K.; Lee, J.Y.; Kemper, M.J.; Kronbichler, A.; Yang, J.W.; Lee, J.M.; Shin, J.I.; Oh, J. Levamisole in Children with Idiopathic Nephrotic Syndrome: Clinical Efficacy and Pathophysiological Aspects. J. Clin. Med. 2019, 8, 860. [Google Scholar] [CrossRef]
- Hebert, L.A.; Parikh, S.; Prosek, J.; Nadasdy, T.; Rovin, B.H. Differential Diagnosis of Glomerular Disease: A Systematic and Inclusive Approach. Am. J. Nephrol. 2013, 38, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats. Nephrology (Carlton). 2018, 23, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Gauckler, P.; Bruchfeld, A. Rituximab in minimal change disease and focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2019, pii: gfz205. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Shang, W. and Wang, Z. The Update of NGAL in Acute Kidney Injury. Curr. Protein Pept. Sci. 2017, 18, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, A.A.; Holscher, T.D.; King, A.J.; Hall, F.W.; Kiosses, W.B.; Tobias, P.S.; Mackman, N.; McKay, D.B. TLR2 Is Constitutively Expressed within the Kidney and Participates in Ischemic Renal Injury through Both MyD88-Dependent and -Independent Pathways. J. Immunol. 2007, 178, 6252–6258. [Google Scholar] [CrossRef]
- Milanesi, S.; Verzola, D.; Cappadona, F.; Bonino, B.; Murugavel, A.; Pontremoli, R.; Garibotto, G.; Viazzi, F. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. J. Cell Physiol. 2019, 234, 10868–10876. [Google Scholar] [CrossRef]
- Oliviera Nascimento, L.; Massari, P.; Wetzler, L. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.J.; Fickentscher, C.; Kruithof, E.K.O.; de Moerloose, P. TLR2 Ligands Induce NF-κB Activation from Endosomal Compartments of Human Monocytes. PLoS ONE 2013, 8, e80743. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karmakar, S.; Babu, S.P.S. TLR2 and TLR4 mediated host immune responses in major infectious diseases: A review. Brazilian J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ferro, M.; Serrano del Castillo, C.; Sánchez-Pernaute, O. Cell Membrane-bound TLR2 and TLR4: Potential Predictors of Active Systemic Lupus Erythematosus and Lupus Nephritis. J. Rheumatol. 2016, 43, 1444–1445. [Google Scholar]
- Liu, Y.; Liao, J.; Zhao, M.; Wu, H.; Yung, S.; Chan, T.M.; Yoshimura, A.; Lu, Q. Increased expression of TLR2 in CD4+ T cells from SLE patients enhances immune reactivity and promotes IL-17 expression through histone modifications. Eur. J. Immunol. 2015, 45, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Saito, A.; Komatsuda, A.; Kaga, H.; Sato, R.; Togashi, M.; Okuyama, S.; Wakui, H.; Takahashi, N. Different Expression Patterns of Toll-Like Receptor mRNAs in Blood Mononuclear Cells of IgA Nephropathy and IgA Vasculitis with Nephritis. Tohoku J. Exp. Med. 2016, 240, 199–208. [Google Scholar] [CrossRef]
- Coppo, R.; Camilla, R.; Amore, A.; Peruzzi, L.; Daprà, V.; Loiacono, E.; Vatrano, S.; Rollino, C.; Sepe, V.; Rampino, T.; et al. Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin. Exp. Immunol. 2010, 159, 73–81. [Google Scholar] [CrossRef]
- Brown, H.J.; Lock, H.R.; Sacks, S.H.; Robson, M.G. TLR2 Stimulation of Intrinsic Renal Cells in the Induction of Immune-Mediated Glomerulonephritis. J. Immunol. 2006, 177, 1925–1931. [Google Scholar] [CrossRef]
- Abbas, I.; Noun, M.; Touboul, D.; Sahali, D.; Brunelle, A.; Ollero, M. Kidney Lipidomics by Mass Spectrometry Imaging: A Focus on the Glomerulus. Int. J. Mol. Sci. 2019, 20, 1623. [Google Scholar] [CrossRef]
- Lionaki, S.; Liapis, G.; Boletis, J.N. Pathogenesis and Management of Acute Kidney Injury in Patients with Nephrotic Syndrome Due to Primary Glomerulopathies. Medicina 2019, 55, 365. [Google Scholar] [CrossRef] [PubMed]
- Komatsuda, A.; Wakui, H.; Iwamoto, K.; Ozawa, M.; Togashi, M.; Masai, R.; Maki, N.; Hatakeyama, T.; Sawada, K. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin. Exp. Immunol. 2008, 152, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.-T.; Hsieh, S.-C.; Chiang, B.-L.; Yu, C.-L.; Lin, S.-C. Altered IL-10 and TNF-α production in peripheral blood mononuclear cells of systemic lupus erythematosus patients after Toll-like receptor 2, 4, or 9 activation. Clin. Exp. Med. 2012, 12, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Tadema, H.; Abdulahad, W.H.; Stegeman, C.A.; Kallenberg, C.G.M.; Heeringa, P. Increased Expression of Toll-Like Receptors by Monocytes and Natural Killer Cells in ANCA-Associated Vasculitis. PLoS ONE 2011, 6, e24315. [Google Scholar] [CrossRef]
- Brown, H.J.; Sacks, S.H.; Robson, M.G. Toll-Like Receptor 2 Agonists Exacerbate Accelerated Nephrotoxic Nephritis. J. Am. Soc. Nephrol. 2006, 17, 1931–1939. [Google Scholar] [CrossRef]
- Ding, L.-H.; Liu, D.; Xu, M.; Wu, M.; Liu, H.; Tang, R.-N.; Ma, K.-L.; Chen, P.-S.; Liu, B.-C. TLR2–MyD88–NF-κB pathway is involved in tubulointerstitial inflammation caused by proteinuria. Int. J. Biochem. Cell Biol. 2015, 69, 114–120. [Google Scholar] [CrossRef]
- Hricik, D.E.; Chung-Park, M.; Sedor, J.R. Glomerulonephritis. N. Engl. J. Med. 1998, 339, 888–899. [Google Scholar] [CrossRef]

| Parameters | NPGN | PGN | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | p-Value * | p-Value ** (NPGN vs Control) | p-Value ** (PGN vs Control) | p-Value ** (NPGN vs PGN) | |
| Age (years) | 41.77 ± 17.61 | 39.5 (19.00–75.00) | 46.00 ± 12.97 | 43.00 (28.00–70.00) | 44.40 ± 12.22 | 45.00 (20.00–61.00) | 0.676 - | 0.709 | 1.000 | 0.653 |
| Urea (mg/dL) (normal range: 15–46) | 55.18 ± 31.53 | 45.12 (17.79–115.66) | 35.36 ± 12.62 | 34.54 (13.03–54.76) | 31.40 ± 6.95 | 32.00 (18.00–42.00) | 0.065 | 0.034 | 0.781 | 0.220 |
| BUN (mg/dL) (normal range: 7–18) | 25.78 ± 14.73 | 21.08 (8.31–54.05) | 16.52 ± 5.89 | 16.14 (6.09–25.59) | 14.67 ± 3.25 | 14.95 (8.41–19.63) | 0.065 | 0.034 | 0.781 | 0.220 |
| Serum creatinine (mg/dL) (normal range: 0.7–1.2) | 1.15 ± 0.61 | 0.9 (0.37–2.30) | 1.02 ± 0.35 | 0.95 (0.54–1.79) | 0.92 ± 0.12 | 0.925 (0.70–1.13) | 0.962 | 1.000 | 1.000 | 1.000 |
| Glomerular filtration rate | 85.44 ± 26.87 | 85.82 (46.80–114.98) | 57.19 ± 23.19 | 56.54 (26.71–100.99) | 125.58 ± 10.26 | 121.08 (114.95–148.19) | <0.001 | <0.001 | <0.001 | 0.047 |
| Serum uric acid (mg/dL) (normal range: 3.6–8.2) | 6.58 ± 1.93 | 6.35 (3.80–11.90) | 6.93 ± 1.53 | 7.60 (4.00–8.60) | 6.22 ± 1.40 | 6.95 (3.70–7.90) | 0.244 | 0.798 | 0.143 | 0.390 |
| Serum IgG (g/L) (normal range: 7–16) | 4.59 ± 2.47 | 4.56 (2.00–13.25) | 6.96±2.10 | 6.56 (3.42–10.84) | 12.71 ± 1.40 | 12.79 (10.06–15.47) | <0.001 | <0.001 | <0.001 | 0.068 |
| Serum IgM (g/L) (normal range: 0.4–2.3) | 1.43 ± 0.87 | 1.05 (0.40–3.00) | 1.33 ± 0.74 | 1.20 (0.50–3.20) | 1.66 ± 0.31 | 1.61 (1.17–2.19) | 0.066 | 0.068 | 0.067 | 1.000 |
| Serum IgA (g/L) (normal range: 0.7–4) | 2.03 ± 0.96 | 2.07 (0.60–3.64) | 3.06 ± 1.44 | 3.10 (0.77–5.69) | 2.39 ± 0.84 | 2.56 (0.92–3.92) | 0.058 | 0.431 | 0.252 | 0.025 |
| Serum total protein (g/dL) (normal range: 6.3–8.3) | 4.91 ± 1.04 | 4.90 (3.20–7.40) | 5.51±0.92 | 5.60 (4.20–7.10) | 7.35 ± 0.58 | 7.35 (6.40–8.20) | <0.001 | <0.001 | <0.001 | 0.314 |
| Serum albumin (g/L) (normal range: 3.40–4.80) | 2.53 ± 0.78 | 2.55 (0.80–3.80) | 2.82 ± 0.94 | 2.96 (0.60–3.90) | 4.18 ± 0.36 | 4.23 (3.50-4.75) | <0.0001 | <0.001 | <0.001 | 0.528 |
| Total protein in a 24 h urine collection test (g/24 h) (max: 0.15) | 5.90 ± 5.31 | 4.45 (1.00–20.00) | 3.85 ± 2.84 | 3.21 (0.40–9.28) | 0.00 ± 0.00 | 0.00 (0.00–0.00) | <0.0001 (2.86) | <0.001 | <0.001 | 0.0725 |
| Serum complement component C3 (g/L) (normal range: 0.9–1.8) | 1.37 ± 0.29 | 1.30 (0.90–2.00) | 1.19 ± 0.29 | 1.20 (0.40–1.70) | 1.28 ± 0.22 | 1.24 (0.95–1.78) | 0.361 | 0.475 | 1.000 | 0.264 |
| Serum complement component C4 (g/L) (normal range: 0.1–0.4) | 0.32 ± 0.10 | 0.28 (0.20–0.55) | 0.28 ± 0.07 | 0.27 (0.11–0.46) | 0.28 ± 0.08 | 0.29 (0.15–0.39) | 0.663 | 0.709 | 1.000 | 0.625 |
| Parameters | NPGN | PGN | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | p-Value * | p-Value ** (NPGN vs Control) | p-Value ** (PGN vs Control) | p-Value ** (NPGN vs PGN) | |
| WBC (103/mm3) (normal range: 4.0–10.0) | 6.57 ± 1.63 | 6.4 (4.38–9.80) | 7.23 ± 1.85 | 7.20 (4.30–9.94) | 6.82 ± 0.42 | 6.72 (6.26–7.61) | 0.251 | 0.339 | 0.998 | 0.179 |
| LYM (103/mm3) (normal range: 1.12–4.73) | 2.01 ± 0.68 | 1.96 (1.20–3.70) | 2.15 ± 0.79 | 1.96 (1.20–3.74) | 2.50 ± 0.56 | 2.54 (1.53–3.70) | 0.053 | 0.030 | 0.136 | 1.000 |
| RBC (106/mm3) (normal range: 4.0–5.8) | 4.72 ± 1.64 | 4.40 (3.66–11.80) | 4.34 ± 0.50 | 4.36 (3.34–5.10) | 5.17 ± 0.43 | 5.12 (4.50–5.80) | <0.001 | <0.001 | <0.001 | 1.000 |
| HGB (g/dL) (normal range: 12–16) | 13.33 ± 1.60 | 13.33 (10.70–16.40) | 12.93 ± 1.54 | 13.10 (9.30–15.10) | 14.30 ± 1.19 | 14.35 (12.50–16.90) | 0.030 | 0.061 | 0.022 | 0.816 |
| PLT (103/mm3) (normal range: 140–400) | 244.05 ± 59.02 | 241.5 (147.00–361.00) | 242.40 ± 69.83 | 214.00 (177.00–410.00) | 279.00 ± 57.05 | 281.50 (186.00–403.00) | 0.084 | 0.110 | 0.066 | 1.00 |
| CD3+ T lymphocytes (%) | 72.26 ± 16.77 | 76.88 (5.210–87.980) | 74.24 ± 6.02 | 74.71 (63.30–85.07) | 72.36 ± 1.76 | 72.38 (69.99–74.75) | 0.175 | 0.114 | 0.265 | 1.000 |
| CD19+ B lymphocytes (%) | 12.32 ± 13.90 | 9.52 (1.66–70.30) | 11.87 ± 3.80 | 11.63 (6.97–19.12) | 10.60 ± 2.00 | 10.80 (6.04–14.52) | 0.302 | 0.690 | 0.596 | 0.183 |
| CD3+/CD4+ lymphocytes (%) | 44.04 ± 9.72 | 44.70 (26.13–63.35) | 42.68 ± 5.96 | 42.84 (34.50–53.26) | 42.00 ± 1.21 | 41.51 (40.54–44.19) | 0.300 | 0.185 | 0.853 | 0.602 |
| CD3+/CD8+ lymphocytes (%) | 28.19 ± 6.71 | 27.35 (18.44–48.68) | 28.10 ± 7.81 | 25.47 (18.13–42.06) | 30.17 ± 1.09 | 29.97 (28.90–33.17) | 0.048 | 0.047 | 0.057 | 1.000 |
| T CD3+/CD4+:T CD3+/CD8+ lymphocyte ratio | 1.69 ± 0.68 | 1.62 (0.73–3.23) | 1.65 ± 0.58 | 1.68 (0.82–2.74) | 1.39 ± 0.06 | 1.40 (1.24–1.47) | 0.136 | 0.094 | 0.193 | 1.000 |
| Parameters | NPGN | PGN | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | p-Value * | p-Value ** (NPGN vs Control) | p-Value ** (PGN vs Control) | p-Value ** (NPGN vs PGN) | |
| CD4+/TLR2+ T lymphocytes (%) | 2.29 ± 1.27 | 2.12 (0.50–4.79) | 16.98 ± 11.18 | 13.30 (5.12–39.05) | 0.82 ± 0.42 | 0.85 (0.17–1.90) | <0.001 | 0.003 | 0.001 | 0.001 |
| CD8+/TLR2+ T lymphocytes (%) | 1.35 ± 1.21 | 0.92 (0.13–4.44) | 20.30 ± 15.39 | 18.43 (2.35–56.60) | 1.21 ± 0.90 | 1.09 (0.25–2.92) | <0.001 | 1.000 | <0.001 | <0.001 |
| CD19+/TLR2+ B lymphocytes (%) | 2.99 ± 2.20 | 2.74 (0.50–9.76) | 19.23 ± 17.93 | 13.91 (3.82–72.00) | 2.71 ± 1.24 | 2.66 (0.16–5.53) | <0.001 | 1.000 | <0.001 | <0.001 |
| CD4 + TLR2 + cells (%) | CD8 + TLR2 + cells (%) | CD19 + TLR2+ cells (%) | ||||
|---|---|---|---|---|---|---|
| NPGN | PGN | NPGN | PGN | NPGN | PGN | |
| Serum urea (mg/dL) | −0.08, p = 0.717 | 0.05 p = 0.863 | −0.25, p = 0.258 | −0.15 p = 0.584 | −0.12, p = 0.589 | −0.17 p = 0.549 |
| BUN (mg/dL) | −0.08, 0.717 | 0.05 0.863 | −0.25, 0.258 | −0.15 0.584 | −0.12, 0.589 | −0.17 0.549 |
| IgG (g/L) | −0.01, p = 0.980 | −0.58 p = 0.026 | −0.28, p = 0.205 | −0.21 p = 0.442 | −0.23, p = 0.314 | −0.56 p = 0.032 |
| IgA (g/L) | −0.26 p = 0.239 | −0.20 p = 0.482 | −0.03 p = 0.912 | 0.09 p = 0.743 | −0.13 p = 0.554 | −0.06 p = 0.822 |
| Total protein (g/dL) | 0.02 p = 0.918 | −0.43 p = 0.111 | −0.14 p = 0.548 | −0.03 p = 0.904 | −0.22 p = 0.319 | −0.36 p = 0.194 |
| Albumin (g/L) | 0.09 p = 0.674 | −0.63 p = 0.012 | −0.11 p = 0.634 | −0.34 p = 0.218 | −0.15 p = 0.517 | −0.39 p = 0.157 |
| Total protein in a 24 h urine collection test (g/24 h) | −0.20 p = 0.364 | 0.64 p = 0.013 | −0.09 p = 0.696 | 0.17 p = 0.549 | 0.16 p = 0.465 | 0.37 p = 0.173 |
| Variable | Frequencies of CD4+/TLR2+ T lymphocytes (%) | Frequencies of CD8+/TLR2+ T lymphocytes (%) | Frequencies of CD19+/TLR2+ B lymphocytes (%) |
|---|---|---|---|
| AUC | 1 | 0.973 | 0.945 |
| SE (AUC) | 0 | 0.031 | 0.043 |
| −95%CI | 1 | 0.932 | 0.880 |
| +95%CI | 1 | 1 | 1 |
| Z statistic | Inf | 15.397 | 10.317 |
| p value * | <0.00001 | <0.00001 | <0.00001 |
| Variable | Frequencies of CD4+/TLR2+ T lymphocytes (%) | Frequencies of CD8+/TLR2+ T lymphocytes (%) | Frequencies of CD19+/TLR2+ B lymphocytes (%) | |||
|---|---|---|---|---|---|---|
| NPGN vs controls | PGN vs controls | NPGN vs controls | PGN vs controls | NPGN vs controls | PGN vs controls | |
| AUC | 0.875 | 1.000 | 0.507 | 0.990 | 0.498 | 0.985 |
| SE (AUC) | 0.567 | 0.000 | 0.903 | 0.016 | 0.090 | 0.020 |
| −95%CI | 0.765 | 1.000 | 0.313 | 0.968 | 0.314 | 0.956 |
| +95%CI | 0.985 | 1.000 | 0.674 | 1.000 | 0.681 | 1.000 |
| Z statistic | 6.619 | Inf | 0.076 | 29.873 | −0.025 | 24.093 |
| p value * | <0.00001 | <0.00001 | 0.4699 | <0.00001 | 0.4899 | <0.00001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertowski, S.; Grywalska, E.; Gosik, K.; Smarz-Widelska, I.; Hymos, A.; Dworacki, G.; Niedźwiedzka-Rystwej, P.; Drop, B.; Roliński, J.; Załuska, W. TLR2 Expression on Select Lymphocyte Subsets as a New Marker in Glomerulonephritis. J. Clin. Med. 2020, 9, 541. https://doi.org/10.3390/jcm9020541
Mertowski S, Grywalska E, Gosik K, Smarz-Widelska I, Hymos A, Dworacki G, Niedźwiedzka-Rystwej P, Drop B, Roliński J, Załuska W. TLR2 Expression on Select Lymphocyte Subsets as a New Marker in Glomerulonephritis. Journal of Clinical Medicine. 2020; 9(2):541. https://doi.org/10.3390/jcm9020541
Chicago/Turabian StyleMertowski, Sebastian, Ewelina Grywalska, Krzysztof Gosik, Iwona Smarz-Widelska, Anna Hymos, Grzegorz Dworacki, Paulina Niedźwiedzka-Rystwej, Bartłomiej Drop, Jacek Roliński, and Wojciech Załuska. 2020. "TLR2 Expression on Select Lymphocyte Subsets as a New Marker in Glomerulonephritis" Journal of Clinical Medicine 9, no. 2: 541. https://doi.org/10.3390/jcm9020541
APA StyleMertowski, S., Grywalska, E., Gosik, K., Smarz-Widelska, I., Hymos, A., Dworacki, G., Niedźwiedzka-Rystwej, P., Drop, B., Roliński, J., & Załuska, W. (2020). TLR2 Expression on Select Lymphocyte Subsets as a New Marker in Glomerulonephritis. Journal of Clinical Medicine, 9(2), 541. https://doi.org/10.3390/jcm9020541






