Inpatient Burden and Mortality of Goodpasture’s Syndrome in the United States: Nationwide Inpatient Sample 2003–2014
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Variables and Outcome of Interest
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and In-Hospital Treatment
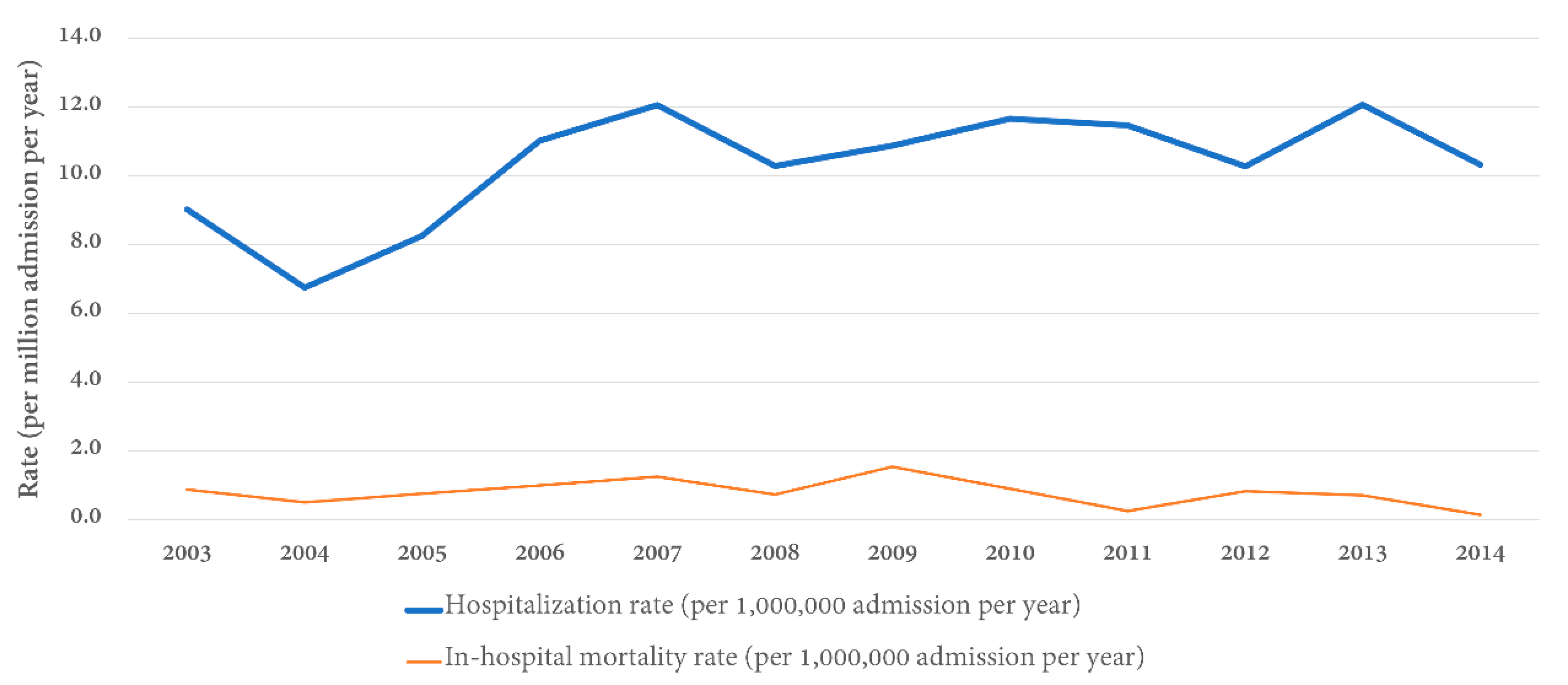
3.2. Inpatient Prevalence of GS
3.3. Organ Failure and In-Hospital Mortality
3.4. Length of Hospital Stay and Hospitalization cost
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gulati, K.; McAdoo, S.P. Anti-Glomerular Basement Membrane Disease. Rheum. Dis. Clin. North. Am. 2018, 44, 651–673. [Google Scholar] [CrossRef]
- Greco, A.; Rizzo, M.I.; De Virgilio, A.; Gallo, A.; Fusconi, M.; Pagliuca, G.; Martellucci, S.; Turchetta, R.; Longo, L.; De Vincentiis, M. Goodpasture’s syndrome: A clinical update. Autoimmun. Rev. 2015, 14, 246–253. [Google Scholar] [CrossRef] [PubMed]
- McAdoo, S.P.; Pusey, C.D. Anti-Glomerular Basement Membrane Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.R.; Salama, A.D. Diagnostic and management challenges in Goodpasture’s (anti-glomerular basement membrane) disease. Nephrol. Dial. Transplant. 2018, 33, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Alchi, B.; Griffiths, M.; Sivalingam, M.; Jayne, D.; Farrington, K. Predictors of renal and patient outcomes in anti-GBM disease: Clinicopathologic analysis of a two-centre cohort. Nephrol. Dial. Transplant. 2015, 30, 814–821. [Google Scholar] [CrossRef]
- Angioi, A.; Cheungpasitporn, W.; Sethi, S.; De Vriese, A.S.; Lepori, N.; Schwab, T.R.; Fervenza, F.C. Familial antiglomerular basement membrane disease in zero human leukocyte antigen mismatch siblings. Clin. Nephrol. 2017, 88, 277–283. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Zacharek, C.C.; Fervenza, F.C.; Cornell, L.D.; Sethi, S.; Herrera Hernandez, L.P.; Nasr, S.H.; Alexander, M.P. Rapidly progressive glomerulonephritis due to coexistent anti-glomerular basement membrane disease and fibrillary glomerulonephritis. Clin. Kidney J. 2016, 9, 97–101. [Google Scholar] [CrossRef][Green Version]
- Tang, W.; McDonald, S.P.; Hawley, C.M.; Badve, S.V.; Boudville, N.C.; Brown, F.G.; Clayton, P.A.; Campbell, S.B.; Zoysa, J.R.; Johnson, D.W. Anti-glomerular basement membrane antibody disease is an uncommon cause of end-stage renal disease. Kidney Int. 2013, 83, 503–510. [Google Scholar] [CrossRef]
- Kluth, D.C.; Rees, A.J. Anti-Glomerular Basement Membrane Disease. J. Am. Soc. Nephrol. 1999, 10, 2446–2453. [Google Scholar]
- Canney, M.; O’Hara, P.V.; McEvoy, C.M.; Medani, S.; Connaughton, D.M.; Abdalla, A.A.; Doyle, R.; Stack, A.G.; O’Seaghdha, C.M.; Clarkson, M.R.; et al. Spatial and Temporal Clustering of Anti-Glomerular Basement Membrane Disease. Clin. J. Am. Soc. Nephrol. 2016, 11, 1392–1399. [Google Scholar] [CrossRef]
- Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2009. Available online: http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf (accessed on 1 February 2020).
- Savage, C.O.; Pusey, C.D.; Bowman, C.; Rees, A.J.; Lockwood, C.M. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. Br. Med. J. (Clin. Res. Ed.). 1986, 292, 301–304. [Google Scholar] [CrossRef]
- Moroni, G.; Ponticelli, C. Rapidly progressive crescentic glomerulonephritis: Early treatment is a must. Autoimmun. Rev. 2014, 13, 723–729. [Google Scholar] [CrossRef]
- Donaghy, M.; Rees, A.J. CIGARETTE SMOKING AND LUNG HAEMORRHAGE IN GLOMERULONEPHRITIS CAUSED BY AUTOANTIBODIES TO GLOMERULAR BASEMENT MEMBRANE. Lancet. 1983, 2, 1390–1393. [Google Scholar] [CrossRef]
- Huart, A.; Josse, A.G.; Chauveau, D.; Korach, J.M.; Heshmati, F.; Bauvin, E.; Cointaul, O.; Kamar, N.; Ribes, D.; Pourrat, J.; et al. Outcomes of patients with Goodpasture syndrome: A nationwide cohort-based study from the French Society of Hemapheresis. J. Autoimmun. 2016, 73, 24–29. [Google Scholar] [CrossRef]
- Taylor, D.M.; Yehia, M.; Simpson, I.J.; Thein, H.; Chang, Y.; De Zoysa, J.R. Anti-glomerular basement membrane disease in Auckland. Int. Med. J. 2012, 42, 672–676. [Google Scholar] [CrossRef]
- Srivastava, A.; Rao, G.K.; Segal, P.E.; Shah, M.; Geetha, D. Characteristics and outcome of crescentic glomerulonephritis in patients with both antineutrophil cytoplasmic antibody and anti-glomerular basement membrane antibody. Clin. Rheumatol. 2013, 32, 1317–1322. [Google Scholar] [CrossRef]
- Pusey, C.D. Anti-glomerular basement membrane disease. Kidney Int. 2003, 64, 1535–1550. [Google Scholar] [CrossRef]
- Levy, J.B.; Turner, A.N.; Rees, A.J.; Pusey, C.D. Long-Term Outcome of Anti-Glomerular Basement Membrane Antibody Disease Treated with Plasma Exchange and Immunosuppression. Ann. Intern. Med. 2001, 134, 1033–1042. [Google Scholar] [CrossRef]
- Lazor, R.; Bigay-Game, L.; Cottin, V.; Cadranel, J.; Decaux, O.; Fellrath, J.M.; Cordier, J.F. Alveolar Hemorrhage in Anti-Basement Membrane Antibody Disease: A Series of 28 cases. Medicine. 2007, 86, 181–193. [Google Scholar] [CrossRef]
- Herbert, D.G.; Buscher, H.; Nair, P. Prolonged venovenous extracorporeal membrane oxygenation without anticoagulation: A case of Goodpasture syndrome-related pulmonary haemorrhage. Crit. Care Resusc. 2014, 16, 69–72. [Google Scholar]
- Balke, L.; Both, M.; Arlt, A.; Rosenberg, M.; Bewig, B. Severe Adult Respiratory Distress Syndrome from Goodpasture syndrome. Survival Using Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2015, 191, 228–229. [Google Scholar] [CrossRef]
- Legras, A.; Mordant, P.; Brechot, N.; Bel, A.; Boussaud, V.; Guillemain, R.; Cholley, B.; Gibault, L.; Le Pimpec-Barthes, F.; Combes, A. Prolonged extracorporeal membrane oxygenation and lung transplantation for isolated pulmonary anti-GBM (Goodpasture) disease. Intensive Care Med. 2015, 41, 1866–1868. [Google Scholar] [CrossRef]
- Shah, M.K.; Hugghins, S.Y. Characteristics and outcomes of patients with Goodpasture’s syndrome. South. Med. J. 2002, 95, 1411–1418. [Google Scholar] [CrossRef]
- Johnson, J.P.; Moore, J., Jr.; Austin, H.A., 3rd; Balow, J.E.; Antonovych, T.T.; Wilson, C.B. Therapy of anti-glomerular basement membrane antibody disease: Analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine. 1985, 64, 219–227. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, J.; Jia, X.Y.; Zhu, S.N.; Jin, Q.Z.; Cheng, X.Y.; Zhao, M.H. Anti-Glomerular Basement Membrane Disease: Outcomes of Different Therapeutic Regimens in a Large Single-Center Chinese Cohort Study. Medicine. 2011, 90, 303–311. [Google Scholar] [CrossRef]
- Booth, A.; Harper, L.; Hammad, T.; Bacon, P.; Griffith, M.; Levy, J.; Savage, C.; Pusey, C.; Jayne, D. Prospective Study of TNFα Blockade with Infliximab in Anti-Neutrophil Cytoplasmic Antibody-Associated Systemic Vasculitis. J. Am. Soc. Nephrol. 2004, 15, 717–721. [Google Scholar] [CrossRef]
- Van Daalen, E.E.; Jennette, J.C.; McAdoo, S.P.; Pusey, C.D.; Alba, M.A.; Poulton, C.J.; Wolterbeek, R.; Nguyen, T.Q.; Goldschmeding, R.; Alchi, B.; et al. Predicting Outcome in Patients with Anti-GBM Glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2018, 13, 63–72. [Google Scholar] [CrossRef]
| All (N = 964) | |
|---|---|
| Clinical characteristics | |
| Age (years) | 54 ± 21 |
| ≤39 | 260 (27) |
| 40–49 | 91 (9) |
| 50–59 | 141 (15) |
| 60–69 | 199 (21) |
| ≥70 | 273 (28) |
| Male sex | 456 (47) |
| Caucasian | 622 (65) |
| Year of hospitalization | |
| 2003–2006 | 281 (29) |
| 2007–2010 | 357 (37) |
| 2011–2014 | 326 (34) |
| Smoking | 95 (10) |
| Hemoptysis | 267 (28) |
| ANCA vasculitis | 84 (9) |
| Granulomatosis with polyangiitis | 54 (6) |
| Microscopic polyangiitis | 30 (3) |
| Sepsis | 62 (6) |
| Treatments | |
| Respiratory support | 216 (23) |
| Invasive mechanical ventilation | 181 (19) |
| Non-invasive ventilation | 49 (5) |
| Renal replacement therapy | 499 (52) |
| Hemodialysis | 494 (51) |
| Peritoneal dialysis | 10 (1) |
| Therapeutic plasmapheresis | 376 (39) |
| Blood transfusion | 391 (41) |
| Outcomes | |
| Number of organ failure | |
| 0 | 230 (24) |
| 1 | 369 (38) |
| 2 | 242 (25) |
| ≥3 | 123 (13) |
| Respiratory failure | 283 (29) |
| Circulatory failure/shock | 53 (6) |
| Renal failure | 597 (62) |
| Liver failure | 10 (1) |
| Hematologic failure | 127 (13) |
| Metabolic failure | 159 (17) |
| Neurological failure | 50 (5) |
| In-hospital death | 74 (8) |
| Resource utilization | |
| Length of stay (days), median (IQR) | 10 (5–18) |
| <5 | 215 (22) |
| 5–9 | 229 (24) |
| 10–14 | 188 (20) |
| ≥15 | 332 (34) |
| Hospitalization cost ($), median (IQR) | 75,831.5 (31,687.3–163,201.0) |
| Year | Total Number of Goodpasture’s Syndrome Patients | Total Number of Admissions | Inpatient Prevalence (per 1,000,000 Admissions) |
|---|---|---|---|
| 2003 | 72 | 7,977,728 | 9.0 |
| 2004 | 54 | 8,004,571 | 6.7 |
| 2005 | 66 | 7,995,048 | 8.3 |
| 2006 | 89 | 8,074,825 | 11.0 |
| 2007 | 97 | 8,043,415 | 12.1 |
| 2008 | 84 | 8,158,381 | 10.3 |
| 2009 | 85 | 7,810,762 | 10.9 |
| 2010 | 91 | 7,800,441 | 11.7 |
| 2011 | 92 | 8,023,590 | 11.5 |
| 2012 | 75 | 7,296,968 | 10.3 |
| 2013 | 86 | 7,119,563 | 12.1 |
| 2014 | 73 | 7,071,762 | 10.3 |
| Total | 964 | 93,377,054 | 10.3 |
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Crude OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | |
| Age (years) | ||||
| ≤39 | 1 (ref) | 1 (ref) | ||
| 40–49 | 0.85 (0.23–3.17) | 0.81 | 0.76 (0.17–3.35) | 0.71 |
| 50–59 | 0.54 (0.15–2.01) | 0.36 | 0.58 (0.13–2.52) | 0.47 |
| 60–69 | 2.34 (1.05–5.22) | 0.04 | 1.68 (0.62–4.54) | 0.31 |
| ≥70 | 4.42 (2.16–9.02) | <0.001 | 3.62 (1.52–8.61) | <0.01 |
| Male | 1.34 (0.83–2.16) | 0.23 | ||
| Caucasian | 1.34 (0.83–2.17) | 0.23 | ||
| Year of admission | ||||
| 2003–2006 | 1 (ref) | 1 (ref) | ||
| 2007–2010 | 1.11 (0.65–1.91) | 0.70 | 0.92 (0.47–1.79) | 0.80 |
| 2011–2014 | 0.46 (0.23–0.90) | 0.02 | 0.23 (0.10–0.55) | 0.001 |
| Smoking | 0.24 (0.06–0.99) | 0.04 | ||
| Hemoptysis | 1.99 (1.22–3.23) | <0.01 | ||
| Granulomatosis with polyangiitis | 1.42 (0.59–3.43) | 0.43 | ||
| Microscopic polyangiitis | 0.41 (0.06–3.03) | 0.38 | ||
| Sepsis | 14.03 (7.85–25.10) | <0.001 | 5.38 (2.53–11.45) | <0.001 |
| Respiratory failure | 10.71 (6.04–19.02) | <0.001 | 7.41 (3.85–14.26) | <0.001 |
| Circulatory failure/shock | 11.72 (6.35–21.66) | <0.001 | 7.85 (3.37–18.26) | <0.001 |
| Renal failure | 3.10 (1.68–5.72) | <0.001 | 2.55 (1.21–5.37) | 0.01 |
| Liver failure | 16.90 (3.71–76.99) | <0.001 | 32.32 (3.51–297.19) | <0.01 |
| Hematologic failure | 1.17 (0.60–2.28) | 0.66 | ||
| Metabolic failure | 1.32 (0.73–2.39) | 0.36 | ||
| Neurological failure | 3.32 (1.59–6.95) | 0.001 | ||
| Invasive mechanical ventilation | 11.26 (6.72–18.86) | <0.001 | ||
| Non-invasive ventilation | 1.74 (0.71–4.23) | 0.22 | ||
| Dialysis | 1.40 (0.87–2.27) | 0.17 | ||
| Therapeutic plasmapheresis | 0.89 (0.54–1.46) | 0.64 | 0.43 (0.22–0.84) | 0.01 |
| Blood transfusion | 0.83 (0.51–1.36) | 0.46 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewput, W.; Thongprayoon, C.; Boonpheng, B.; Ungprasert, P.; Bathini, T.; Chewcharat, A.; Srivali, N.; Vallabhajosyula, S.; Cheungpasitporn, W. Inpatient Burden and Mortality of Goodpasture’s Syndrome in the United States: Nationwide Inpatient Sample 2003–2014. J. Clin. Med. 2020, 9, 455. https://doi.org/10.3390/jcm9020455
Kaewput W, Thongprayoon C, Boonpheng B, Ungprasert P, Bathini T, Chewcharat A, Srivali N, Vallabhajosyula S, Cheungpasitporn W. Inpatient Burden and Mortality of Goodpasture’s Syndrome in the United States: Nationwide Inpatient Sample 2003–2014. Journal of Clinical Medicine. 2020; 9(2):455. https://doi.org/10.3390/jcm9020455
Chicago/Turabian StyleKaewput, Wisit, Charat Thongprayoon, Boonphiphop Boonpheng, Patompong Ungprasert, Tarun Bathini, Api Chewcharat, Narat Srivali, Saraschandra Vallabhajosyula, and Wisit Cheungpasitporn. 2020. "Inpatient Burden and Mortality of Goodpasture’s Syndrome in the United States: Nationwide Inpatient Sample 2003–2014" Journal of Clinical Medicine 9, no. 2: 455. https://doi.org/10.3390/jcm9020455
APA StyleKaewput, W., Thongprayoon, C., Boonpheng, B., Ungprasert, P., Bathini, T., Chewcharat, A., Srivali, N., Vallabhajosyula, S., & Cheungpasitporn, W. (2020). Inpatient Burden and Mortality of Goodpasture’s Syndrome in the United States: Nationwide Inpatient Sample 2003–2014. Journal of Clinical Medicine, 9(2), 455. https://doi.org/10.3390/jcm9020455








