Towards Understanding Therapeutic Failures in Masquelet Surgery: First Evidence that Defective Induced Membrane Properties are Associated with Clinical Failures
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Histology and Immunohistochemical Analysis
2.3. Isolation and Characterization of Mesenchymal Stromal Cells (MSC) from IM Fragments
2.4. Real-Time PCR Analysis
2.5. Statistics
3. Results
3.1. Demographic and Clinical Features of Patients
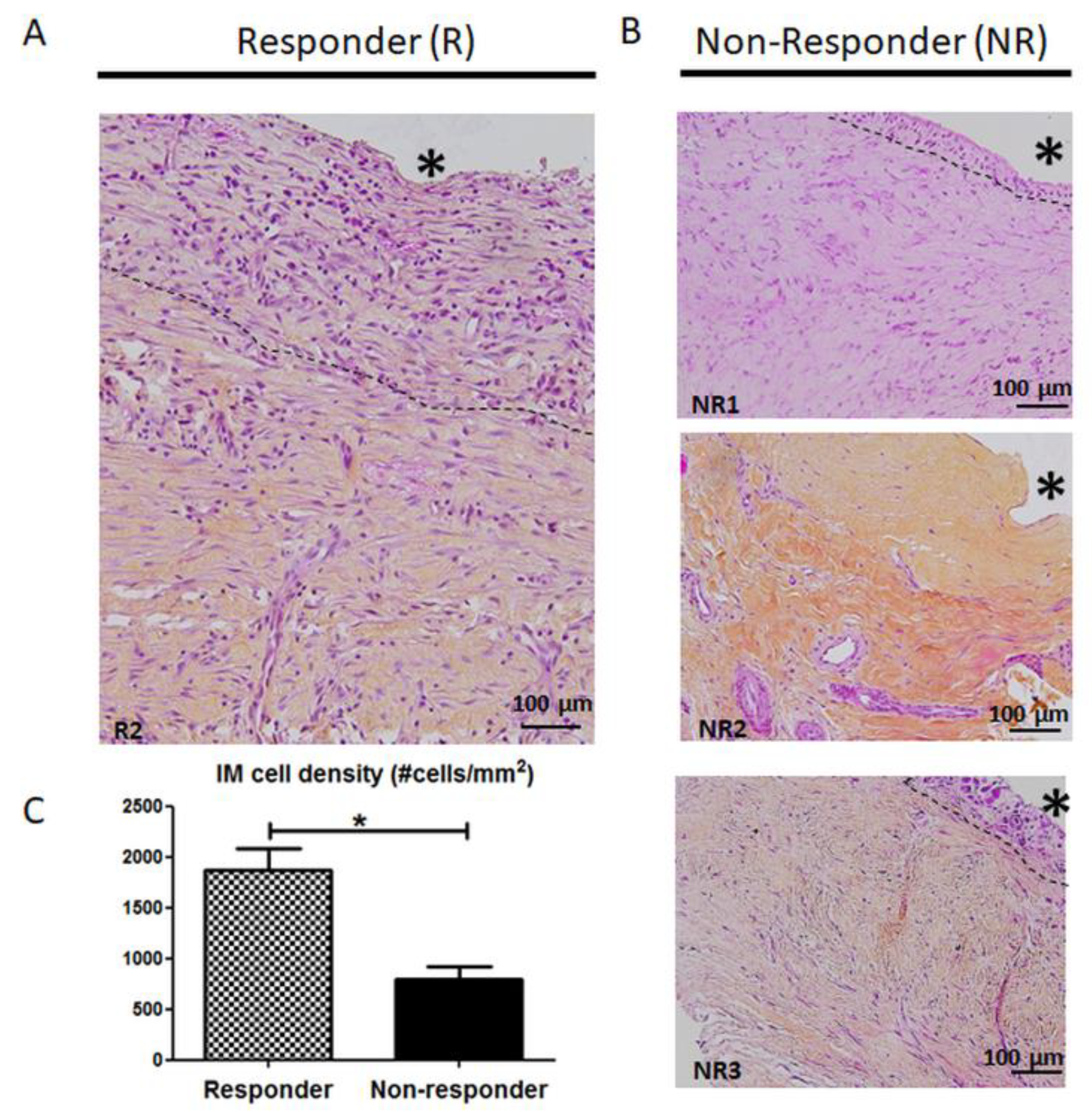
3.2. Histology and Cellularity are Disrupted in Non-Responder Induced Membranes (IM)
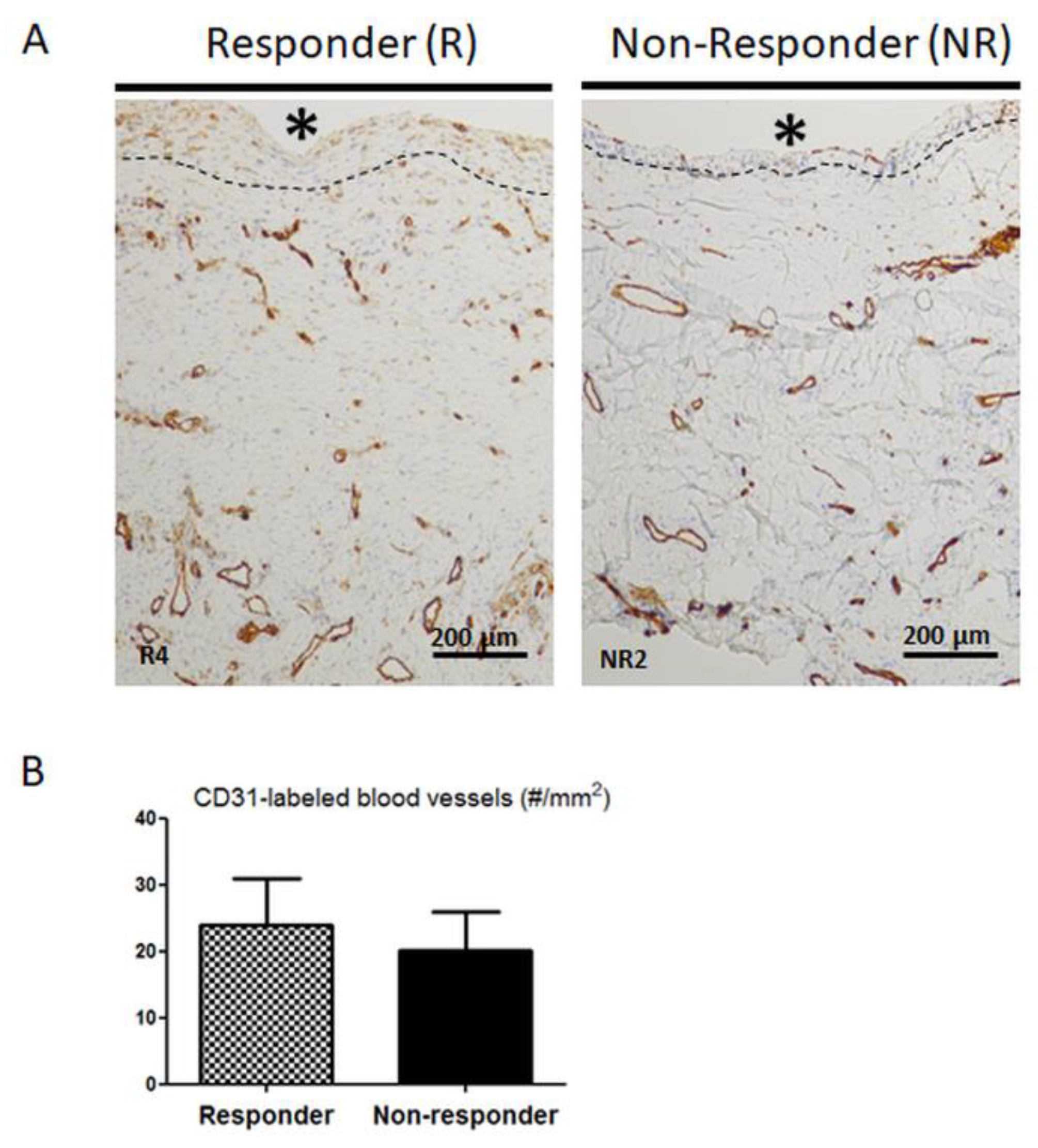
3.3. Distribution and Number of CD31-Positive Blood Vessels are not Altered in Non-Responder Induced Membranes
3.4. Mesenchymal Stromal Cell Content is Altered in Non-Responder Membranes
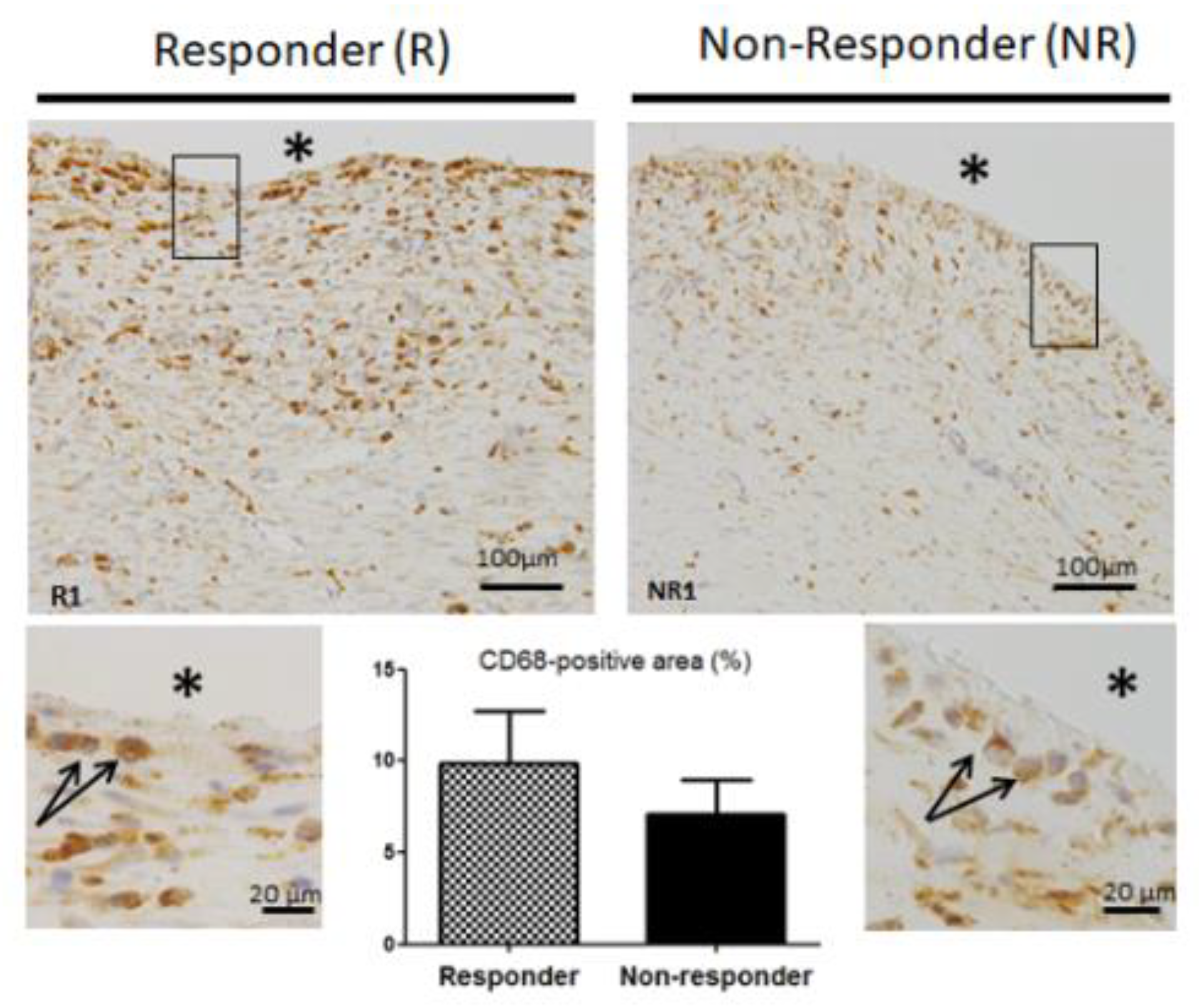
3.5. CD68 Pan Macrophage Marker Localization and Gene Expression are Similar in both Responder and Non-Responder Induced Membranes
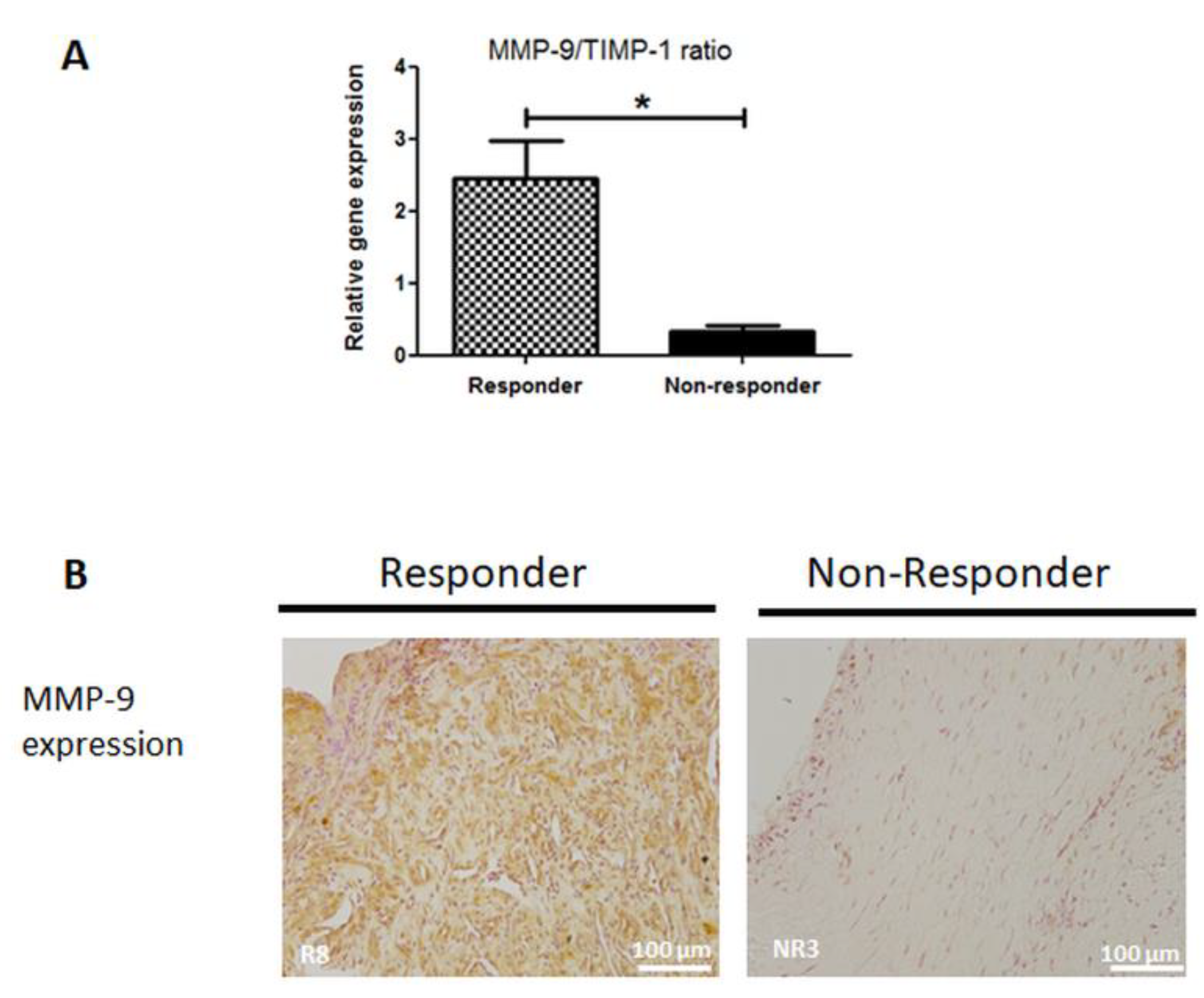
3.6. MMP-9 Expression is Disrupted in Non-Responder Induced Membranes
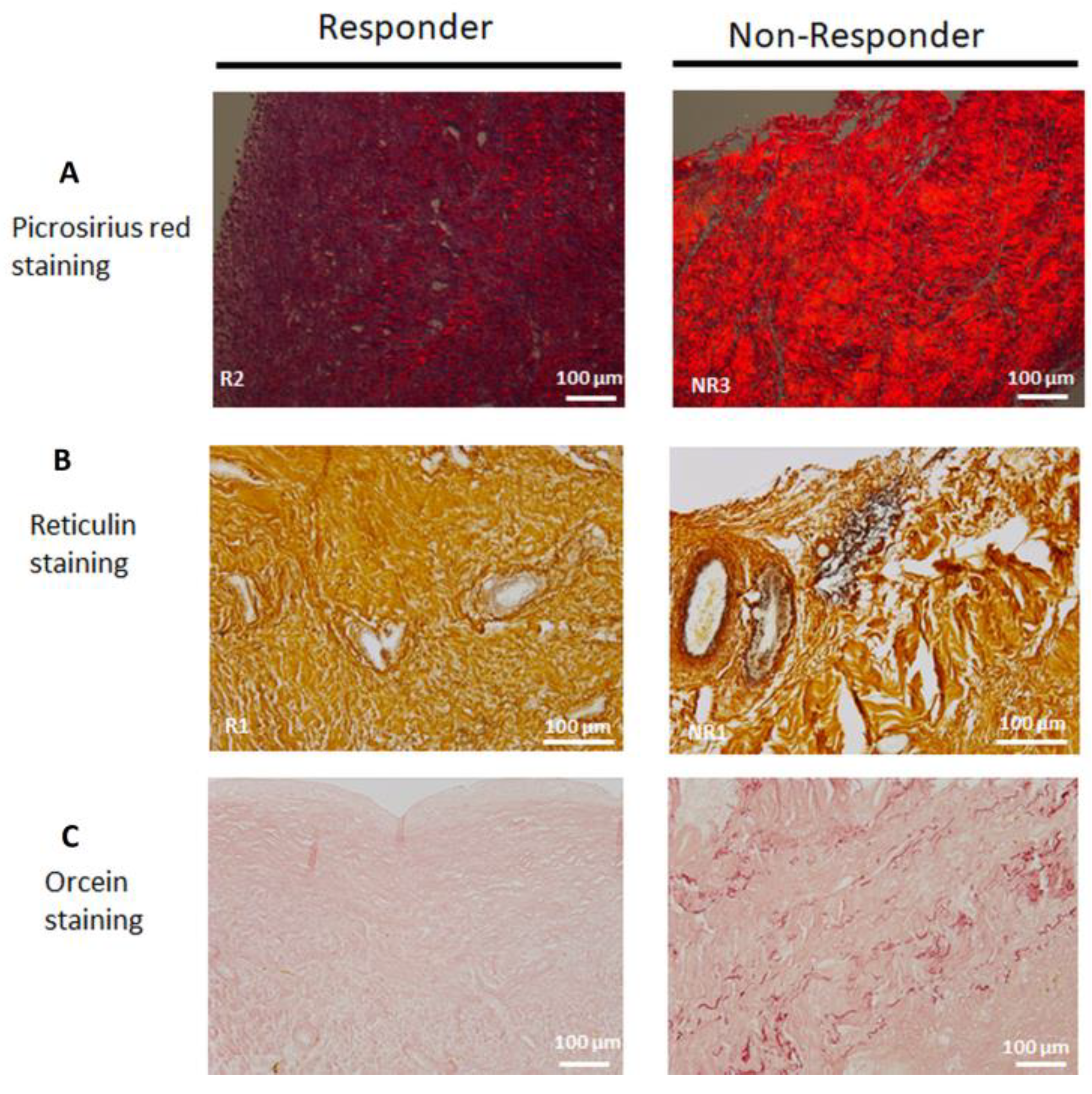
3.7. Collagen Matrix Organization is Disrupted in Non-Responder Induced Membranes
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Gene Symbol | Accession Number | 5’3’-Primer Sequence | Product Size (bp) | Annealing Temperature (°C) | Primers Concentrations (µM) | |
| REFERENCE GENES | ribosomal protein, large, P0 | rplp0 3’ set | NM_001002 | F GGCGACCTGGAAGTCCAACTAC | 113 | 54 | 0.5 |
| R CGGATCTGCTGCATCTGCTTG | |||||||
| rplp0 5’ set | NM_001002 | F GCATCTACAACCCTGAAGTGCTTG | 93 | 57 | 0.5 | ||
| R GCAGACAGACACTGGCAACATTG | |||||||
| peptidylpropyl isomerase A | ppia | XM_193409 | F TATCTGCACTGCCAAGACTGAGTG | 126 | 57 | 0.5 | |
| R CTTCTTGCTGGTCTTGCCATTCC | |||||||
| tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta | ywhaz | NM_145690 | F CAGCACGCTAATAATGCAATTACTG | 129 | 54 | 0.5 | |
| R AATGAGGCAGACAAAAGTTGGAAG | |||||||
| TARGET GENES | monocyte chemoattractant protein 1 | mcp1 | NM_002982 | F AGAAGAATCACCAGCAGCAAGTG | 86 | 55 | 0.5 |
| R TGCTTGGGGTCAGCACAGAT | |||||||
| transforming growth factor beta 2 | tgfβ2 | NM_001135599.3 | F GCCCGTATTTATGGAGTTCAGACAC | 93 | 58 | 0.5 | |
| R CGCAGCAAGGAGAAGCAGATG | |||||||
| metalloproteinase inhibitor 1 | timp | NM_003254.2 | F GGGGCTTCACCAAGACCTACAC | 108 | 58 | 0.5 | |
| R GGTCCGTCCACAAGCAATGAG | |||||||
| runt related transcription factor | runx2 | NM_001024630.3 | F GCCTTCCACTCTCAGTAAGA | 122 | 57 | 0.4 | |
| R GCATTCGTGGGTTGGAGAA | |||||||
| CD68 molecule | cd68 | NM_001251.2 | F GGTTGTCTACCTGAGCTACA | 74 | 56 | 0.4 | |
| R CCGAGAATGTCCACTGTG | |||||||
| von Willebrand factor | vwf | NM_000552.4 | F CAAGGTGGGAAGCTGTAAG | 105 | 53 | 0.5 | |
| R TCCTGCACATCGTTGATG | |||||||
| matrix metalloproteinase-9 | mmp9 | NM_004994.2 | F GGGAAGATGCTGCTGTTCA | 127 | 59 | 0.5 |
References
- Wiese, A.; Pape, H.C. Bone Defects Caused by High-energy Injuries, Bone Loss, Infected Nonunions, and Nonunions. Orthop. Clin. North Am. 2010, 41, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chimutengwende-Gordon, M.; Mbogo, A.; Khan, W.; Wilkes, R. Limb reconstruction after traumatic bone loss. Injury 2017, 48, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Zhong, Z.; Peng, Y.; Lin, C.; Cao, S.; Yang, Y.; Wang, G. Masquelet technique versus Ilizarov bone transport for reconstruction of lower extremity bone defects following posttraumatic osteomyelitis. Injury 2017, 48, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. North Am. 2010, 41, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.; Kishi, T.; Schneider, L.; Fitoussi, F.; Masquelet, A.-C. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop. Traumatol. Surg. Res. 2012, 98, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pelissier, P.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef]
- Christou, C.; Oliver, R.A.; Yu, Y.; Walsh, W.R. The Masquelet technique for membrane induction and the healing of ovine critical sized segmental defects. PLoS ONE 2014, 9, e114122. [Google Scholar] [CrossRef]
- Zwetyenga, N.; Catros, S.; Emparanza, A.; Deminiere, C.; Siberchicot, F.; Fricain, J.-C. Mandibular reconstruction using induced membranes with autologous cancellous bone graft and HA-betaTCP: Animal model study and preliminary results in patients. Int. J. Oral Maxillofac. Surg. 2009, 38, 1289–1297. [Google Scholar] [CrossRef]
- Wang, X.; Wei, F.; Luo, F.; Huang, K.; Xie, Z. Induction of granulation tissue for the secretion of growth factors and the promotion of bone defect repair. J. Orthop. Surg. Res. 2015, 10, 147. [Google Scholar] [CrossRef]
- Cuthbert, R.J.; Churchman, S.M.; Tan, H.B.; McGonagle, D.; Jones, E.; Giannoudis, P.V. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone 2013, 57, 484–492. [Google Scholar] [CrossRef]
- Tang, Q.; Tong, M.; Zheng, G.; Shen, L.; Shang, P.; Liu, H. Masquelet’s induced membrane promotes the osteogenic differentiation of bone marrow mesenchymal stem cells by activating the Smad and MAPK pathways. Am. J. Transl. Res. 2018, 10, 1211–1219. [Google Scholar] [PubMed]
- Morelli, I.; Drago, L.; George, D.A.; Gallazzi, E.; Scarponi, S.; Romanò, C.L. Masquelet technique: Myth or reality? A systematic review and meta-analysis. Injury 2016, 47 (Suppl. 6), S68–S76. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Harwood, P.J.; Tosounidis, T.; Kanakaris, N.K. Restoration of long bone defects treated with the induced membrane technique: Protocol and outcomes. Injury 2016, 47 (Suppl. 6), S53–S61. [Google Scholar] [CrossRef]
- El-Alfy, B.S.; Ali, A.M. Management of segmental skeletal defects by the induced membrane technique. Indian J. Orthop. 2015, 49, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Giotikas, D.; Tarazi, N.; Spalding, L.; Nabergoj, M.; Krkovic, M. Results of the Induced Membrane Technique in the Management of Traumatic Bone Loss in the Lower Limb: A Cohort Study. J. Orthop. Trauma 2019, 33, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Siboni, R.; Joseph, E.; Blasco, L.; Barbe, C.; Bajolet, O.; Diallo, S.; Ohl, X. Management of septic non-union of the tibia by the induced membrane technique. What factors could improve results? Orthop. Traumatol. Surg. Res. 2018, 104, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Pugniere, P.; Banzet, S.; Chaillou, T.; Mouret, C.; Peinnequin, A. Pitfalls of reverse transcription quantitative polymerase chain reaction standardization: Volume-related inhibitors of reverse transcription. Anal. Biochem. 2011, 415, 151–157. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034-1. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Tarchala, M.; Harvey, E.J.; Barralet, J. Biomaterial-Stabilized Soft Tissue Healing for Healing of Critical-Sized Bone Defects: The Masquelet Technique. Adv. Healthc. Mater. 2016, 5, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Aho, O.-M.; Lehenkari, P.; Ristiniemi, J.; Lehtonen, S.; Risteli, J.; Leskelä, H.-V. The mechanism of action of induced membranes in bone repair. J. Bone Jt. Surg. Am. 2013, 95, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Aurégan, J.-C.; Bégué, T.; Rigoulot, G.; Glorion, C.; Pannier, S. Success rate and risk factors of failure of the induced membrane technique in children: A systematic review. Injury 2016, 47 (Suppl. 6), S62–S67. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ode, G.; Hoelscher, G.; Ingram, J.; Bethea, S.; Bosse, M.J. Osteogenic, stem cell and molecular characterisation of the human induced membrane from extremity bone defects. Bone Jt. Res. 2016, 5, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Azi, M.L.; Teixeira, A.D.; Cotias, R.B.; Joeris, A.; Kfuri, M. Induced-Membrane Technique in the Management of Posttraumatic Bone Defects. JBJS Essent. Surg. Tech. 2019, 9, e22. [Google Scholar] [CrossRef]
- Newman, A.C.; Nakatsu, M.N.; Chou, W.; Gershon, P.D.; Hughes, C.C.W. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell 2011, 22, 3791–3800. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Tang, L.; Jennings, T.A.; Eaton, J.W. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc. Natl. Acad. Sci. USA 1998, 95, 8841–8846. [Google Scholar] [CrossRef]
- Kao, W.J.; McNally, A.K.; Hiltner, A.; Anderson, J.M. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J. Biomed. Mater. Res. 1995, 29, 1267–1275. [Google Scholar] [CrossRef]
- Wyatt, L.E.; Sinow, J.D.; Wollman, J.S.; Sami, D.A.; Miller, T.A. The influence of time on human breast capsule histology: Smooth and textured silicone-surfaced implants. Plast. Reconstr. Surg. 1998, 102, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, W.; Nassiri, S.; Kwan, T.; Dang, C.; Liu, W.; Spiller, K.L. Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 721–742. [Google Scholar] [CrossRef] [PubMed]
- Jham, B.C.; Nikitakis, N.G.; Scheper, M.A.; Papadimitriou, J.C.; Levy, B.A.; Rivera, H. Granulomatous foreign-body reaction involving oral and perioral tissues after injection of biomaterials: A series of 7 cases and review of the literature. J. Oral Maxillofac. Surg. 2009, 67, 280–285. [Google Scholar] [CrossRef]
- Wanat, K.A.; Rosenbach, M.; Zoiber, A.F.; Zhang, P.J.; Schaffer, A. E-cadherin is expressed by mono-and multinucleated histiocytes in cutaneous sarcoidal and foreign body granulomas. Am. J. Derm. 2014, 36, 651–654. [Google Scholar] [CrossRef]
- Nagaoka, I.; Hirota, S. Increased expression of matrix metalloproteinase-9 in neutrophils in glycogen-induced peritoneal inflammation of guinea pigs. Inflamm. Res. 2000, 49, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C.; Thompson, Z.; Miclau, T.; Werb, Z.; Helms, J.A. Altered fracture repair in the absence of MMP9. Development 2003, 130, 4123–4133. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.Y.; Lieu, S.; Yang, F.; Lang, J.; Lu, C.; Werb, Z.; Hu, D.; Miclau, T.; Marcucio, R.; et al. MMP9 regulates the cellular response to inflammation after skeletal injury. Bone 2013, 52, 111–119. [Google Scholar] [CrossRef]
- MacLauchlan, S.; Skokos, E.A.; Meznarich, N.; Zhu, D.H.; Raoof, S.; Shipley, J.M.; Senior, R.M.; Bornstein, P.; Kyriakides, T.R. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J. Leukoc. Biol. 2009, 85, 617–626. [Google Scholar] [CrossRef]
- Haubruck, P.; Heller, R.; Apitz, P.; Kammerer, A.; Alamouti, A.; Daniel, V.; Schmidmaier, G.; Moghaddam, A. Evaluation of matrix metalloproteases as early biomarkers for bone regeneration during the applied Masquelet therapy for non-unions. Injury 2018, 49, 1732–1738. [Google Scholar] [CrossRef]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharm. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Gindraux, F.; Loisel, F.; Bourgeois, M.; Oudina, K.; Melin, M.; de Billy, B.; Sergent, P.; Leclerc, G.; Petite, H.; Auber, F.; et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet’s technique is performed later. Eur. J. Trauma Emerg. Surg. 2019, 1–12. [Google Scholar] [CrossRef]
| Patient Identification | Age (yrs) | Gender (F/M) | Smoker (Y/N) | Bone Defect Location | Bone Defect Lenght (cm) | Fracture Fixation | Infection (Y/N) | Duration Spacer in Site (wks) | |
|---|---|---|---|---|---|---|---|---|---|
| Responder (R) | R1 | 30 | M | N | Left tibia | 12 | Ex.Fix | N | 20 |
| R2 | 65 | M | N | Left radius | 7 | Plate | Y | 12 | |
| R3 | 87 | M | N | Left tibia | 5 | Nail | Y | 6 | |
| R4 | 40 | M | N | Left femur | 9 | Nail | N | 8 | |
| R5 | 46 | M | Y | Left tibia | 8 | K-wires | Y | 8 | |
| R6 | 55 | M | N | Left tibia | 4 | Ex.Fix | Y | 10 | |
| R7 | 42 | M | N | Right tibia | 15 | Ex. Fix | Y | 8 | |
| R8 | 40 | M | Y | Right radius | 6 | Plate | Y | 8 | |
| Non responder (NR) | NR1 | 47 | F | N | Left tibia | 10 | Ex. Fix | Y | 20 |
| NR2 | 40 | M | N | Left femur | 9 | Nail | N | 40 | |
| NR3 | 60 | M | Y | Right humerus | 7 | Plate | Y | 9 | |
| Mean +/− SEM | Responder | 50.63 ± 6.40 | 8.25 ± 1.30 | 10.00 ± 1.55 | |||||
| Non-responder | 49.00 ± 5.85 | 8.66 ± 0.88 | 23.00 ± 9.07 | ||||||
| P-value | 0.88 | 0.85 | 0.047 | ||||||
| Patient Identification | Presence or Absence of IM-Derived MSC (CD45- CD90+ CD73+ CD105+ Cells) | |
|---|---|---|
| Responder (R) | R1 | Presence |
| R2 | Not tested | |
| R3 | Presence | |
| R4 | Presence | |
| R5 | Presence | |
| R6 | Presence | |
| R7 | Presence | |
| R8 | Presence | |
| Non-Responder (NR) | NR1 | Not tested |
| NR2 | Absence | |
| NR3 | Absence |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durand, M.; Barbier, L.; Mathieu, L.; Poyot, T.; Demoures, T.; Souraud, J.-B.; Masquelet, A.-C.; Collombet, J.-M. Towards Understanding Therapeutic Failures in Masquelet Surgery: First Evidence that Defective Induced Membrane Properties are Associated with Clinical Failures. J. Clin. Med. 2020, 9, 450. https://doi.org/10.3390/jcm9020450
Durand M, Barbier L, Mathieu L, Poyot T, Demoures T, Souraud J-B, Masquelet A-C, Collombet J-M. Towards Understanding Therapeutic Failures in Masquelet Surgery: First Evidence that Defective Induced Membrane Properties are Associated with Clinical Failures. Journal of Clinical Medicine. 2020; 9(2):450. https://doi.org/10.3390/jcm9020450
Chicago/Turabian StyleDurand, Marjorie, Laure Barbier, Laurent Mathieu, Thomas Poyot, Thomas Demoures, Jean-Baptiste Souraud, Alain-Charles Masquelet, and Jean-Marc Collombet. 2020. "Towards Understanding Therapeutic Failures in Masquelet Surgery: First Evidence that Defective Induced Membrane Properties are Associated with Clinical Failures" Journal of Clinical Medicine 9, no. 2: 450. https://doi.org/10.3390/jcm9020450
APA StyleDurand, M., Barbier, L., Mathieu, L., Poyot, T., Demoures, T., Souraud, J.-B., Masquelet, A.-C., & Collombet, J.-M. (2020). Towards Understanding Therapeutic Failures in Masquelet Surgery: First Evidence that Defective Induced Membrane Properties are Associated with Clinical Failures. Journal of Clinical Medicine, 9(2), 450. https://doi.org/10.3390/jcm9020450





