Outcomes of Vitrectomy for Long-Duration Macular Hole
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| MH | macular hole |
| FTMH | full thickness macular hole |
| ERM | epiretinal membrane |
| PPV | pars plana vitrectomy |
| ILM | internal limiting membrane |
| AL | axial length |
| BCVA | best corrected visual acuity |
| VA | visual acuity |
| OCT | optical coherence tomography |
| EZ | ellipsoid zone |
| RPE | retinal pigment epithelium |
| logMAR | the Logarithm of the Minimum Angle of Resolution |
| IP | group of patients that underwent PPV with systematic ILM peeling |
| IPEP | group of patients that underwent vitrectomy with an ERM and ILM peeling |
| IF | group of patients that underwent vitrectomy with inverted flap technique |
References
- Johnson, R.N.; Gass, J.D. Idiopathic macular holes: Observation, stages of formation, and implications for surgical intervention. Ophthalmology 1988, 95, 917–924. [Google Scholar] [CrossRef]
- Gass, J.D. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am. J. Ophthalmol. 1995, 119, 752–759. [Google Scholar] [CrossRef]
- Kanski, J.; Bowling, B. Kanski’s Clinical Ophtalmology, 8th ed.; W.B. Saunders Ltd.: London, UK, 2015. [Google Scholar]
- Huang, L.; Levinson, D.; Levine, J.P.; Mian, U.; Tsui, I. Optical coherence tomography findings in idiopathic macular holes. J. Ophtalm. 2011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duker, J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Stalmans, P. The International Vitre-omacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef]
- Blain, P.; Paques, M.; Massin, P.; Erginay, A.; Spielmann, A.C.; Santiago, P.Y.; Gaudric, A. Epiretinal membranes surrounding idiopathic macular holes. Retina 1998, 18, 316–321. [Google Scholar] [CrossRef]
- Ip, M.S.; Baker, B.J.; Duker, J.S.; Reichel, E.; Baumal, C.R.; Gangnon, R.; Puliafito, C.A. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch. Ophthalmol 2002, 120, 29–35. [Google Scholar] [CrossRef]
- Brooks, H.L. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000, 107, 1939–1948. [Google Scholar] [CrossRef]
- Baumann, C.; Kaye, S.; Iannetta, D.; Sultan, Z.; Dwivedi, R.; Pearce, I. Effect of inverted internal limiting membrane flap on closure rate, postoperative visual acuity, and restoration of outer retinal layers in primary idiopathic macular hole surgery. Retina 2019. [Google Scholar] [CrossRef]
- Kang, S.W. Types of macular hole closure and their clinical implications. Br. J. Ophthalmol. 2003, 87, 1015–1019. [Google Scholar] [CrossRef]
- Jaycock, P.D.; Bunce, C.; Xing, W.; Thomas, D.; Poon, W.; Gazzard, G.; Williamson, T.H.; Laidlaw, D.A. Outcomes of macular hole surgery: Implications for surgical management and clinical governance. Eye 2005, 19, 879–884. [Google Scholar] [CrossRef]
- Willis, A.W.; Garcia-Cosio, J.F. Macular hole surgery. Comparison of longstanding versus recent macular holes. Ophthalmology 1996, 103, 1811–1814. [Google Scholar] [CrossRef]
- Thompson, J.T.; Sjaarda, R.N.; Lansing, M.B. The results of vitreous surgery for chronic macular holes. Retina 1997, 17, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Joondeph, B.C. Macular Hole Repair Using an ILM Patch. Available online: http://retinatoday.com/2016/08/macular-hole-repair-using-an-ilm-patch (accessed on 1 July 2017).
- Yun, C.; Oh, J.; Hwang, S.-Y.; Togloom, A.; Kim, S.-W.; Huh, K. Morphologic characteristics of chronic macular hole on optical coherence tomography. Retina 2012, 32, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Ezra, E.; Wells, J.; Gray, R.H.; Kinsella, F.M.P.; Orr, G.M.; Grego, J.; Arden, G.B.; Gregor, Z.J. Incidence of idiopathic fullthickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology 1998, 105, 353–359. [Google Scholar] [CrossRef]
- Wendel, R.T.; Patel, A.C.; Kelly, N.E.; Salzano, T.C.; Wells, J.W.; Novack, G.D. Vitreous surgery for macular holes. Ophthalmology 1993, 100, 1671–1676. [Google Scholar] [CrossRef]
- Lois, N.; Burr, J.; Norrie, J.; Vale, L.; Cook, J.; McDonald, A.; Boachie, C.; Ternent, L.; McPherson, G. Full-thickness Macular Hole and Internal Limiting Membrane Peeling. Internal limiting membrane peeling versus no peeling for idiopathic full-thickness macular hole: A pragmatic randomized controlled trial. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1586–1592. [Google Scholar] [CrossRef]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 2010, 117, 2018–2025. [Google Scholar] [CrossRef]
- Bae, K.; Lee, S.M.; Kang, S.W.; Kim, E.S.; Yu, S.Y.; Kim, K.T. Atypical epiretinal tissue in full-thickness macular holes: Pathogenic and prognostic significance. Br. J. Ophthalmol. 2018. [Google Scholar] [CrossRef]
- Lai, T.T.; Chen, S.N.; Yang, C.M. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: Clinical and surgical findings. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 629–638. [Google Scholar] [CrossRef]
- De Giacinto, C.; Pastore, M.R.; Cirigliano, G.; Tognetto, D. Macular Hole in Myopic Eyes: A Narrative Review of the Current Surgical Techniques. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Tornambe, P.E.; Poliner, L.S.; Cohen, R.G. Definition of macular hole surgery end points: Elevated/open, flat/open, flat/closed. Retina 1998, 18, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Benson, W.E.; Cruickshanks, K.C.; Fong, D.S.; Williams, G.A.; Bloome, M.A.; Frambach, D.A.; Kreiger, A.E.; Murphy, R.P. Surgical management of macular holes: A report by the American Academy of Ophthalmology. Ophthalmology 2001, 108, 1328–1335. [Google Scholar] [CrossRef]
- Haritoglou, C.; Gass, C.A.; Schaumberger, M.; Ehrt, O.; Gandorfer, A.; Kampik, A. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am. J. Ophthalmol. 2001, 132, 363–368. [Google Scholar] [CrossRef]
- Yooh, H.S.; Brooks, H.L.; Capone, A.; L’hernault, N.L.; Grossniklaus, H.E. Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am. J. Ophthalmol. 1996, 122, 67–75. [Google Scholar]
- Yan, X.; Andresen, P.; Lumi, X.; Chen, Q.; Petrovski, G. Expression of Progenitor Cell Markers in the Glial-Like Cells of Epiretinal Membranes of Different Origins. J. Ophthalmol. 2018. [Google Scholar] [CrossRef]
- Abdelkader, E.; Lois, N. Internal limiting membrane peeling in vitreo-retinal surgery. Surv. Ophthalmol. 2008, 53, 368–396. [Google Scholar] [CrossRef]
- Sheidow, T.G.; Blinder, K.J.; Holekamp, N.; Joseph, D.; Shah, G.; Grand, M.G.; Thomas, M.A.; Bakal, J.; Sharma, S. Outcome results in macular hole surgery: An evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology 2003, 110, 1697–1701. [Google Scholar] [CrossRef]
- Mitamura, Y.; Mitamura-Aizawa, S.; Katome, T.; Naito, T.; Hagiwara, A.; Kumagai, K.; Yamamoto, S. Photoreceptor impairment and restoration on optical coherence tomographic image. J. Ophthalmol. 2013. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; Arias, L.; Araiz, J.; García-Arumí, J.; Montero, J.A.; Piñero, D.P. Spectral-domain optical coherence tomography study of macular structure as prognostic and determining factor for macular hole surgery outcome. Retina 2013, 33, 1117–1122. [Google Scholar] [CrossRef]
- Kishi, S.; Kamei, Y.; Shimizu, K. Tractional elevation of Henle’s fiber layer in idiopathic macular holes. Am. J. Ophthalmol. 1995, 120, 486–496. [Google Scholar] [CrossRef]
- Poliner, L.S.; Tornambe, P.E. Retinal pigment epitheliopathy after macular hole surgery. Ophthalmology 1992, 99, 1671–1677. [Google Scholar] [CrossRef]
- Frangieh, G.T.; Green, W.R.; Engel, H.M. A histopathologic study of macular cysts and holes. Retina 1981, 1, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Guyer, D.R.; Green, W.R.; de Bustros, S.; Fine, S.L. Histopathologic features of idiopathic macular holes and cysts. Ophthalmology 1990, 97, 1045–1051. [Google Scholar] [CrossRef]
- Bu, S.C.; Kuijer, R.; van der Worp, R.J.; Huiskamp, E.A.; de Lavalette, R.; Victor, W.; Li, X.R.; Hooymans, J.M.; Los, L.I. Glial cells and collagens in epiretinal membranes associated with idiopathic macular holes. Retina 2014, 34, 897–906. [Google Scholar] [CrossRef]
| Groups | Eyes N = 50 (%) | Patients N = 48 (%) | Female Patients N = 33 (%) | Male Patients N = 15 (%) | Age Mean ± SD Range (Years) |
|---|---|---|---|---|---|
| IP | 17 (34.0) | 15 (31.2) | 9 (27.3) | 6 (40.0) | 68.8 ± 5.4 61–76 |
| IPEP | 12 (24.0) | 12 (25.0) | 9 (27.3) | 3 (20.0) | 67.6 ± 7.0 57–81 |
| IF | 21 (42.0) | 21 (43.8) | 15 (45.4) | 6 (40.0) | 69.8 ± 7.1 60–87 |
| Groups | Smaller Diameter MH (µm) Mean ± SD (Range) | Larger Diameter MH (µm) Mean ± SD (Range) |
|---|---|---|
| IP | 299.2 ± 151.6 (45–572) | 806.0 ± 272.0 (300–1513) |
| IPEP | 383.0 ± 149.4 (226–732) | 903.2 ± 210.7 (532–1281) |
| IF | 458.1 ± 130.8 (148–707) | 950.6 ± 305.6 (280–1543) |
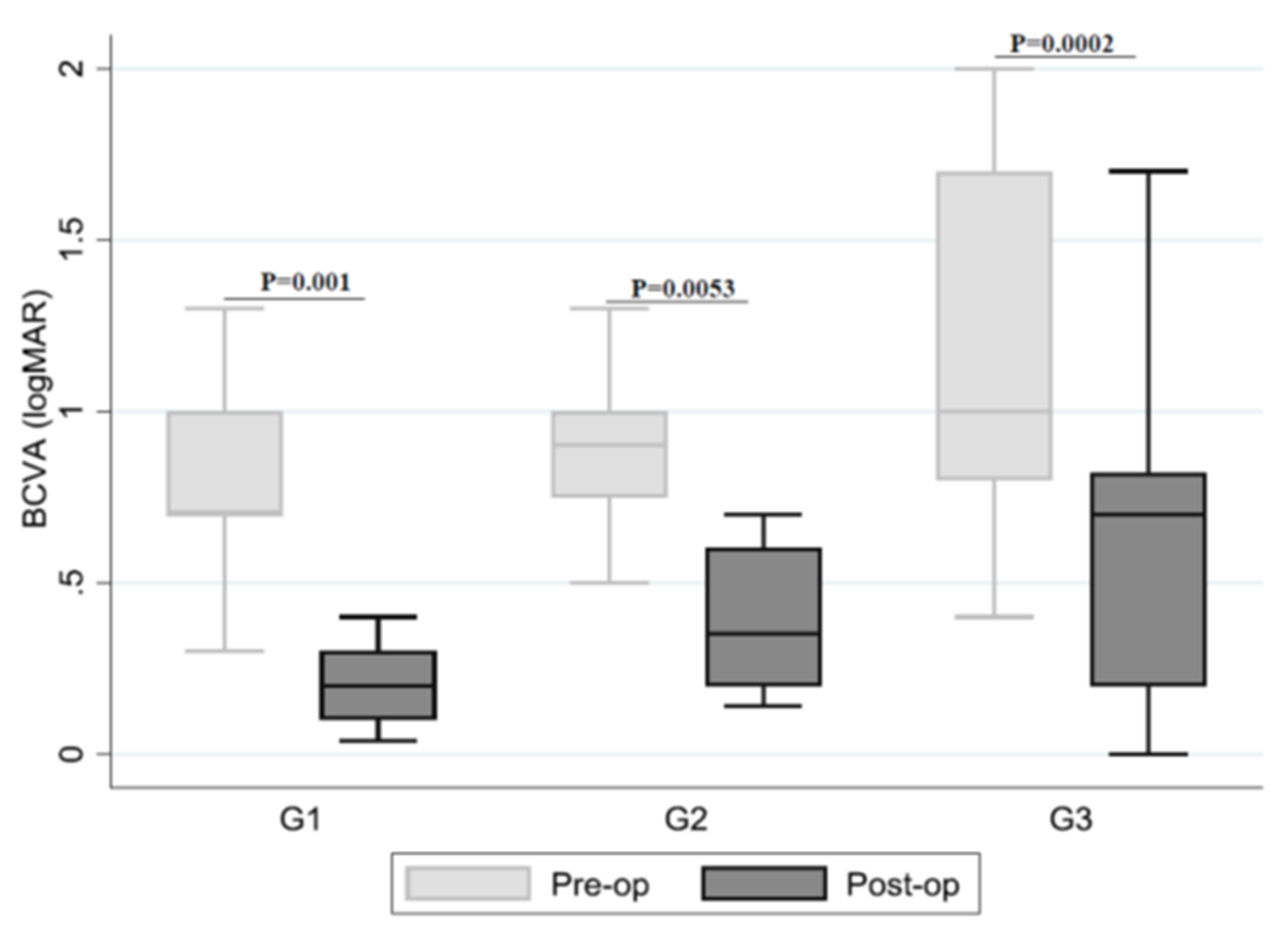
| Groups | BCVA Pre-op Logarithm of the Minimum Angle of Resolution (logMAR) Mean ± SD, Median (IQR), Range | BCVA Post-op (logMAR) Mean ± SD, Median (IQR), Range | Anatomic MH Closure N (%) | p-Value |
|---|---|---|---|---|
| IP | 0.8 ± 0.4 0.7 (0.7–1.0) 0.3–2.0 | 0.3 ± 0.3 0.2 (0.1–0.3) 0.4–1.0 | 17/17 (100.0%) | 0.001 |
| IPEP | 1.0 ± 0.4 0.9 (0.7–1.0) 0.5–2.0 | 0.4 ± 0.3 0.4 (0.2–0.6) 0.1–1.3 | 8/12 (66.7%) | 0.0053 |
| IF | 1.2 ± 0.5 1.0 (0.8–1.7) 0.4–2.0 | 0.6 ± 0.5 0.7 (0.2–0.8) 0.0–1.7 | 20/21 (95.2%) | 0.0002 |
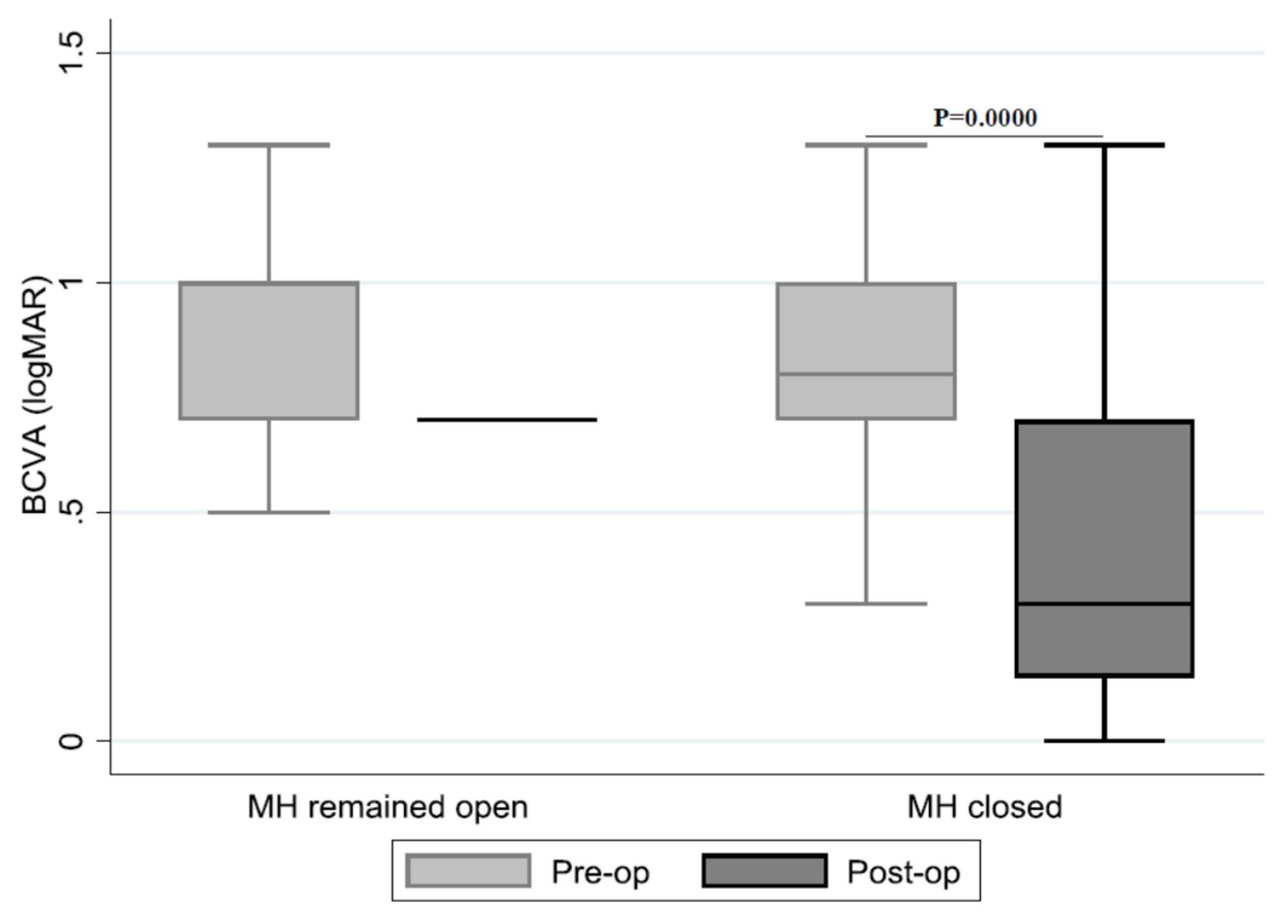
| (Pre-op)–(Post-op) logMAR Difference | (%) | MH Remained Open (Frequency (%)) | MH Closed (Frequency (%)) |
|---|---|---|---|
| <0 | 3 (6.0) | 1 (20.0) | 2 (4.4) |
| 0–0.2 | 10 (20.0) | 2 (40.0) | 8 (17.8) |
| 0.2–0.4 | 9 (18.0) | 1 (20.0) | 8 (17.8) |
| >0.4 | 28 (56.0) | 1 (20.0) | 27 (60.0) |
| (Pre-op)–(Post-op) logMAR Difference (n) | ||||
| Smaller MH Diameter (µm) | <0 N(%) | 0–0.2 N(%) | 0.2–0.4 N(%) | >0.4 N(%) |
| 0–250 | 1 (33.3) | 2 (20.0) | 2 (22.2) | 6 (21.4) |
| 250–400 | 0 (0.0) | 2 (20.0) | 4 (44.4) | 10 (35.7) |
| >400 | 2 (66.7) | 6 (60.0) | 3 (33.3) | 12 (42.9) |
| (Pre-op)–(Post-op) logMAR Difference (n) | ||||
| Large MH Diameter (µm) | <0 | 0–0.2 | 0.2–0.4 | >0.4 |
| 0–250 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 250–400 | 0 (0.0) | 1 (16.7) | 2 (33.3) | 0 (0.0) |
| >400 | 2 (100.0) | 5 (83.3) | 4 (66.7) | 17 (100.0) |
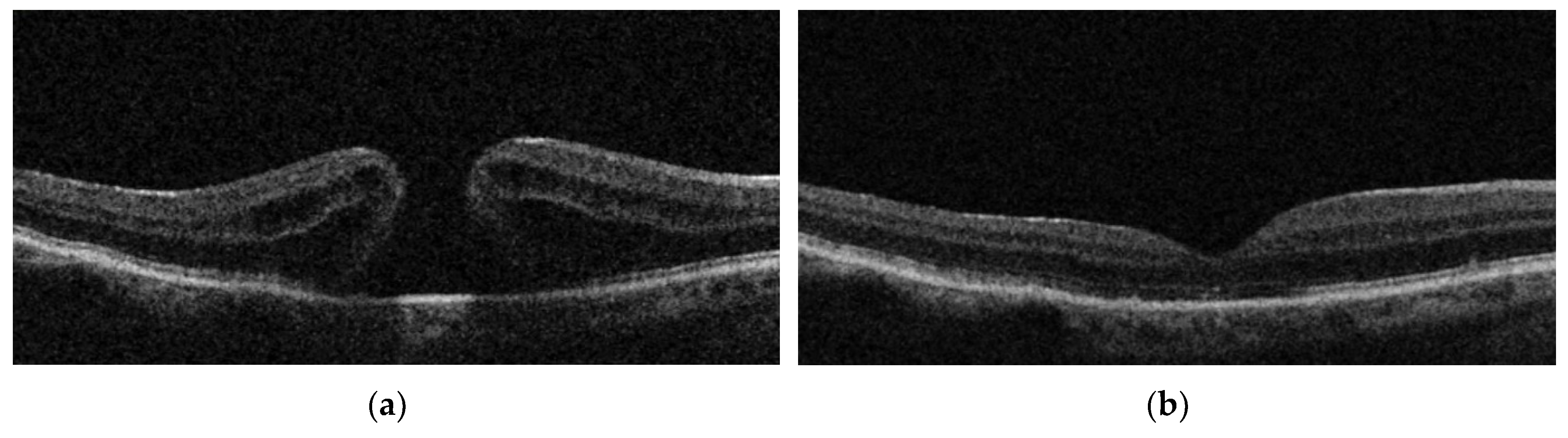
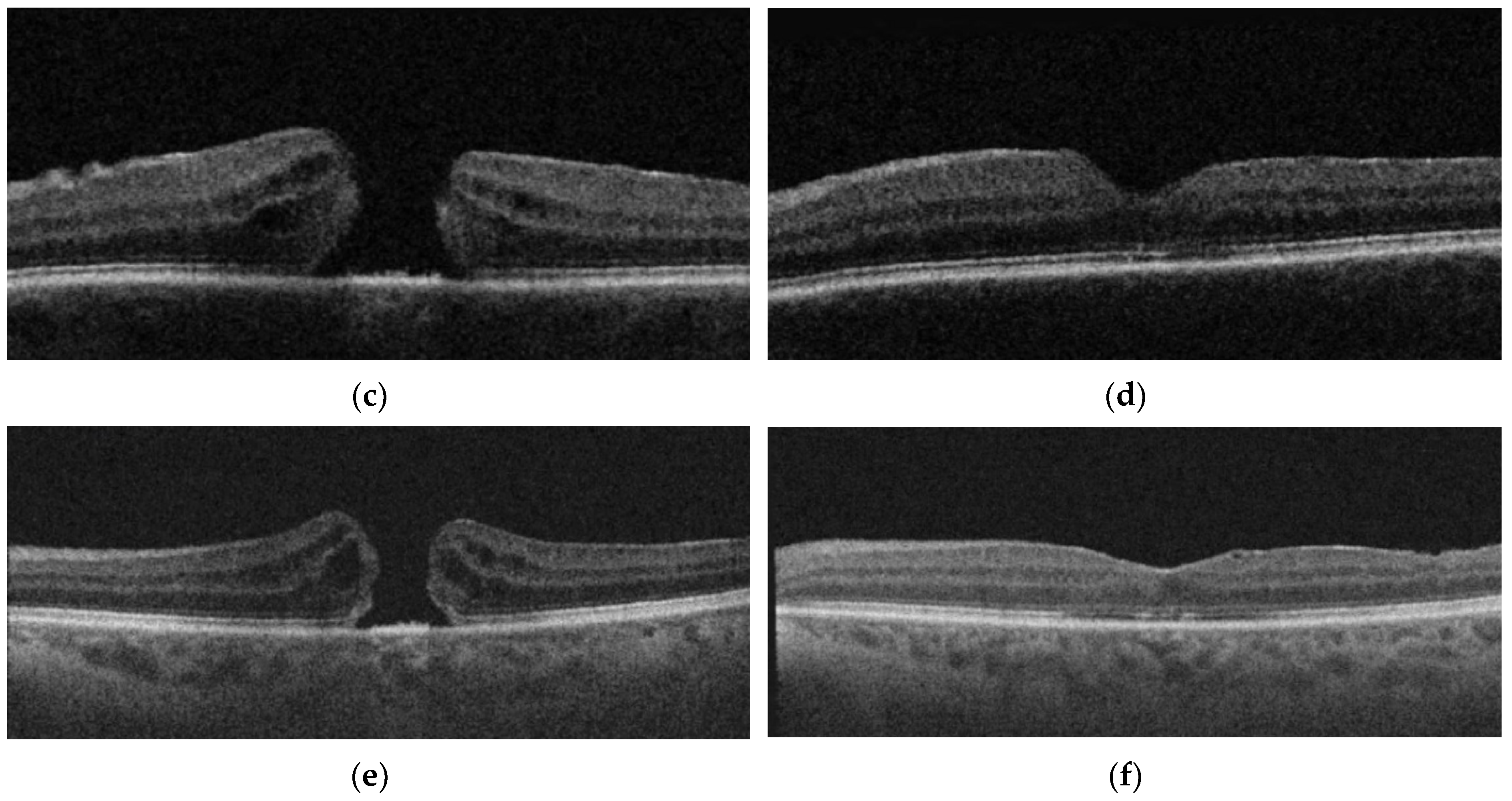
| Groups | Integrity of Ellipsoid Zone (%) N | Integrity of RPE (%) | Characteristics Post-op |
|---|---|---|---|
| IP | 88.2% (15/17) | 100% | 5.9% (1/17) Irregularity of inner retinal layers 5.9% (1/17) ERM 5.9% (1/17) Intraretinal edema + hyperreflective dots |
| IPEP | 41.6% (5/12) | 100% | 8.3% (1/12) Subretinal fluid |
| IF | 23.8% (5/21) | 100% | 4.8% (1/21) Irregularity inner retinal layers 4.8% (1/21) ERM 4.8% (1/21) Intraretinal pseudocysts |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumi, X.; Mahnic, M.; Petrovski, B.É.; Petrovski, G. Outcomes of Vitrectomy for Long-Duration Macular Hole. J. Clin. Med. 2020, 9, 444. https://doi.org/10.3390/jcm9020444
Lumi X, Mahnic M, Petrovski BÉ, Petrovski G. Outcomes of Vitrectomy for Long-Duration Macular Hole. Journal of Clinical Medicine. 2020; 9(2):444. https://doi.org/10.3390/jcm9020444
Chicago/Turabian StyleLumi, Xhevat, Mina Mahnic, Beáta Éva Petrovski, and Goran Petrovski. 2020. "Outcomes of Vitrectomy for Long-Duration Macular Hole" Journal of Clinical Medicine 9, no. 2: 444. https://doi.org/10.3390/jcm9020444
APA StyleLumi, X., Mahnic, M., Petrovski, B. É., & Petrovski, G. (2020). Outcomes of Vitrectomy for Long-Duration Macular Hole. Journal of Clinical Medicine, 9(2), 444. https://doi.org/10.3390/jcm9020444





