Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Measurements at Baseline
2.3. Assessment of Physical Activity
2.4. Endpoints of the Study
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
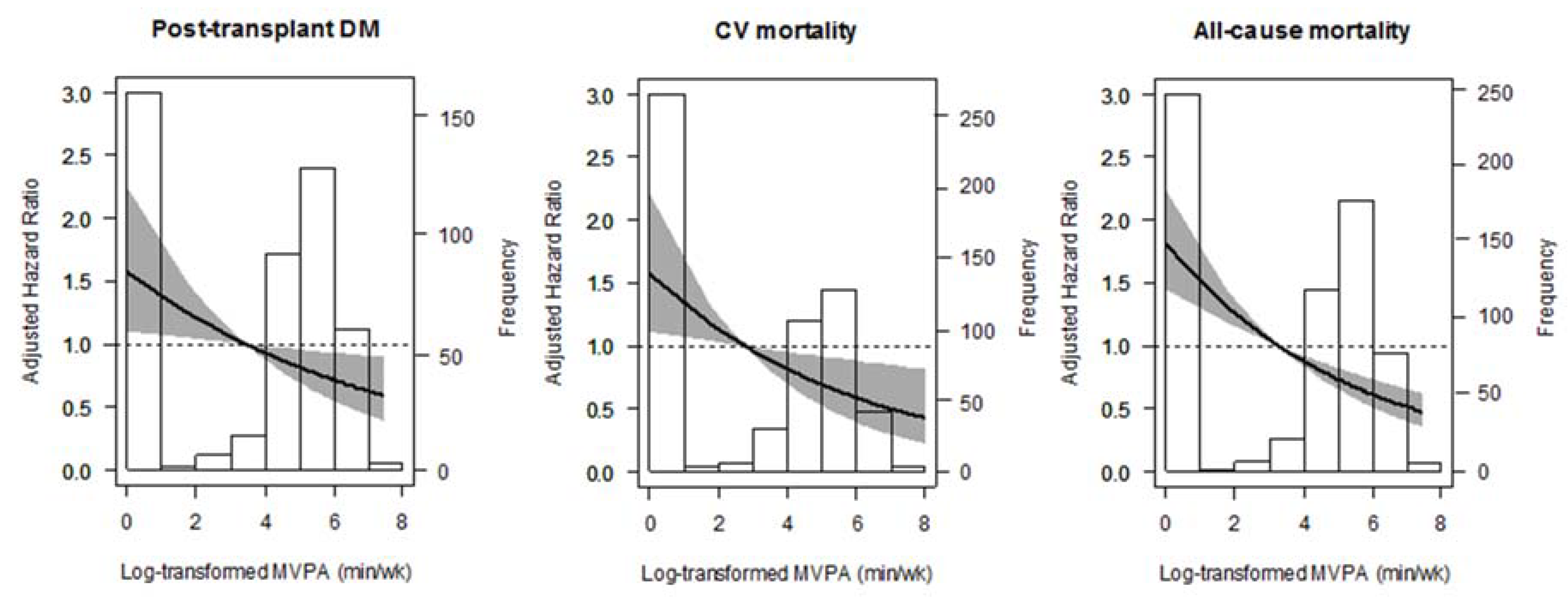
3.2. Post-Transplant Diabetes Mellitus
3.3. Cardiovascular and All-Cause Mortality
3.4. Additional Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ponton, P.; Rupolo, G.P. Quality-of-life change after kidney transplantation. Transplant. Proc. 2001, 33, 1887–1889. [Google Scholar] [CrossRef]
- Neale, J. Cardiovascular risk factors following renal transplant. World J. Transplant. 2015, 5, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S. New-onset diabetes after transplantation—Should it be a factor in choosing an immunosuppressant regimen for kidney transplant recipients. Nephrol. Dial. Transplant. 2008, 23, 1816–1818. [Google Scholar] [CrossRef] [PubMed]
- Irons, B.K.; Mazzolini, T.A.; Greene, R.S. Delaying the Onset of Type 2 Diabetes Mellitus in Patients with Prediabetes. Pharmacotherapy 2004, 24, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Patrick, J. Skerrett. Physical activity and all-cause mortality: What is the dose-response relation? Med. Sci. Sports Exerc. 2001, 33, S459–S474. [Google Scholar] [CrossRef] [PubMed]
- Calella, P.; Hernández-Sánchez, S.; Garofalo, C.; Ruiz, J.R.; Carrero, J.J.; Bellizzi, V. Exercise training in kidney transplant recipients: A systematic review. J. Nephrol. 2019, 32, 567–579. [Google Scholar] [CrossRef]
- Zelle, D.M.; Klaassen, G.; Van Adrichem, E.; Bakker, S.J.L.; Corpeleijn, E.; Navis, G. Physical inactivity: A risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 2017, 13, 152. [Google Scholar] [CrossRef]
- Macdonald, J.H.; Kirkman, D.; Jibani, M. Kidney Transplantation: A Systematic Review of Interventional and Observational Studies of Physical Activity on Intermediate Outcomes. Adv. Chronic Kidney Dis. 2009, 16, 482–500. [Google Scholar] [CrossRef]
- Bellizzi, V.; Cupisti, A.; Capitanini, A.; Calella, P.; D’Alessandro, C. Physical activity and renal transplantation. Kidney Blood Press. Res. 2014, 39, 212–219. [Google Scholar] [CrossRef]
- Takahashi, A.; Hu, S.L.; Bostom, A. Physical Activity in Kidney Transplant Recipients: A Review. Am. J. Kidney Dis. 2018, 72, 433–443. [Google Scholar] [CrossRef]
- Kang, A.W.; Garber, C.E.; Eaton, C.B.; Risica, P.M. Physical Activity and Cardiovascular Risk among Kidney Transplant Patients. Med. Sci. Sport. Exerc. 2019, 51, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Kok, T.; Dontje, M.L.; Danchell, E.I.; Navis, G.; Van Son, W.J.; Bakker, S.J.L.; Corpeleijn, E. The role of diet and physical activity in post-transplant weight gain after renal transplantation. Clin. Transplant. 2013, 27, E484–E490. [Google Scholar] [CrossRef] [PubMed]
- Orazio, L.; Hickman, I.; Armstrong, K.; Johnson, D.; Banks, M.; Isbel, N. Higher Levels of Physical Activity Are Associated With a Lower Risk of Abnormal Glucose Tolerance in Renal Transplant Recipients. J. Ren. Nutr. 2009, 19, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Zelle, D.M.; Corpeleijn, E.; Stolk, R.P.; de Greef, M.H.G.; Gans, R.O.B.; van der Heide, J.J.H.; Navis, G.; Bakker, S.J.L. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 898–905. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Janaudis-Ferreira, T.; Mathur, S.; Deliva, R.; Howes, N.; Patterson, C.; Räkel, A.; So, S.; Wickerson, L.; White, M.; Avitzur, Y.; et al. Exercise for Solid Organ Transplant Candidates and Recipients: A Joint Position Statement of the Canadian Society of Transplantation and CAN-RESTORE. Transplantation 2019, 103, e220–e238. [Google Scholar] [CrossRef]
- Dontje, M.L.; de Greef, M.H.G.; Krijnen, W.P.; Corpeleijn, E.; Kok, T.; Bakker, S.J.L.; Stolk, R.P.; van der Schans, C.P. Longitudinal measurement of physical activity following kidney transplantation. Clin. Transplant. 2014, 28, 394–402. [Google Scholar] [CrossRef]
- Nielens, H.; Lejeune, T.M.; Lalaoui, A.; Squifflet, J.P.; Pirson, Y.; Goffin, E. Increase of physical activity level after successful renal transplantation: A 5 year follow-up study. Nephrol. Dial. Transplant. 2001, 16, 134–140. [Google Scholar] [CrossRef]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.P.; Sehgal, A.R.; Strogatz, D.; Conti, D.; Siminoff, L.A. Prevalence and determinants of physical activity and fluid intake in kidney transplant recipients. Clin. Transplant. 2010, 24, E69–E81. [Google Scholar] [CrossRef]
- Lund, T.; Labriola, M.; Christensen, K.B.; Bültmann, U.; Villadsen, E. Physical work environment risk factors for long term sickness absence: Prospective findings among a cohort of 5357 employees in Denmark. Br. Med. J. 2006, 332, 449–451. [Google Scholar] [CrossRef]
- Huai, P.; Xun, H.; Reilly, K.H.; Wang, Y.; Ma, W.; Xi, B. Physical activity and risk of hypertension a meta-analysis of prospective cohort studies. Hypertension 2013, 62, 1021–1026. [Google Scholar] [CrossRef]
- Larsson, C.A.; Krøll, L.; Bennet, L.; Gullberg, B.; Råstam, L.; Lindblad, U. Leisure time and occupational physical activity in relation to obesity and insulin resistance: A population-based study from the Skaraborg Project in Sweden. Metabolism. 2012, 61, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.; Engberink, M.F.; Brink, E.J.; Van den Berg, E.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Joosten, M.M.; Gans, R.O.B.; Navis, G.; et al. Dietary acid load and metabolic acidosis in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, E.; Engberink, M.F.; Brink, E.J.; Van Baak, M.A.; Gans, R.O.B.; Navis, G.; Bakker, S.J.L. Dietary protein, blood pressure and renal function in renal transplant recipients. Br. J. Nutr. 2013, 109, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.; Johanna, M.; Geleijnse, E.; Brink, J.; van Baak, M.A.; van der Heide, J.J.H.; Gans, R.O.B.; Navis, G.; Bakker, S.J.L. Sodium intake and blood pressure in renal transplant recipients. Nephrol. Dial. Transplant. 2012, 27, 3352–3359. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Kramer, H.J.; Griffin, K.A.; Vellanki, K.; Leehey, D.J.; Bansal, V.K.; Markossian, T.W.; Levey, A.; Stevens, L.; Schmid, C.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Oterdoom, L.H.; Gansevoort, R.T.; Schouten, J.P.; de Jong, P.E.; Gans, R.O.B.; Bakker, S.J.L. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis 2009, 207, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wendel-Vos, G.C.W.; Schuit, A.J.; Saris, W.H.M.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Byambasukh, O.; Snieder, H.; Corpeleijn, E. The relation between leisure time, commuting and occupational physical activity with blood pressure in 125,402 adults: The Lifelines cohort. J. Am. Heart Assoc. 2019, 8, e0814313. [Google Scholar]
- Davidson, J.; Wilkinson, A.; Dantal, J.; Dotta, F.; Haller, H.; Hernandez, D.; Kasiske, B.L.; Kiberd, B.; Krentz, A.; Legendre, C.; et al. New-onser diabetes after transplantation: 2003 International Consensus Guidelines. Transplantation 2003, 75, SS3–SS24. [Google Scholar] [CrossRef]
- Sharif, A.; Hecking, M.; De Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Osté, M.C.J.; Corpeleijn, E.; Navis, G.J.; Keyzer, C.A.; Soedamah-Muthu, S.S.; Van Den Berg, E.; Postmus, D.; De Borst, M.H.; Kromhout, D.; Bakker, S.J.L. Mediterranean style diet is associated with low risk of new-onset diabetes after renal transplantation. BMJ Open Diabetes Res. Care 2017, 5, e000283. [Google Scholar] [CrossRef] [PubMed]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Spaderna, H.; Zahn, D.; Pretsch, J.; Connor, S.L.; Zittermann, A.; Schulze Schleithoff, S.; Bramstedt, K.A.; Smits, J.M.A.; Weidner, G. Dietary habits are related to outcomes in patients with advanced heart failure awaiting heart transplantation. J. Card. Fail. 2013, 19, 240–250. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Shah, S. Prevention of Cardiovascular Disease: Guideline for Assessment and Management of Cardiovascular Risk; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Weir, M.R.; Fink, J.C. Risk for posttransplant diabetes mellitus with current immunosuppressive medications. Am. J. Kidney Dis. 1999, 34, 1–13. [Google Scholar] [CrossRef]
- Sharif, A.; Moore, R.; Baboolal, K. Influence of lifestyle modification in renal transplant recipients with postprandial hyperglycemia. Transplantation 2008, 85, 353–358. [Google Scholar] [CrossRef]
- Sullivan, P.W.; Morrato, E.H.; Ghushchyan, V.; Wyatt, H.R.; Hill, J.O. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care 2005, 28, 1599. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Katz, R.; Mozaffarian, D.; Dalrymple, L.S.; De Boer, I.; Sarnak, M.; Shlipak, M.; Siscovick, D.; Kestenbaum, B. Physical activity and rapid decline in kidney function among older adults. Arch. Intern. Med. 2009, 169, 2116–2123. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Littman, A.J.; Duncan, G.E.; Weiss, N.S.; Sachs, M.C.; Ruzinski, J.; Kundzins, J.; Rock, D.; de Boer, I.H.; Ikizler, T.A.; et al. Physical Activity and Change in Estimated GFR among Persons with CKD. J. Am. Soc. Nephrol. 2014, 25, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.P.; Sehgal, A.R.; Strogatz, D.; Yucel, R.; Conti, D.; Siminoff, L.A. Longitudinal analysis of physical activity, fluid intake, and graft function among kidney transplant recipients. Transpl. Int. 2009, 22, 990–998. [Google Scholar] [CrossRef]
- Koivisto, V.A.; Yki-Järvinen, H.; DeFronzo, R.A. Physical training and insulin sensitivity. Diabetes. Metab. Rev. 1986, 1, 445–481. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.D.; Stefanick, M.L.; Williams, P.T.; Haskell, W.L. The Effects on Plasma Lipoproteins of a Prudent Weight-Reducing Diet, With or Without Exercise, in Overweight Men and Women. N. Engl. J. Med. 1991, 325, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Osté, M.C.J.; Gomes-Neto, A.W.; Corpeleijn, E.; Gans, R.O.B.; de Borst, M.H.; van den Berg, E.; Soedamah-Muthu, S.S.; Kromhout, D.; Navis, G.J.; Bakker, S.J.L. Dietary Approach to Stop Hypertension (DASH) diet and risk of renal function decline and all-cause mortality in renal transplant recipients. Am. J. Transplant. 2018, 10, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Byambasukh, O.; Zelle, D.; Corpeleijn, E. Physical Activity, Fatty Liver, and Glucose Metabolism Over the Life Course. Am. J. Gastroenterol. 2019, 114, 907–915. [Google Scholar] [CrossRef]
- Crandall, J.; Schade, D.; Ma, Y.; Fujimoto, W.Y.; Barrett-Connor, E.; Fowler, S.; Dagogo-Jack, S.; Andres, R. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1075–1081. [Google Scholar]
- Wagenmakers, R.; Van Den Akker-Scheek, I.; Groothoff, J.W.; Zijlstra, W.; Bulstra, S.K.; Kootstra, J.W.J.; Wendel-Vos, G.C.W.; Van Raaij, J.J.A.M.; Stevens, M. Reliability and validity of the short questionnaire to assess health-enhancing physical activity (SQUASH) in patients after total hip arthroplasty. BMC Musculoskelet. Disord. 2008, 9, 141. [Google Scholar] [CrossRef]
- Arends, S.; Hofman, M.; Kamsma, Y.P.T.; van der Veer, E.; Houtman, P.M.; Kallenberg, C.G.M.; Spoorenberg, A.; Brouwer, E. Daily physical activity in ankylosing spondylitis: Validity and reliability of the IPAQ and SQUASH and the relation with clinical assessments. Arthritis Res. Ther. 2013, 15, R99. [Google Scholar] [CrossRef]


| Variable | Total (n = 650) | No-MVPA (n = 246) | MVPA-1 (n = 201) | MVPA-2 (n = 203) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 52.6 ± 12.8 | 54.1 ± 11.8 | 51.9 ± 13.4 | 51.6 ± 13.3 | 0.08 |
| Male gender (%, n) | 56.3 (366) | 56.1 (138) | 55.7 (112) | 57.1 (116) | 0.96 |
| Current smoking (%, n) | 12.3 (80) | 15.3 (36) | 12.4 (25) | 9.5 (19) | 0.20 |
| Occupational status: Employed * (%, n) | 49.5 (322) | 44.7 (110) | 51.2 (103) | 53.7 (109) | 0.14 |
| Alcohol use (g/day) | 2.61 (0.1–11.1) | 1.45 (0.1–9.7) | 2.51 (0.1–9.7) | 3.95 (0.1–14.1) | 0.01 |
| Total energy intake (kcal/d) | 2174.9 ± 640.7 | 2114.7± 720.4 | 2247.5 ± 598.7 | 2173.2 ± 573.9 | 0.11 |
| Non-occupational MVPA (min/week) | 90 (0–240) | 0 | 120 (60–150) | 360 (260–540) | − |
| Anthropometric measures | |||||
| Body mass index (kg/m2) | 26.7 ± 4.84 | 27.9 ± 5.49 | 25.7 ± 4.26 | 26.1 ± 4.23 | 0.001 |
| Waist circumference, men (cm) | 101.1 ± 13.4 | 104.0 ± 13.7 | 98.9 ± 13.5 | 99.7 ± 12.4 | 0.01 |
| Waist circumference, women (cm) | 95.0 ± 15.8 | 99.7 ± 12.4 | 91.5 ± 14.6 | 93.1 ± 14.1 | 0.01 |
| Creatinine excretion (mmol/24h) | 11.7 ± 3.49 | 11.2 ± 3.80 | 11.8 ± 3.29 | 12.2 ± 3.23 | 0.01 |
| Lipids and blood pressure | |||||
| Total cholesterol (mmol/L) | 5.14 ± 1.11 | 5.18 ± 1.18 | 5.17 ± 1.10 | 5.07 ± 1.02 | 0.48 |
| Triglyceride (mmol/L) | 1.68 (1.2–2.33) | 1.85 (1.3–2.6) | 1.67 (1.2–2.4) | 1.59 (1.2–2.05) | 0.001 |
| HDL-C in men (mmol/L) | 1.27 ± 0.41 | 1.23 ± 0.41 | 1.27 ± 0.36 | 1.32 ± 0.45 | 0.21 |
| HDL-C in women (mmol/L) | 1.56 ± 0.51 | 1.39 ± 0.43 | 1.62 ± 0.54 | 1.70 ± 0.53 | 0.001 |
| Systolic blood pressure (mm Hg) | 136.2 ± 17.3 | 138.3 ± 18.5 | 135.6 ± 16.7 | 134.2 ± 16.3 | 0.04 |
| Diastolic blood pressure (mm Hg) | 82.8 ± 10.9 | 83.1 ± 11.2 | 82.5 ± 11.3 | 82.6 ± 10.3 | 0.77 |
| Cardiovascular medication use | |||||
| Antihypertensive (%, n) | 88 (572) | 92.7 (228) | 81.1 (163) | 89.2 (181) | 0.001 |
| A2 antagonist (%, n) | 14.8 (96) | 15.4 (38) | 12.9 (26) | 15.8 (32) | 0.68 |
| ACE inhibitor (%, n) | 32.2 (209) | 32.1 (79) | 30.3 (61) | 34.0 (69) | 0.74 |
| RAAS blockers (%, n) | 47.8 (311) | 49.2 (121) | 44.8 (90) | 49.3 (100) | 0.58 |
| Beta-blockers (%, n) | 63.2 (411) | 63.4 (156) | 62.2 (125) | 64.0 (130) | 0.93 |
| Calcium channel blockers (%, n) | 24.5 (159) | 26.0 (64) | 20.4 (41) | 26.6 (54) | 0.27 |
| Diuretics (%, n) | 40.0 (260) | 52.0 (128) | 27.4 (50) | 37.9 (77) | 0.001 |
| Vitamin K antagonist (%, n) | 11.4 (74) | 13 (32) | 10.4 (21) | 10.3 (21) | 0.60 |
| mTOR inhibitor (%, n) | 1.8 (12) | 3.3 (8) | 1 (2) | 1 (2) | 0.60 |
| Anti-diabetic drugs (%, n) | 14.8 (96) | 18.7 (46) | 14.4 (29) | 10.3 (21) | 0.045 |
| Statin (%, n) | 51.8 (337) | 54.9 (135) | 52.7 (106) | 47.3 (96) | 0.27 |
| Glucose metabolism | |||||
| Fasting plasma glucose (mmol/L) | 5.67 ± 1.82 | 5.78 ± 192 | 5.76 ± 2.13 | 5.46 ± 1.28 | 0.13 |
| Heamoglobin A1C (%) | 5.94 ± 0.78 | 6.03 ± 0.77 | 5.94 ± 0.90 | 5.83 ± 0.65 | 0.021 |
| Kidney function | |||||
| eGFR (mL/min/1.73m2) | 52.0 ± 20.2 | 49.9 ± 22.1 | 53.8 ± 18.7 | 52.9 ± 18.8 | 0.09 |
| Albumin excretion (mg/24h) | 267.3 ± 734.6 | 307.2 ± 777.5 | 175.1 ± 378.5 | 308.7 ± 917.5 | 0.11 |
| Proteinuria (%, n) | 21.5 (140) | 28.0 (69) | 16.9 (34) | 18.2 (37) | 0.01 |
| Primary renal disease (%, n) | 0.01 | ||||
| Glomerulosclerosis | 28.8 (187) | 30.1 (74) | 28.4 (57) | 27.6 (56) | |
| Glomerulonephritis | 7.7 (50) | 5.7 (14) | 8.0 (16) | 9.9 (20) | |
| Tubulointerstitial nephritis | 11.8 (77) | 9.8 (24) | 12.9 (26) | 13.3 (27) | |
| Polycystic kidney disease | 20.9 (136) | 20.7 (51) | 19.9 (40) | 22.2 (45) | |
| Renal hypodysplasia | 3.5 (23) | 4.1 (10) | 3.0 (6) | 3.4 (7) | |
| Renavascular diseases | 5.7 (37) | 7.7 (19) | 4.0 (8) | 4.9 (10) | |
| Diabetes mellitus | 4.6 (30) | 6.5 (16) | 5.5 (11) | 1.5 (3) | |
| Others | 16.9 (110) | 15.4 (38) | 18.4 (37) | 17.2 (35) | |
| Duration of dialysis before the transplantation (months) | 25 (8–48) | 29 (11–51) | 19 (4–49) | 25 (9–43) | 0.51 |
| Transplant characteristics | |||||
| Transplant vintage (months) | 14.0 (2.0–39.5) | 17.0 (2.0–41.0) | 12.0 (2.0–44.8) | 16.0 (0.5–41.0) | 0.49 |
| Cold ischemia time (h) | 15.2 (2.8–21.1) | 16.4 (3.6–22.0) | 15.1 (2.6–21.3) | 13.6 (2.5–20.5) | 0.10 |
| Living donor (%, n) | 34.8 (226) | 26.4 (65) | 37.3 (75) | 42.4 (86) | 0.001 |
| Pre-emptive transplant (%, n) | 16.6 (108) | 13.4 (33) | 20.9 (42) | 16.3 (33) | 0.11 |
| Acute rejection | 27.2 (177) | 27.2 (67) | 27.9 (56) | 26.6 (54) | 0.96 |
| Immunosuppressive medication | |||||
| Calcineurin inhibitor (%, n) | 58.3 (379) | 59.8 (147) | 59.2 (119) | 55.7 (113) | 0.52 |
| Proliferation inhibitor (%, n) | 82.6 (537) | 80.1 (197) | 84.1 (169) | 84.2 (171) | 0.62 |
| Prednisolone dose (mg) | 10.0 (7.5–10.0) | 10.0 (7.5–10.0) | 10.0 (7.5–10.0) | 10.0 (7.5–10.0) | 0.48 |
| Physical Activity | MVPA (cont.) | No-MVPA (Ref) | MVPA-1 | MVPA-2 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| Post-transplant DM | |||||||
| No. of events | 50/502 | 23 | 14 | 13 | |||
| Model 1 | 0.88 (0.79–0.97) | 0.01 | 1.00 | 0.57 (0.29–1.10) | 0.09 | 0.49 (0.25–0.96) | 0.04 |
| Model 2 | 0.88 (0.79–0.98) | 0.02 | 1.00 | 0.61 (0.31–1.20) | 0.15 | 0.49 (0.25–0.96) | 0.04 |
| Model 3 | 0.88 (0.79–0.98) | 0.02 | 1.00 | 0.55 (0.28–1.07) | 0.08 | 0.48 (0.24–0.95) | 0.04 |
| Model 4 | 0.87 (0.79–0.97) | 0.01 | 1.00 | 0.57 (0.29–1.12) | 0.10 | 0.46 (0.22–0.94) | 0.03 |
| Model 5 | 0.89 (0.80–0.99) | 0.03 | 1.00 | 0.59 (0.30–1.26) | 0.11 | 0.52 (0.26–1.03) | 0.06 |
| Model 6 | 0.91 (0.82–1.01) | 0.09 | 1.00 | 0.70 (0.36–1.40) | 0.31 | 0.60 (0.29–1.22) | 0.16 |
| Model 7 | 0.88 (0.79–0.99) | 0.03 | 1.00 | 0.63 (0.31–1.25) | 0.19 | 0.50 (0.25–1.03) | 0.06 |
| Model 8 | 0.87 (0.79–0.97) | 0.01 | 1.00 | 0.55 (0.29–1.08) | 0.08 | 0.47 (0.24–0.93) | 0.03 |
| Model 9 | 0.91 (0.82–1.01) | 0.12 | 1.00 | 0.72 (0.36–1.41) | 0.34 | 0.59 (0.30–1.19) | 0.14 |
| Model 10 | 0.87 (0.78–0.97) | 0.01 | 1.00 | 0.58 (0.32–1.25) | 0.11 | 0.44 (0.21–0.92) | 0.03 |
| Cardiovascular mortality | |||||||
| No. of events | 53/589 | 26 | 14 | 13 | |||
| Model 1 | 0.84 (0.74–0.94) | 0.01 | 1.00 | 0.45 (0.22–0.94) | 0.03 | 0.34 (0.15–0.77) | 0.01 |
| Model 2 | 0.84 (0.75–0.95) | 0.01 | 1.00 | 0.49 (0.23–1.02) | 0.06 | 0.35 (0.16–0.80) | 0.01 |
| Model 3 | 0.86 (0.76–0.96) | 0.01 | 1.00 | 0.51 (0.25–1.05) | 0.07 | 0.40 (0.18–0.91) | 0.03 |
| Model 4 | 0.87 (0.77–0.98) | 0.02 | 1.00 | 0.56 (0.26–1.21) | 0.14 | 0.43 (0.19–0.94) | 0.046 |
| Model 5 | 0.84 (0.74–0.94) | 0.001 | 1.00 | 0.45 (0.21–0.93) | 0.03 | 0.36 (0.16–0.81) | 0.01 |
| Model 6 | 0.85 (0.76–0.96) | 0.001 | 1.00 | 0.49 (0.23–1.02) | 0.06 | 0.38 (0.17–0.86) | 0.02 |
| Model 7 | 0.85 (0.75–0.96) | 0.01 | 1.00 | 0.51 (0.23–1.11) | 0.09 | 0.40 (0.17–0.92) | 0.03 |
| Model 8 | 0.87 (0.77–0.98) | 0.02 | 1.00 | 0.55 (0.26–1.16) | 0.12 | 0.44 (0.19–0.99) | 0.051 |
| All-cause mortality | |||||||
| No. of events | 129/650 | 76 | 27 | 26 | |||
| Model 1 | 0.84 (0.78–0.89) | <0.001 | 1.00 | 0.39 (0.25–0.61) | <0.001 | 0.37 (0.24–0.58) | <0.001 |
| Model 2 | 0.85 (0.79–0.91) | <0.001 | 1.00 | 0.43 (0.27–0.67) | <0.001 | 0.40 (0.26–0.63) | <0.001 |
| Model 3 | 0.85 (0.79–0.91) | <0.001 | 1.00 | 0.41 (0.27–0.64) | <0.001 | 0.41 (0.26–0.64) | <0.001 |
| Model 4 | 0.83 (0.77–0.89) | <0.001 | 1.00 | 0.41 (0.21–0.64) | <0.001 | 0.35 (0.22–0.58) | <0.001 |
| Model 5 | 0.83 (0.78–0.89) | <0.001 | 1.00 | 0.39 (0.25–0.61) | <0.001 | 0.37 (0.23–0.58) | <0.001 |
| Model 6 | 0.85 (0.79–0.91) | <0.001 | 1.00 | 0.42 (0.27–0.66) | <0.001 | 0.41 (0.26–0.65) | <0.001 |
| Model 7 | 0.84 (0.78–0.89) | <0.001 | 1.00 | 0.40 (0.25–0.63) | <0.001 | 0.37 (0.23–0.59) | <0.001 |
| Model 8 | 0.86 (0.80–0.92) | <0.001 | 1.00 | 0.45 (0.29–0.70) | <0.001 | 0.44 (0.28–0.69) | <0.001 |
| Physical Activity | MVPA (cont.) | No-MVPA | MVPA > 0 | ||||
|---|---|---|---|---|---|---|---|
| HR^ (95% CI) | p-value | N * | Reference | N * | HR^^ (95% CI) | p-Value | |
| Post-transplant DM | |||||||
| Non-occupational PA | 0.87 (0.74–1.03) | 0.113 | 10 | 1.00 | 10 | 0.46 (0.18–1.13) | 0.076 |
| Total PA | 0.91 (0.78–1.06) | 0.212 | 8 | 1.00 | 12 | 0.48 (0.20–1.20) | 0.056 |
| Cardiovascular mortality | |||||||
| Non-occupational PA | 0.63 (0.48–0.83) | 0.001 | 12 | 1.00 | 3 | 0.11 (0.11–0.42) | 0.001 |
| Total PA | 0.75 (0.63–0.91) | 0.003 | 9 | 1.00 | 6 | 0.23 (0.10–0.58) | 0.051 |
| All-cause mortality | |||||||
| Non-occupational PA | 0.76 (0.66–0.87) | <0.01 | 25 | 1.00 | 11 | 0.21 (0.14–0.51) | <0.01 |
| Total PA | 0.82 (0.74–0.92) | 0.001 | 19 | 1.00 | 17 | 0.30 (0.18–0.58) | <0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byambasukh, O.; Osté, M.C.J.; Gomes-Neto, A.W.; van den Berg, E.; Navis, G.; Bakker, S.J.L.; Corpeleijn, E. Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 415. https://doi.org/10.3390/jcm9020415
Byambasukh O, Osté MCJ, Gomes-Neto AW, van den Berg E, Navis G, Bakker SJL, Corpeleijn E. Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients. Journal of Clinical Medicine. 2020; 9(2):415. https://doi.org/10.3390/jcm9020415
Chicago/Turabian StyleByambasukh, Oyuntugs, Maryse C. J. Osté, António W. Gomes-Neto, Else van den Berg, Gerjan Navis, Stephan J. L. Bakker, and Eva Corpeleijn. 2020. "Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients" Journal of Clinical Medicine 9, no. 2: 415. https://doi.org/10.3390/jcm9020415
APA StyleByambasukh, O., Osté, M. C. J., Gomes-Neto, A. W., van den Berg, E., Navis, G., Bakker, S. J. L., & Corpeleijn, E. (2020). Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients. Journal of Clinical Medicine, 9(2), 415. https://doi.org/10.3390/jcm9020415





