The Bed Nucleus of the Stria Terminalis as a Brain Correlate of Psychological Inflexibility in Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Procedure
2.3. Measures
2.3.1. Socio-Demographic Characteristics and Clinical History
2.3.2. MRI Assessments
2.3.3. Main Outcome Measure
2.3.4. Clinical Measures
2.3.5. Third-Wave Cognitive Measures
2.4. Data Analyses
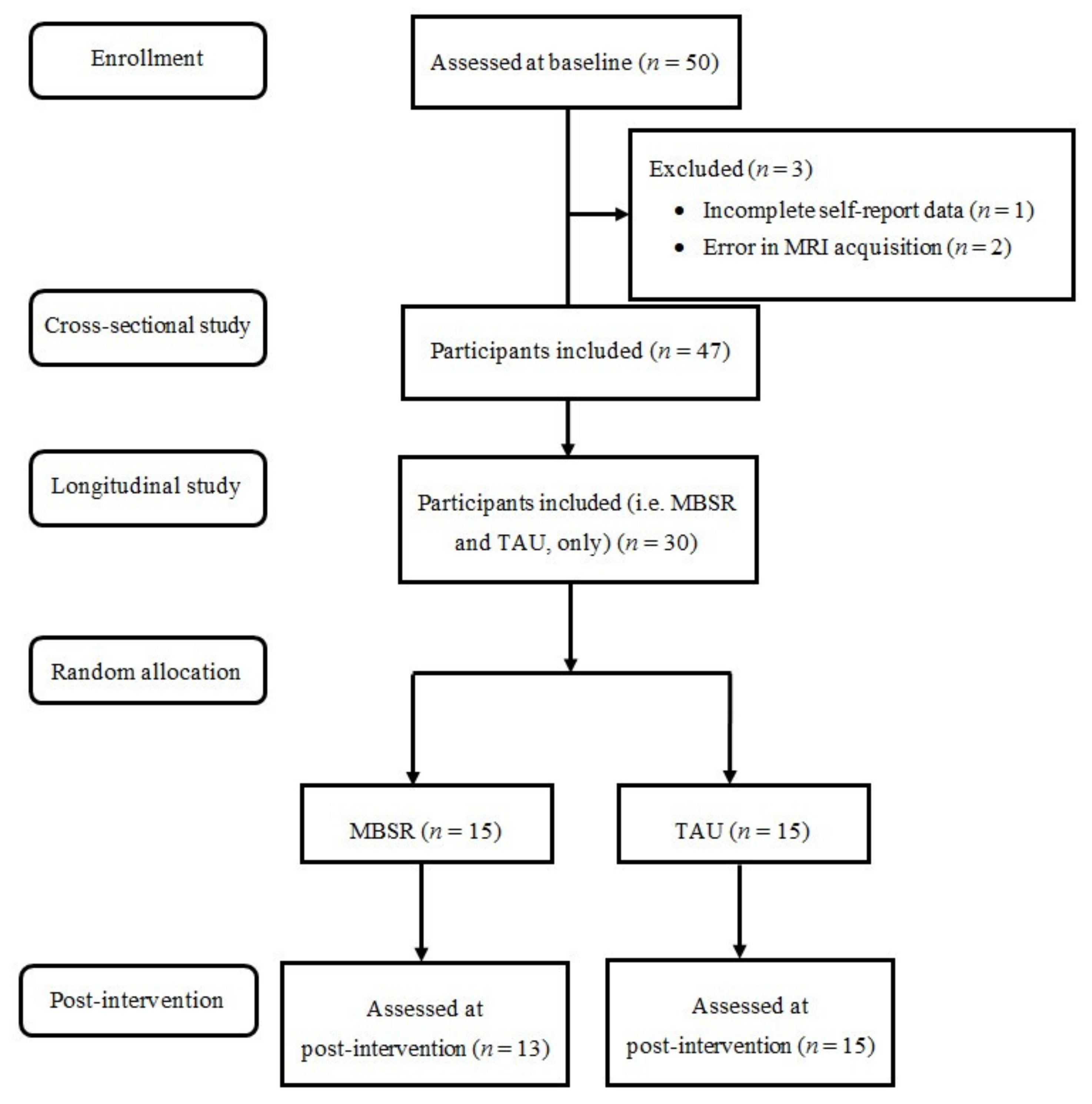
3. Results
3.1. Sample Descriptive Statistics
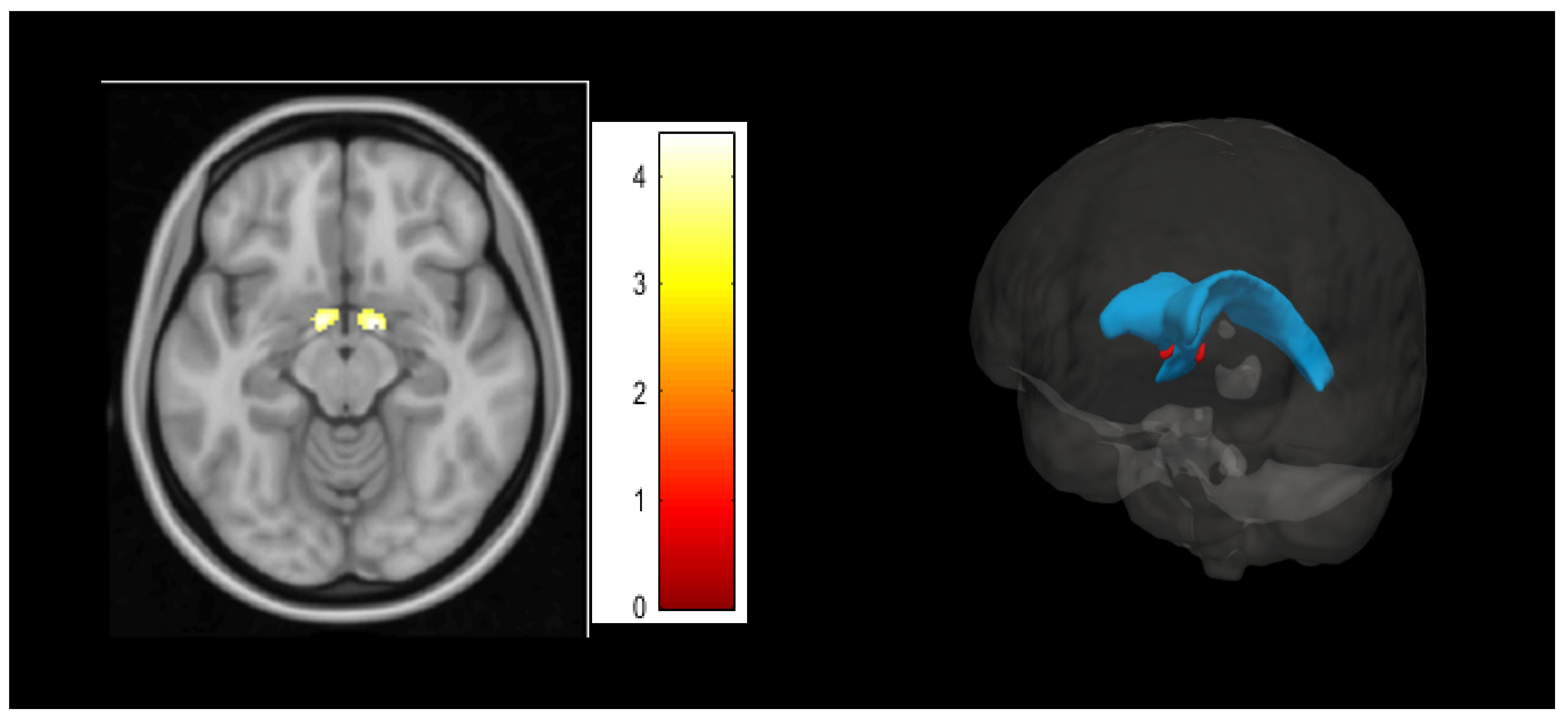
3.2. Psychological Inflexibility Clusters in Voxel-Based Morphometry Analyses
3.3. Association between BNST Cluster with Clinical and Third-Wave Cognitive Measures
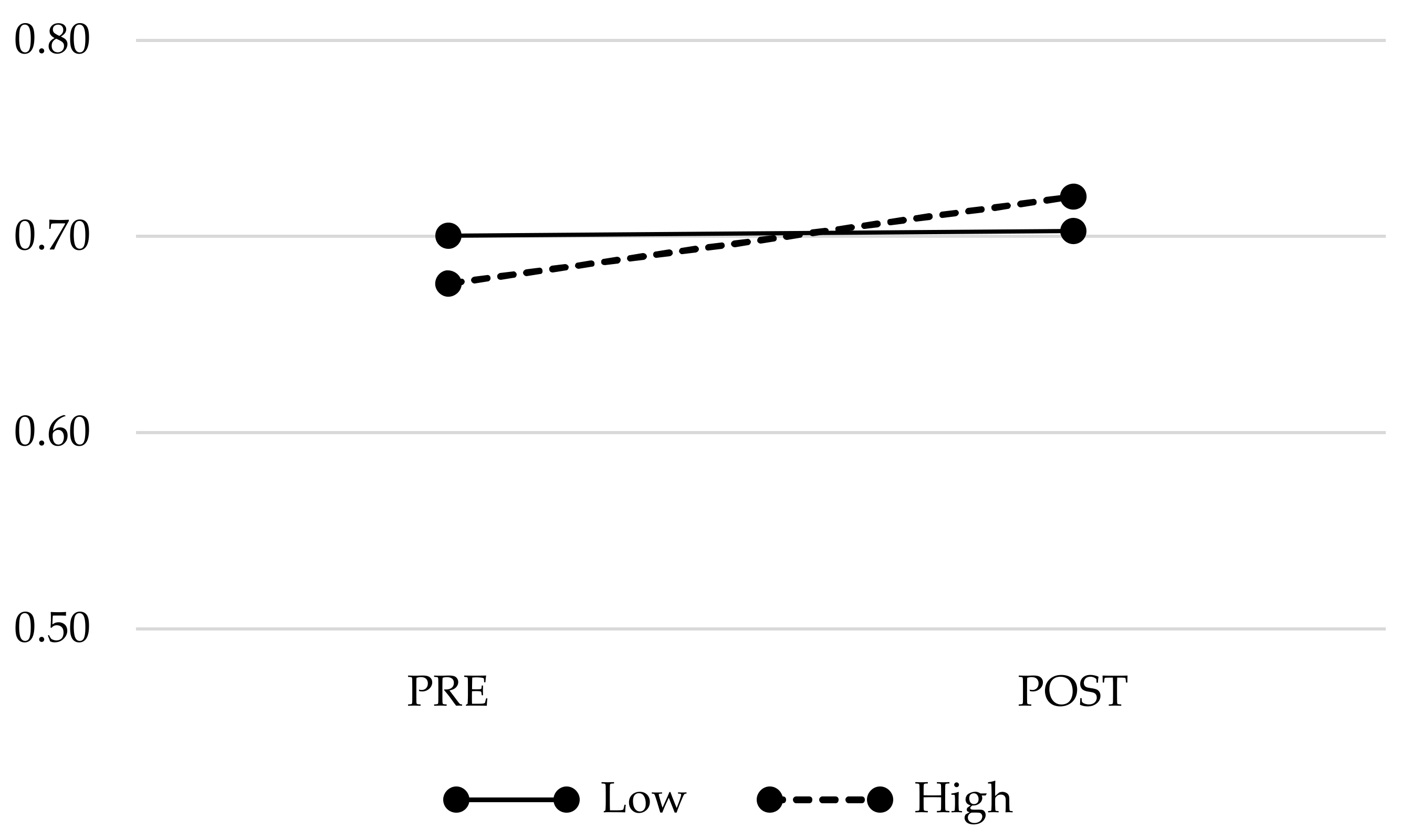
3.4. Changes in PIPS-Related Brain Volumes Across Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiesa, A.; Serretti, A.; Jakobsen, J.C. Mindfulness: Top-down or bottom-up emotion regulation strategy? Clin. Psychol. Rev. 2013, 33, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, B.K.; Lazar, S.W.; Gard, T.; Schuman-Olivier, Z.; Vago, D.R.; Ott, U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci. 2011, 6, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Marchand, W.R. Neural mechanisms of mindfulness and meditation: Evidence from neuroimaging studies. World J. Radiol. 2014, 6, 471. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.W.; Goodman, R.J.; Inzlicht, M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Soc. Cogn. Affect. Neurosci. 2013, 8, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.D.; Way, B.M.; Eisenberger, N.I.; Lieberman, M.D. Neural correlates of dispositional mindfulness during affect labeling. Psychosom. Med. 2007, 69, 560–565. [Google Scholar] [CrossRef]
- Dickenson, J.; Berkman, E.T.; Arch, J.; Lieberman, M.D. Neural correlates of focused attention during a brief mindfulness induction. Soc. Cogn. Affect. Neurosci. 2013, 8, 40–47. [Google Scholar] [CrossRef]
- Lu, H.; Song, Y.; Xu, M.; Wang, X.; Li, X.; Liu, J. The brain structure correlates of individual differences in trait mindfulness: A voxel-based morphometry study. Neuroscience 2014, 272, 21–28. [Google Scholar] [CrossRef]
- Murakami, H.; Nakao, T.; Matsunaga, M.; Kasuya, Y.; Shinoda, J.; Yamada, J.; Ohira, H. The structure of mindful brain. PLoS One 2012, 7, e46377. [Google Scholar] [CrossRef]
- Taren, A.A.; Creswell, J.D.; Gianaros, P.J. Dispositional mindfulness co-varies with smaller amygdala and caudate volumes in community adults. PLoS One 2013, 8, e64574. [Google Scholar] [CrossRef]
- Zhuang, K.; Bi, M.; Li, Y.; Xia, Y.; Guo, X.; Chen, Q.; Du, X.; Wang, K.; Wei, D.; Yin, H.; et al. A distinction between two instruments measuring dispositional mindfulness and the correlations between those measurements and the neuroanatomical structure. Sci. Rep. 2017, 7, 6252. [Google Scholar] [CrossRef] [PubMed]
- Gotink, R.A.; Meijboom, R.; Vernooij, M.W.; Smits, M.; Hunink, M.G.M. 8-week Mindfulness Based Stress Reduction induces brain changes similar to traditional long-term meditation practice—A systematic review. Brain Cogn. 2016, 108, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, B.K.; Carmody, J.; Vangel, M.; Congleton, C.; Yerramsetti, S.M.; Gard, T.; Lazar, S.W. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011, 191, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Young, K.S.; van der Velden, A.M.; Craske, M.G.; Pallesen, K.J.; Fjorback, L.; Roepstorff, A.; Parsons, C.E. The impact of mindfulness-based interventions on brain activity: A systematic review of functional magnetic resonance imaging studies. Neurosci. Biobehav. Rev. 2018, 84, 424–433. [Google Scholar] [CrossRef]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G.; Landau, L. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change; Guilford Press: New York, NY, USA, 1999. [Google Scholar]
- McCracken, L.M.; Morley, S. The psychological flexibility model: A basis for integration and progress in psychological approaches to chronic pain management. J. Pain 2014, 15, 221–234. [Google Scholar] [CrossRef]
- Rodero, B.; Pereira, J.P.; Pérez-Yus, M.C.; Casanueva, B.; Serrano-Blanco, A.; Rodrigues da Cunha Ribeiro, M.J.; Luciano, J.V.; Garcia-Campayo, J. Validation of a Spanish version of the psychological inflexibility in pain scale (PIPS) and an evaluation of its relation with acceptance of pain and mindfulness in sample of persons with fibromyalgia. Health Qual. Life Outcomes 2013, 11, 62. [Google Scholar] [CrossRef]
- Trompetter, H.R.; Bohlmeijer, E.T.; Van Baalen, B.; Kleen, M.; Köke, A.; Reneman, M.; Schreurs, K.M.G. The Psychological Inflexibility in Pain Scale (PIPS) exploration of psychometric properties in a heterogeneous chronic pain sample. Eur. J. Psychol. Assess. 2014, 30, 289–295. [Google Scholar] [CrossRef]
- Kashdan, T.B.; Rottenberg, J. Psychological flexibility as a fundamental aspect of health. Clin. Psychol. Rev. 2010, 30, 865–878. [Google Scholar] [CrossRef]
- Luciano, J.V.; Guallar, J.A.; Aguado, J.; López-Del-Hoyo, Y.; Olivan, B.; Magallón, R.; Alda, M.; Serrano-Blanco, A.; Gili, M.; Garcia-Campayo, J. Effectiveness of group acceptance and commitment therapy for fibromyalgia: A 6-month randomized controlled trial (EFFIGACT study). Pain 2014, 155, 693–702. [Google Scholar] [CrossRef]
- McCracken, L.M.; Velleman, S.C. Psychological flexibility in adults with chronic pain: A study of acceptance, mindfulness, and values-based action in primary care. Pain 2010, 148, 141–147. [Google Scholar] [CrossRef]
- Vowles, K.E.; McCracken, L.M.; Sowden, G.; Ashworth, J. Psychological flexibility in coping with chronic pain: Further examination of the brief pain coping inventory-2. Clin. J. Pain 2014, 30, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Lekander, M.; Sorjonen, K.; Olsson, G.L. The Psychological Inflexibility in Pain Scale (PIPS) —Statistical properties and model fit of an instrument to assess change processes in pain related disability. Eur. J. Pain 2010, 14, 771.e1–771.e14. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Renöfält, J.; Olsson, G.L.; Bond, F.W.; Melin, L. Avoidance and cognitive fusion—Central components in pain related disability? Development and preliminary validation of the Psychological Inflexibility in Pain Scale (PIPS). Eur. J. Pain 2008, 12, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Feliu-Soler, A.; Montesinos, F.; Gutiérrez-Martínez, O.; Scott, W.; McCracken, L.M.; Luciano, J.V. Current status of acceptance and commitment therapy for chronic pain: A narrative review. J. Pain Res. 2018, 11, 2145–2159. [Google Scholar] [CrossRef]
- Montero-Marín, J.; Navarro-Gil, M.; Puebla-Guedea, M.; Luciano, J.V.; Van Gordon, W.; Shonin, E.; García-Campayo, J. Efficacy of “attachment-based compassion therapy” in the treatment of fibromyalgia: A randomized controlled trial. Front. Psychiatry 2018, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Aranda, A.; Feliu-Soler, A.; Montero-Marín, J.; García-Campayo, J.; Andrés-Rodríguez, L.; Borràs, X.; Rozadilla-Sacanell, A.; Peñarrubia-Maria, M.T.; Angarita-Osorio, N.; McCracken, L.M.; et al. A randomized controlled efficacy trial of mindfulness-based stress reduction compared with an active control group and usual care for fibromyalgia: The EUDAIMON study. Pain 2019, 160, 2508–2523. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Kemani, M.; Jensen, K.; Kosek, E.; Kadetoff, D.; Sorjonen, K.; Ingvar, M.; Olsson, G.L. Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. Eur. J. Pain 2013, 17, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Why Voxel-based morphometry should be used. Neuroimage 2001, 14, 1238–1243. [Google Scholar] [CrossRef]
- Feliu-Soler, A.; Borràs, X.; Peñarrubia-María, M.T.; Rozadilla-Sacanell, A.; D’Amico, F.; Moss-Morris, R.; Howard, M.A.; Fayed, N.; Soriano-Mas, C.; Puebla-Guedea, M.; et al. Cost-utility and biological underpinnings of Mindfulness-Based Stress Reduction (MBSR) versus a psychoeducational programme (FibroQoL) for fibromyalgia: A 12-month randomised controlled trial (EUDAIMON study). BMC Complement. Altern. Med. 2016, 16, 81. [Google Scholar] [CrossRef]
- Bennett, R.M.; Friend, R.; Jones, K.D.; Ward, R.; Han, B.K.; Ross, R.L. The revised fibromyalgia impact questionnaire (FIQR): Validation and psychometric properties. Arthritis Res. Ther. 2009, 11, R120. [Google Scholar] [CrossRef]
- Luciano, J.V.; Aguado, J.; Serrano-Blanco, A.; Calandre, E.P.; Rodriguez-Lopez, C.M. Dimensionality, reliability, and validity of the revised fibromyalgia impact questionnaire in two spanish samples. Arthritis Care Res. 2013, 65, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Williamson, G. Perceived stress in a probability sample of the United States. In The Social Psychology of Health: The Claremont Symposium on Applied Social Psychology; Spacapan, S., Oskamp, S., Eds.; SAGE Publications: Thousand Oaks, CA, USA, 1988. [Google Scholar]
- Remor, E. Psychometric properties of a European Spanish version of the Perceived Stress Scale (PSS). Span. J. Psychol. 2006, 9, 86–93. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validation of the Spanish version of the Pain Catastrophizing Scale in fibromyalgia. Med. Clin. 2008, 131, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Baer, R.A.; Smith, G.T.; Hopkins, J.; Krietemeyer, J.; Toney, L. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006, 13, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Luciano, J.V.; Cebolla, A.; Serrano-Blanco, A.; Soler, J.; García-Campayo, J. Bifactor analysis and construct validity of the five facet mindfulness questionnaire (FFMQ) in non-clinical Spanish samples. Front. Psychol. 2015, 6, 404. [Google Scholar] [CrossRef]
- Raes, F.; Pommier, E.; Neff, K.D.; Van Gucht, D. Construction and factorial validation of a short form of the Self-Compassion Scale. Clin. Psychol. Psychother. 2011, 18, 250–255. [Google Scholar] [CrossRef]
- Garcia-Campayo, J.; Navarro-Gil, M.; Andrés, E.; Montero-Marin, J.; López-Artal, L.; Demarzo, M.M.P. Validation of the Spanish versions of the long (26 items) and short (12 items) forms of the Self-Compassion Scale (SCS). Health Qual. Life Outcomes 2014, 12, 4. [Google Scholar] [CrossRef]
- Ashburner, J.; Barnes, G.; Chen, C.; Daunizeau, J.; Flandin, G.; Friston, K.; Penny, W. SPSM12 Manual; Wellcome Trust Centre for Neuroimaging: London, UK, 2014. [Google Scholar]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0; IBM Corp.: New York, NY, USA, 2016. [Google Scholar]
- Mai, J.K.; Paxinos, G.; Voss, T. Atlas of the Human Brain, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Theiss, J.D.; Ridgewell, C.; McHugo, M.; Heckers, S.; Blackford, J.U. Manual segmentation of the human bed nucleus of the stria terminalis using 3 T MRI. Neuroimage 2017, 146, 288–292. [Google Scholar] [CrossRef]
- Motzkin, J.C.; Philippi, C.L.; Oler, J.A.; Kalin, N.H.; Baskaya, M.K.; Koenigs, M. Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex 2015, 64, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Lebow, M.A.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.K.; Depue, B.E. New frontiers in anxiety research: The translational potential of the bed nucleus of the stria terminalis. Front. Psychiatry 2019, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Forray, M.I.; Gysling, K. Role of Noradrenergic Projections to the bed nucleus of the stria terminalis in the Regulation of the Hypothalamic-Pituitary-Adrenal Axis. Brain Res. Brain Res. Rev. 2004, 47, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Gungor, N.Z.; Paré, D. Functional heterogeneity in the bed nucleus of the stria terminalis. J. Neurosci. 2016, 36, 8038–8049. [Google Scholar] [CrossRef]
- Hammack, S.E.; Cooper, M.A.; Lezak, K.R. Overlapping neurobiology of learned helplessness and conditioned defeat: Implications for PTSD and mood disorders. Neuropharmacology 2012, 62, 565–575. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Neblett, R.; Kishino, N.; Ray, C.T. Fear-avoidance beliefs and chronic pain. J. Orthop. Sports Phys. Ther. 2016, 46, 38–43. [Google Scholar] [CrossRef]
- Meier, M.L.; Stämpfli, P.; Vrana, A.; Humphreys, B.K.; Seifritz, E.; Hotz-Boendermaker, S. Neural correlates of fear of movement in patients with chronic low back pain vs. Pain-free individuals. Front. Hum. Neurosci. 2016, 10, 386. [Google Scholar] [CrossRef]
- Elman, I.; Borsook, D. Threat response system: Parallel brain processes in pain vis-à-vis fear and anxiety. Front. Psychiatry 2018, 9, 29. [Google Scholar] [CrossRef]
- Gatchel, R.; Neblett, R. Pain catastrophizing: What clinicians need to know. Pract. Pain Manag. 2017, 15, 70–75. [Google Scholar]
- Quartana, P.J.; Campbell, C.M.; Edwards, R.R. Pain catastrophizing a critical review. Expert Rev. Neurother. 2009, 9, 745–758. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef]
- Malfliet, A.; Coppieters, I.; Van Wilgen, P.; Kregel, J.; De Pauw, R.; Dolphens, M.; Ickmans, K. Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review. Eur. J. Pain 2017, 21, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.; Mist, S.D.; Casselberry, M.A.; Ali, A.; Christopher, M.S. Fibromyalgia impact and mindfulness characteristics in 4986 people with fibromyalgia. Explor. J. Sci. Heal. 2015, 11, 304–309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bergomi, C.; Tschacher, W.; Kupper, Z. The assessment of mindfulness with self-report measures: Existing scales and open issues. Mindfulness 2013, 4, 191–202. [Google Scholar] [CrossRef]
- Kanai, R.; Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011, 12, 231–242. [Google Scholar] [CrossRef]
| Variables | N = 47 |
|---|---|
| Gender, n (female %) | 47 (100%) |
| Age, M (SD) | 51.60 (7.61) |
| Marital status, n (%) | |
| Single | 2 (4.3%) |
| Married/living with a partner | 36 (76.6%) |
| Separated/divorced | 6 (12.8%) |
| Widowed | 3 (6.4%) |
| Living arrangement, n (%) | |
| Living alone | 3 (6.4%) |
| Living with partner | 44 (93.6%) |
| Education level, n (%) | |
| Illiterate | 2 (4.3%) |
| Did not graduate from primary school | 1 (2.1%) |
| Primary school | 22 (44.8%) |
| Secondary school | 20 (42.6%) |
| University | 2 (4.3%) |
| Employment status, n (%) | |
| Homemaker | 3 (6.4%) |
| Paid employment | 19 (40.4%) |
| Paid employment but in sick leave | 1 (2.1%) |
| Unemployed with subsidy | 4 (8.5%) |
| Unemployed without subsidy | 10 (21.3%) |
| Retired/pensioner | 6 (12.8%) |
| Temporal disability | 0 (0.0%) |
| Others | 4 (8.5%) |
| Clinical features | |
| Years of FM diagnosis, M (SD) | 12.05 (8.76) |
| Current episode of depression, n (%) | 27 (57.4%) |
| Previous episode(s) of depression, n (%) | 20 (42.6%) |
| Dysthymia, n (%) | 6 (12.8%) |
| Daily FM-related medication | |
| Analgesics, n (%) | 10 (21.3%) |
| Anti-inflammatory, n (%) | 7 (14.9%) |
| Opioids, n (%) | 14 (29.8%) |
| Antiepileptic, n (%) | 6 (12.8%) |
| Muscle relaxant, n (%) | 1 (2.1%) |
| Antidepressants, n (%) | 19 (40.4%) |
| Anxiolytics, n (%) | 17 (36.2%) |
| Main Variables | M (SD) | BNST Cluster Corr. (p) | BNST Theiss Corr. (p) | BNST Motzkin Corr. (p) |
|---|---|---|---|---|
| PIPS (12–84) | 53.89 (16.51) | 0.54 (<0.001) | 0.26 (0.089) | 0.31 (0.036) |
| FIQR (0–100) | 60.99 (21.79) | 0.47 (<0.001) | 0.32 (0.033) | 0.35 (0.018) |
| HADS-A (0–21) | 10.21 (4.27) | 0.24 (0.106) | 0.14 (n.s.) | 0.14 (n.s.) |
| HADS-D (0–21) | 7.91 (5.21) | 0.50 (<0.001) | 0.33 (0.026) | 0.40 (0.007) |
| PSS (0–40) | 21.43 (10.64) | 0.54 (<0.001) | 0.37 (0.012) | 0.41 (0.005) |
| PCS (0–52) | 21.72 (13.45) | 0.43 (0.003) | 0.22 (n.s.) | 0.26 (0.088) |
| FFMQ-Observing (8–40) | 24.79 (6.22) | 0.35 (0.019) | 0.04 (n.s.) | 0.08 (n.s.) |
| FFMQ-Describing (8–40) | 27.17 (8.26) | −0.26 (0.089) | −0.19 (n.s.) | −0.22 (n.s.) |
| FFMQ-Acting with awareness (8–40) | 26.09 (8.59) | −0.30 (0.047) | −0.32 (0.032) | −0.33 (0.031) |
| FFMQ-Non judging (8–40) | 25.53 (8.62) | −0.49 (0.001) | −0.33 (0.031) | −0.37 (0.015) |
| FFMQ-Non reacting (7–35) | 20.65 (5.95) | −0.33 (0.028) | −0.27 (0.082) | −0.284 (0.061) |
| SCS (1–5) | 3.06 (0.99) | −0.34 (0.026) | −0.24 (0.123) | −0.27 (0.074) |
| MBSR (n = 13) | TAU (n = 15) | Group × Time p (ηp2) | |||
|---|---|---|---|---|---|
| PRE | POST | PRE | POST | ||
| BNST | 0.68 (0.05) | 0.72 (0.05) | 0.69 (0.05) | 0.71 (0.07) | n.s. (0.03) |
| PIPS | 57.69 (17.23) | 52.77 (14.04) | 51.53 (16.42) | 50.53 (16.08) | n.s. (0.03) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feliu-Soler, A.; Martínez-Zalacaín, I.; Pérez-Aranda, A.; Borràs, X.; Andrés-Rodríguez, L.; Sanabria-Mazo, J.P.; Fayed, N.; Stephan-Otto, C.; Núñez, C.; Soriano-Mas, C.; et al. The Bed Nucleus of the Stria Terminalis as a Brain Correlate of Psychological Inflexibility in Fibromyalgia. J. Clin. Med. 2020, 9, 374. https://doi.org/10.3390/jcm9020374
Feliu-Soler A, Martínez-Zalacaín I, Pérez-Aranda A, Borràs X, Andrés-Rodríguez L, Sanabria-Mazo JP, Fayed N, Stephan-Otto C, Núñez C, Soriano-Mas C, et al. The Bed Nucleus of the Stria Terminalis as a Brain Correlate of Psychological Inflexibility in Fibromyalgia. Journal of Clinical Medicine. 2020; 9(2):374. https://doi.org/10.3390/jcm9020374
Chicago/Turabian StyleFeliu-Soler, Albert, Ignacio Martínez-Zalacaín, Adrián Pérez-Aranda, Xavier Borràs, Laura Andrés-Rodríguez, Juan P. Sanabria-Mazo, Nicolás Fayed, Christian Stephan-Otto, Christian Núñez, Carles Soriano-Mas, and et al. 2020. "The Bed Nucleus of the Stria Terminalis as a Brain Correlate of Psychological Inflexibility in Fibromyalgia" Journal of Clinical Medicine 9, no. 2: 374. https://doi.org/10.3390/jcm9020374
APA StyleFeliu-Soler, A., Martínez-Zalacaín, I., Pérez-Aranda, A., Borràs, X., Andrés-Rodríguez, L., Sanabria-Mazo, J. P., Fayed, N., Stephan-Otto, C., Núñez, C., Soriano-Mas, C., & Luciano, J. V. (2020). The Bed Nucleus of the Stria Terminalis as a Brain Correlate of Psychological Inflexibility in Fibromyalgia. Journal of Clinical Medicine, 9(2), 374. https://doi.org/10.3390/jcm9020374







