Primary Hyperparathyroidism in Sickle Cell Disease: An Unknown Complication of the Disease in Adulthood
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Biological Characteristics
3.3. Bone Mineral Density
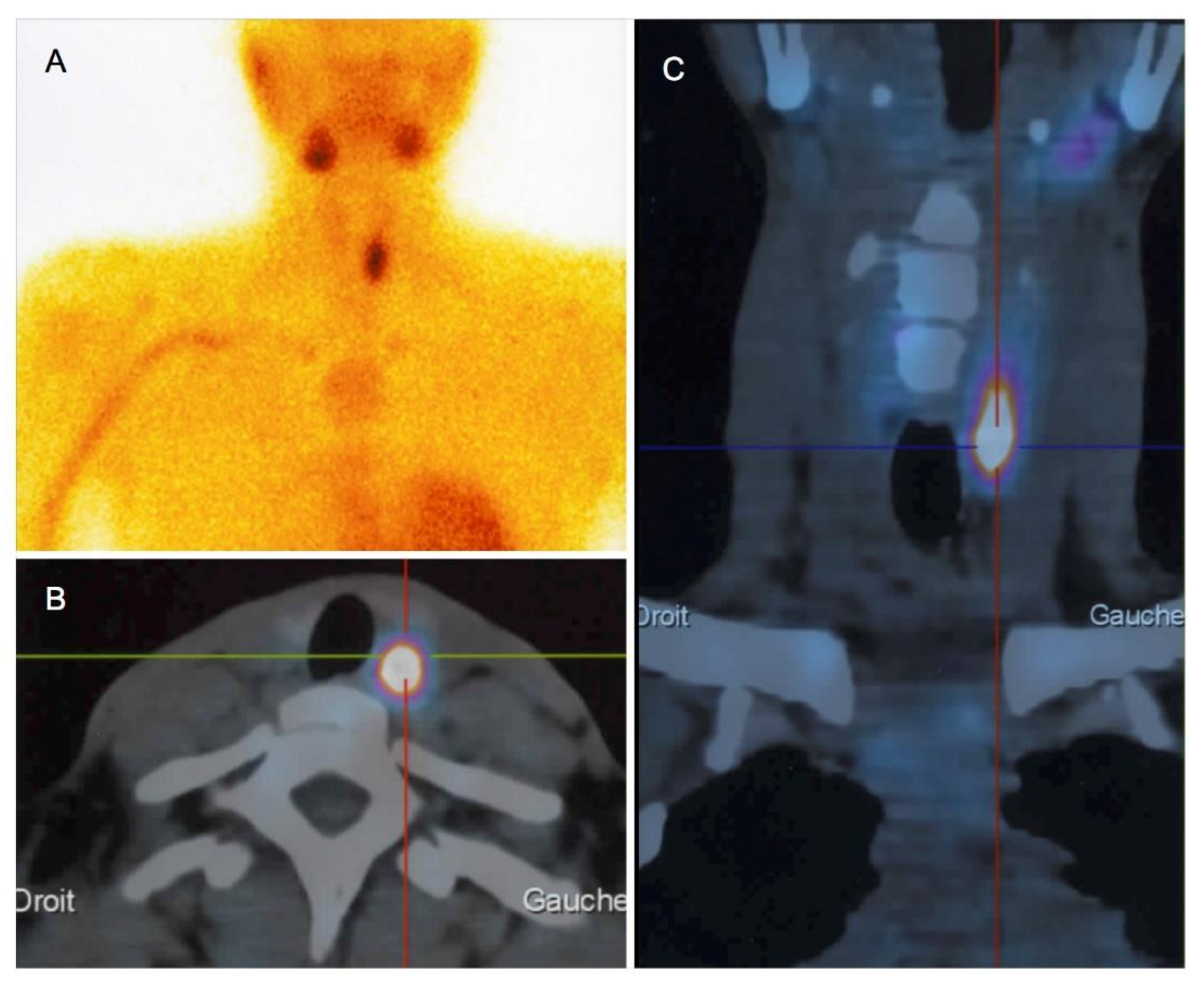
3.4. Parathyroid Imaging
3.5. Genetic Abnormalities
3.6. pHPT Treatment
3.7. Follow-Up
4. Discussion
4.1. General Considerations
4.2. Bone Involvement
4.3. Treatment
4.4. Pathophysiology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wermers, R.A.; Khosla, S.; Atkinson, E.J.; Achenbach, S.J.; Oberg, A.L.; Grant, C.S.; Melton, L. Incidence of Primary Hyperparathyroidism in Rochester, Minnesota, 1993–2001: An Update on the Changing Epidemiology of the Disease. J. Bone Miner. Res. 2006, 21, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Mosekilde, L. Cohort study on effects of parathyroid surgery on multiple outcomes in primary hyperparathyroidism. BMJ 2003, 327, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Trombetti, A.; Herrmann, F.R.; Triponez, F.; Meier, C.; Robert, J.H.; Rizzoli, R. Age and gender distribution of primary hyperparathyroidism and incidence of surgical treatment in a European country with a particularly high life expectancy. Swiss Med. Wkly. 2009, 139, 400–404. [Google Scholar] [PubMed]
- Yeh, M.W.; Ituarte, P.H.G.; Zhou, H.C.; Nishimoto, S.; Liu, I.L.; Harari, A.; Haigh, P.I.; Adams, A.L. Incidence and Prevalence of Primary Hyperparathyroidism in a Racially Mixed Population. J. Clin. Endocrinol. Metab. 2013, 98, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Kandil, E.; Tsai, H.L.; Somervell, H.; Dackiw, A.P.; Tufano, R.P.; Tufaro, A.P.; Kowalski, J.; Zeiger, M.A. African Americans present with more severe primary hyperparathyroidism than non-African Americans. Surgery 2008, 144, 1023–1027. [Google Scholar] [CrossRef]
- Starker, L.F.; Akerström, T.; Long, W.D.; Delgado-Verdugo, A.; Donovan, P.; Udelsman, R.; Lifton, R.P.; Carling, T. Frequent Germ-Line Mutations of the MEN1, CASR, and HRPT2/CDC73 Genes in Young Patients with Clinically Non-familial Primary Hyperparathyroidism. Horm. Cancer 2012, 3, 44–51. [Google Scholar] [CrossRef]
- Thakker, R.V.; Newey, P.J.; Walls, G.V.; Bilezikian, J.; Dralle, H.; Ebeling, P.R.; Melmed, S.; Sakurai, A.; Tonelli, F.; Brandi, M.L. Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1). J. Clin. Endocrinol. Metab. 2012, 97, 2990–3011. [Google Scholar] [CrossRef]
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Alyaarubi, S.; Abish, S.; Gale, M.; Albuquerque, P.; Jabado, N. Primary Hyperparathyroidism Mimicking Vaso-occlusive Crises in Sickle Cell Disease. Pediatrics 2006, 118, e537–e539. [Google Scholar] [CrossRef]
- Muthu, J.; Ali, M. Amelioration of Sickle Cell Pain after Parathyroidectomy in Two Patients with Concurrent Hyperparathyroidism: An Interesting Finding. Case Rep. Med. 2016, 2016, 3263951. [Google Scholar] [CrossRef]
- Arlet, J.-B.; Courbebaisse, M.; Chatellier, G.; Eladari, D.; Souberbielle, J.-C.; Friedlander, G.; de Montalembert, M.; Prié, D.; Pouchot, J.; Ribeil, J.-A. Relationship between vitamin D deficiency and bone fragility in sickle cell disease: A cohort study of 56 adults. Bone 2013, 52, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Arlet, J.-B.; Ribeil, J.-A.; Chatellier, G.; Eladari, D.; De Seigneux, S.; Souberbielle, J.-C.; Friedlander, G.; de Montalembert, M.; Pouchot, J.; Prié, D.; et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: A prospective observational cohort study. BMC Nephrol. 2012, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Cadiot, G.; Houillier, P.; Allouch, A.; Paillard, M.; Mignon, M. Oral calcium tolerance test in the early diagnosis of primary hyperparathyroidism and multiple endocrine neoplasia type 1 in patients with the Zollinger-Ellison syndrome. Gut 1996, 39, 273–278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arlet, J.-B.; Comarmond, C.; Habibi, A.; Stankovic, K.; Ribeil, J.-A.; Lopez Sublet, M.; Affo, L.; Chantalat Auger, C.; Bouldouyre, M.-A.; Gellen-Dautremer, J.; et al. Prevalence and Characteristics of Hepatitis C Virus Infection in Adult Sickle Cell Disease Patients Living in France. J. Infect. Dis. Epidemiol. 2016, 2, 20. [Google Scholar] [CrossRef]
- Gomes, E.; Castetbon, K.; Goulet, V. Mortalité liée à la drépanocytose en France: Âge de décès et causes associées (1979–2010). Bull. Epidémiol. Hebd. 2015, 8, 142–150. [Google Scholar]
- Yu, N.; Donnan, P.T.; Murphy, M.J.; Leese, G.P. Epidemiology of primary hyperparathyroidism in Tayside, Scotland, UK. Clin. Endocrinol. 2009, 71, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Alhefdhi, A.; Schneider, D.F.; Sippel, R.; Chen, H. Recurrent and persistence primary hyperparathyroidism occurs more frequently in patients with double adenomas. J. Surg. Res. 2014, 190, 198–202. [Google Scholar] [CrossRef]
- Mallick, R.; Nicholson, K.J.; Yip, L.; Carty, S.E.; McCoy, K.L. Factors associated with late recurrence after parathyroidectomy for primary hyperparathyroidism. Surgery 2020, 167, 160–165. [Google Scholar] [CrossRef]
- Maruani, G.; Cornire, N.; Nicolet, L.; Baron, S.; Courbebaisse, M.; Renaud, S.; Houillier, P. Primary hyperparathyroidism. Rev. Med. Interne 2013, 34, 605–613. [Google Scholar] [CrossRef]
- Nam, M.; Jeong, H.-S.; Shin, J.H. Differentiation of parathyroid carcinoma and adenoma by preoperative ultrasonography. Acta Radiol. 2017, 58, 670–675. [Google Scholar] [CrossRef]
- Mazzaglia, P.J.; Berber, E.; Kovach, A.; Milas, M.; Esselstyn, C.; Siperstein, A.E. The changing presentation of hyperparathyroidism over 3 decades. Arch. Surg. 2008, 143, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Segal, J.B.; Ashar, B.H.; Leung, S.; Ahmed, S.; Siddique, S.; Rice, T.; Lanzkron, S. High prevalence and correlates of low bone mineral density in young adults with sickle cell disease. Am. J. Hematol. 2006, 81, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Garadah, T.S.; Hassan, A.B.; Jaradat, A.A.; Diab, D.E.; Kalafalla, H.O.; Kalifa, A.K.; Sequeira, R.P.; Alawadi, A.H. Predictors of abnormal bone mass density in adult patients with homozygous sickle-cell disease. Clin. Med. Insights Endocrinol. Diabetes 2015, 8, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Arlet, J.-B.; Pouchot, J. Vitamin D deficiency and bone fragility in sickle cell disease. In Handbook of Nutrition and Diet in Therapy of Bone Diseases; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 53–66. [Google Scholar]
- De Luna, G.; Ranque, B.; Courbebaisse, M.; Ribeil, J.-A.; Khimoud, D.; Dupeux, S.; Silvera, J.; Offredo, L.; Pouchot, J.; Arlet, J.-B. High bone mineral density in sickle cell disease: Prevalence and characteristics. Bone 2018, 110, 199–203. [Google Scholar] [CrossRef]
- Reid, L.J.; Muthukrishnan, B.; Patel, D.; Seckl, J.R.; Gibb, F.W. Predictors of Nephrolithiasis, Osteoporosis, and Mortality in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3692–3700. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Khan, A.A.; Potts, J.T. Guidelines for the Management of Asymptomatic Primary Hyperparathyroidism: Summary statement from the third international workshop. J. Clin. Endocrinol. Metab. 2009, 94, 335–339. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Nguyen, D.T.; Yeh, M.W. Who Benefits from Treatment of Primary Hyperparathyroidism? Surg. Clin. N. Am. 2019, 99, 667–679. [Google Scholar] [CrossRef]
- Rao, D.S.; Honasoge, M.; Divine, G.W.; Phillips, E.R.; Lee, M.W.; Ansari, M.R.; Talpos, G.B.; Parfitt, A.M. Effect of Vitamin D nutrition on parathyroid adenoma weight: Pathogenetic and clinical implications. J. Clin. Endocrinol. Metab. 2000, 85, 1054–1058. [Google Scholar] [CrossRef]
- Shah, V.N.; Shah, C.S.; Bhadada, S.K.; Rao, D.S. Effect of 25 (OH) D replacements in patients with primary hyperparathyroidism (PHPT) and coexistent vitamin D deficiency on serum 25(OH) D, calcium and PTH levels: A meta-analysis and review of literature. Clin. Endocrinol. 2014, 80, 797–803. [Google Scholar] [CrossRef]
- Grubbs, E.G.; Rafeeq, S.; Jimenez, C.; Phillips, E.R.; Lee, M.W.; Ansari, M.R.; Talpos, G.B.; Parfitt, A.M. Preoperative vitamin D replacement therapy in primary hyperparathyroidism: Safe and beneficial? Surgery 2008, 144, 852–859. [Google Scholar] [CrossRef]
- Oztürk, M.; Ustek, D.; Akbas, F.; Kösem, M.; Abaci, N.; Alagöl, F.; Oztürk, G.; Kotan, C. The presence of erythropoietin receptor in parathyroid cells. J. Endocrinol. Investig. 2007, 30, RC35–RC37. [Google Scholar] [CrossRef] [PubMed]
- Foldes, J.; Wilson, P.; Saeed, S.M.; Buck, K.; Parfitt, A.M.; Kleerekoper, M. Parathyroid stimulation after bleeding in man. Acta Endocrinol. 1992, 127, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Qari, M.H.; Dier, U.; Mousa, S.A. Biomarkers of inflammation, growth factor, and coagulation activation in patients with sickle cell disease. Clin. Appl. Thromb. Hemost. 2012, 18, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Cucina, A.; Coluccia, P.; Petramala, L.; Cotesta, D.; Polistena, A.; Zinnamosca, L.; Letizia, C.; Rosato, L.; Cavallaro, A.; et al. Role of growth factors on human parathyroid adenoma cell proliferation. World J. Surg. 2010, 34, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.; Fernandez, J.; Lacour, B.; Price, M.; Monet, J.D.; Garay, R.P.; Drüeke, T. Increased potassium permeability in erythrocytes from patients with hyperparathyroidism. Horm. Metab. Res. 1986, 18, 642–646. [Google Scholar] [CrossRef] [PubMed]
| Demographic parameters Age at diagnosis (years) | 41 (31.5–49.2) |
| Female | 18 (64%) |
| Body mass index (kg/m2) | 21.8 (19.4–25.1) |
| Homozygous (SS) sickle cell disease | 22 (79%) |
| Sub-Saharan African origin | 23 (82%) |
| SCD treatment | |
| Hydroxyurea * | 15/27 (56%) |
| Median dosage (mg/day) | 1000 (800–1500) |
| Exchange transfusion * | 6/27 (22%) |
| Folic acid | 27/27 (100%) |
| Vitamin D intake | 22/27 (81%) |
| pHPT clinical complication | |
| Asymptomatic | 16 (57%) |
| Kidney stone | 3 (11%) |
| Gastric ulcer | 2 (7%) |
| Bone mineral density (n = 16) | |
| Low BMD (< −2.5 < T-score < −1 SD) | 2 (12.5%) |
| Very low BMD (≤−2.5 SD) | 7 (44%) |
| Lumbar spine T-score | −1.1 (−3.05; +0.45) |
| Femoral neck T-score | −0.8 (−1.2; +2) |
| Distal radius T-score (n = 5) | −2.6 (−5.1; −1) |
| n | ||
|---|---|---|
| Serum total calcium level (mmol/L) | 2.62 (2.60–2.78) | 28 |
| Serum ionized calcium (mmol/L) | 1.38 (1.35–1.42) | 18 |
| Serum phosphate level (mmol/L) | 0.90 (0.81–0.99) | 28 |
| Hypophosphatemia * | 8 (29%) | 28 |
| PTH (pg/mL) | 105 (69–137) | 27 ** |
| Increased PTH level * | 21 (78%) | 27 |
| Serum creatinine level (µmol/L) | 58.5 (50.5–70.2) | 28 |
| eGFR (mL/min/1.73 m2) | 110.5 (93.7–129.2) | 28 |
| 25(OH)D (ng/mL) | 25.9 (12.7–48.2) | 24 |
| 1,25(OH)2D (pg/mL) | 85 (53–121) | 15 |
| Hemoglobin (g/dL) | 8.45 (7.7–10) | 28 |
| HbF (%) | 8 (4–15) | 24 |
| MCV (fL) | 91.5 (79.7–107.5) | 28 |
| Reticulocyte count (G/L) | 200 (115–252) | 27 |
| Total bilirubin (µmol/L) | 30.5 (19–43) | 24 |
| LDH (UI/L) | 444 (305–716) | 26 |
| Neutrophil count (G/L) | 3.8 (2.2–4.3) | 28 |
| CTX (nmol/L) (normal < 1.8) | 4.3 (2.6–7.1) | 12 |
| Osteocalcin (ng/mL) (normal range 14–32) | 35 (23–38) | 13 |
| Patients | Controls | p | |
|---|---|---|---|
| n = 16 | n = 32 | ||
| Age | 40 (34–49) | 35 (31–43) | 0.31 |
| Female * | 11 (69) | 21 (66) | |
| Homozygous SCD | 12 (75) | 21 (66) | |
| Body mass index (kg/m2) | 21.9 (19.6–25.8) | 22.1 (20.3–25.2) | 0.64 |
| Hemoglobin (g/dl) | 9 (7.7–10.7) | 8.8 (7.5–10.5) | 0.9 |
| HbF (%) | 7.8 (5–15) | 8 (5.5–16) | 0.8 |
| Reticulocytes (G/L) | 183 (88–240) | 190 (100–250) | 0.8 |
| Neutrophil count (G/L) | 3.6 (2.2–4.4) | 4 (2.4–4.5) | 0.7 |
| Lumbar spine T-score | −1.1 (−3.05; +0.45) | −0.1 (−2.1; +0.72) | 0.38 |
| Femoral neck T-score | −0.8 (−1.2; +2) | 0.4 (−0.65; +1.25) | 0.68 |
| Low BMD (<−2.5 < T-score < −1 SD) | 2 (12.5) | 10 (31) | 0.073 |
| Very low BMD (≤−2.5 SD) | 7 (44) | 4 (12.5) | 0.027 |
| 25(OH)D (ng/mL) | 26 (14–68) | 15 (10–25) | 0.05 |
| Before Surgery | After Surgery | p | |
|---|---|---|---|
| Calcemia (mmol/L) | 2.65 (2.54–2.86) | 2.23 (2.13–2.3) | 0.0025 |
| Phosphatemia (mmol/L) | 0.89 (0.82–0.96) | 1.2 (0.96–1.28) | 0.056 |
| PTH (pg/mL) | 106 (93–145) | 34 (31–50) | 0.004 |
| eGFR (ml/min/1.73 m2) | 103 (91–131) | 104 (85–124) | 0.26 |
| Hemoglobin (g/dL) | 7.9 (6.75–9.7) | 8.7 (7.8–9.6) | 0.38 |
| Reticulocyte count (G/L) | 208 (135–285) | 154 (122–237) | 0.50 |
| Surgery (n = 14) | No Surgery (n = 14) | p | |
|---|---|---|---|
| Age at diagnosis (years) | 46 (30.5–51.5) | 39 (34.2–44.7) | 0.59 |
| Female (%) | 11 (79) | 7 (50) | 0.24 |
| SCD genotype SS | 12 (86) | 10 (71) | 0.65 |
| Body mass index (kg/m2) | 21.6 (19.5–23.7) | 22.3 (19.5–26) | 0.68 |
| Low BMD (T-score < −1 SD) | 6/9 (67) | 2/7 (29) | 0.66 |
| Calcemia (mmol/L) | 2.65 (2.54–2.86) | 2.62 (2.6–2.71) | 0.98 |
| PTH (pg/mL) | 106 (93–145) | 88.5 (59.5–129.7) | 0.19 |
| Hemoglobin (g/dL) | 7.9 (6.7–9.7) | 9.2 (8.2–10.4) | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denoix, E.; Bomahou, C.; Clavier, L.; Ribeil, J.-A.; Lionnet, F.; Bartolucci, P.; Courbebaisse, M.; Pouchot, J.; Arlet, J.-B. Primary Hyperparathyroidism in Sickle Cell Disease: An Unknown Complication of the Disease in Adulthood. J. Clin. Med. 2020, 9, 308. https://doi.org/10.3390/jcm9020308
Denoix E, Bomahou C, Clavier L, Ribeil J-A, Lionnet F, Bartolucci P, Courbebaisse M, Pouchot J, Arlet J-B. Primary Hyperparathyroidism in Sickle Cell Disease: An Unknown Complication of the Disease in Adulthood. Journal of Clinical Medicine. 2020; 9(2):308. https://doi.org/10.3390/jcm9020308
Chicago/Turabian StyleDenoix, Elsa, Charlène Bomahou, Lorraine Clavier, Jean-Antoine Ribeil, François Lionnet, Pablo Bartolucci, Marie Courbebaisse, Jacques Pouchot, and Jean-Benoît Arlet. 2020. "Primary Hyperparathyroidism in Sickle Cell Disease: An Unknown Complication of the Disease in Adulthood" Journal of Clinical Medicine 9, no. 2: 308. https://doi.org/10.3390/jcm9020308
APA StyleDenoix, E., Bomahou, C., Clavier, L., Ribeil, J.-A., Lionnet, F., Bartolucci, P., Courbebaisse, M., Pouchot, J., & Arlet, J.-B. (2020). Primary Hyperparathyroidism in Sickle Cell Disease: An Unknown Complication of the Disease in Adulthood. Journal of Clinical Medicine, 9(2), 308. https://doi.org/10.3390/jcm9020308




