Changes in Hepcidin Serum Levels Correlate with Clinical Improvement in Idiopathic Restless Legs Syndrome Patients
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Assessment of the Severity and Impact of RLS on the Quality of Life
2.3. Blood Workup
2.4. Assessment of Hepcidin
2.5. Assessment of Sleep Parameters and Mood Status
2.6. Statistical Analyses
3. Results
3.1. Demographic Data and Subjective Sleep Scales
3.2. Hepcidin Levels
3.3. RLS severity is Associated with Hepcidin Levels after Treatment
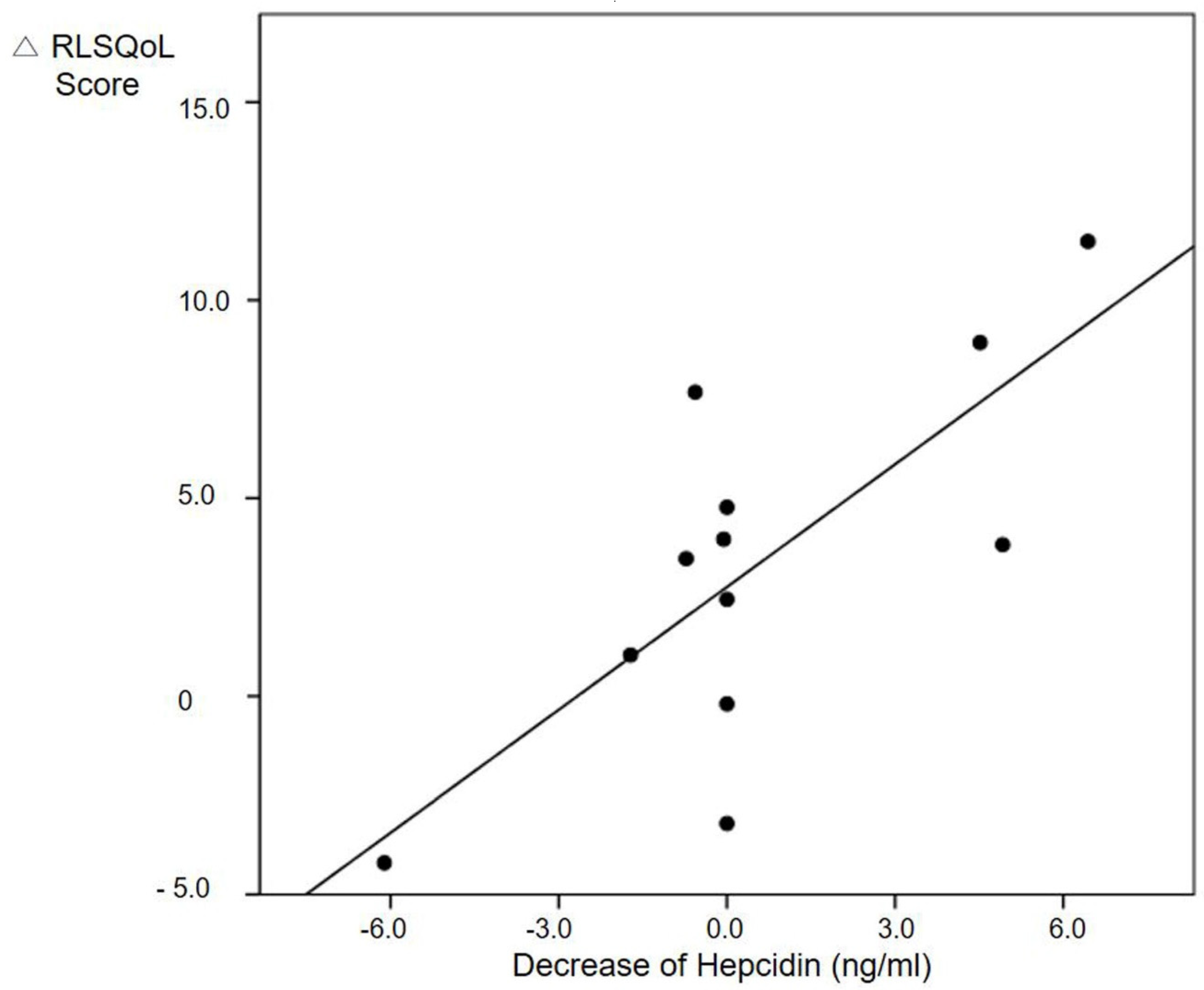
3.4. Quality of Life is Associated with Hepcidin Levels After Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abetz, L.; Allen, R.; Follet, A.; Washburn, T.; Earley, C.; Kirsch, J.; Knight, H. Evaluating the quality of life of patients with restless legs syndrome. Clin. Ther. 2004, 26, 925–935. [Google Scholar] [CrossRef]
- Connor, J.R. Pathophysiology of restless legs syndrome: Evidence for iron involvement. Curr. Neurol. Neurosci. Rep. 2008, 8, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borreguero, D.; Silber, M.H.; Winkelman, J.W.; Högl, B.; Bainbridge, J.; Buchfuhrer, M.; Hadjigeorgiou, G.; Inoue, Y.; Manconi, M.; Oertel, W.; et al. Guidelines for the first-line treatment of restless legs syndrome/Willis–Ekbom disease, prevention and treatment of dopaminergic augmentation: A combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Allen, R.P.; Earley, C.J. Lower molecular weight intravenous iron dextran for restless legs syndrome. Sleep Med. 2013, 14, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Ponnuru, P.; Wang, X.-S.; Patton, S.M.; Allen, R.P.; Earley, C.J. Profile of altered brain iron acquisition in restless legs syndrome. Brain 2011, 134, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Wang, X.-S.; Allen, R.P.; Beard, J.L.; Wiesinger, J.A.; Felt, B.T.; Earley, C.J. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain 2009, 132, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B. Restless legs syndrome/Willis–Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria-history, rationale, description, and significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef]
- Walters, A.S.; LeBrocq, C.; Dhar, A.; Hening, W.; Rosen, R.; Allen, R.P.; Trenkwalder, C. International Restless Legs Syndrome Study Group Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003, 4, 121–132. [Google Scholar] [CrossRef]
- Woloshin, S.; Schwartz, L.M. Giving Legs to Restless Legs: A Case Study of How the Media Helps Make People Sick. PLoS Med. 2006, 3, e170. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Högl, B.; Benes, H.; Kohnen, R. Augmentation in restless legs syndrome is associated with low ferritin. Sleep Med. 2008, 9, 572–574. [Google Scholar] [CrossRef]
- Sun, E.R.; Chen, C.A.; Ho, G.; Earley, C.J.; Allen, R.P. Iron and the Restless Legs Syndrome. Sleep 1998, 21, 381–387. [Google Scholar] [CrossRef]
- Grebenchtchikov, N.; Geurts-Moespot, A.J.; Kroot, J.J.; Den Heijer, M.; Tjalsma, H.; Swinkels, D.W.; Sweep, F.G.J. High-sensitive radioimmunoassay for human serum hepcidin. Br. J. Haematol. 2009, 146, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Laarakkers, C.M.M.; Wiegerinck, E.T.; Klaver, S.; Kolodziejczyk, M.; Gille, H.; Hohlbaum, A.M.; Tjalsma, H.; Swinkels, D.W. Improved Mass Spectrometry Assay For Plasma Hepcidin: Detection and Characterization of a Novel Hepcidin Isoform. PLoS ONE 2013, 8, e75518. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Kim, K.-W.; Yoon, S.-Y.; Kim, S.-H.; Lee, S.-H. Serum Pro-hepcidin Could Reflect Disease Activity in Patients with Rheumatoid Arthritis. J. Korean Med. Sci. 2010, 25, 348–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clardy, S.L.; Wang, X.; Boyer, P.J.; Earley, C.J.; Allen, R.P.; Connor, J.R. Is ferroportin–hepcidin signaling altered in restless legs syndrome? J. Neurol. Sci. 2006, 247, 173–179. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Chenini, S.; Vialaret, J.; Delaby, C.; Guiraud, L.; Gabelle, A.; Lopez, R.; Hirtz, C.; Jaussent, I.; Lehmann, S. Association between serum hepcidin level and restless legs syndrome. Mov. Disord. 2018, 33, 618–627. [Google Scholar] [CrossRef]
- Yang, J.G.; Kim, D.H.; Lee, J.H.; Park, K.H.; Jung, K.Y.; Shin, W.C.; Cho, Y.W. The reliability and validity of the Korean versions of the international restless legs scale and the restless legs syndrome quality of life questionnaire. J. Korean Neurol. Assoc. 2010, 28, 263–269. [Google Scholar]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Bastien, C.H. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C. Faculty Opinions recommendation of Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Post-Publ. Peer Rev. Biomed. Lit. 2004, 306, 2090–2093. [Google Scholar]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, L.B.; Walters, A.S.; Paueksakon, P. Restless legs syndrome—Theoretical roles of inflammatory and immune mechanisms. Sleep Med. Rev. 2012, 16, 341–354. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Walters, A.S. Restless legs syndrome is associated with irritable bowel syndrome and small intestinal bacterial overgrowth. Sleep Med. 2011, 12, 610–613. [Google Scholar] [CrossRef]
- Wang, Q.; Du, F.; Qian, Z.-M.; Ge, X.H.; Zhu, L.; Yung, W.H.; Yang, L.; Ke, Y. Lipopolysaccharide Induces a Significant Increase in Expression of Iron Regulatory Hormone Hepcidin in the Cortex and Substantia Nigra in Rat Brain. Endocrinology 2008, 149, 3920–3925. [Google Scholar] [CrossRef]
- Varım, C.; Acar, B.A.; Uyanık, M.S.; Acar, T.; Alagoz, N.; Nalbant, A.; Kaya, T.; Ergenc, H. Association between the neutrophil-to-lymphocyte ratio, a new marker of systemic inflammation, and restless legs syndrome. Singap. Med. J. 2016, 57, 514–516. [Google Scholar] [CrossRef]
- Didato, G.; Di Giacomo, R.; Rosa, G.J.; Dominese, A.; De Curtis, M.; Lanteri, P. Restless Legs Syndrome across the Lifespan: Symptoms, Pathophysiology, Management and Daily Life Impact of the Different Patterns of Disease Presentation. Int. J. Environ. Res. Public Health 2020, 17, 3658. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; Van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; Heijer, M.D.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef] [PubMed]
- Chenini, S.; Delaby, C.; Rassu, A.-L.; Barateau, L.; Vialaret, J.; Hirtz, C.; Dupuy, A.M.; Lehmann, S.; Jaussent, I.; Dauvilliers, Y. Hepcidin and ferritin levels in restless legs syndrome: A case-control study. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, S.; Haschka, D.; Nairz, M.; Seifert, M.; Volani, C.; Lutz, O.; Weiss, G. Dopamine promotes cellular iron accumulation and oxidative stress responses in macrophages. Biochem. Pharmacol. 2018, 148, 193–201. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patient (N = 18) | Control (N = 15) | p-Value |
|---|---|---|---|
| Age (y) | 47.3 ± 12.1 | 38.8 ± 12.9 | 0.061 |
| Female sex | 12 (66.7) | 9 (60.0) | 0.692 |
| BMI (kg/m2) | 22.3 ± 3.0 | 21.3 ± 5.5 | 0.762 |
| Weekly sleep duration (hour) | 5.4 ± 1.8 | 6.4 ± 0.9 | 0.067 |
| Sleep efficiency (%) | 78.6 ± 22.3 | 90.3 ± 13.9 | 0.229 |
| Sleep latency (min) | 51.4 ± 46.4 | 30.1 ± 30.8 | 0.117 |
| PSQI score | 10.0 ± 5.2 | 5.7 ± 3.4 | 0.009 * |
| Poor sleep quality (PSQI > 5) | 14 (77.8) | 7 (46.7) | 0.064 |
| Epworth Sleepiness Scale | 6.8 ± 5.0 | 5.0 ± 3.0 | 0.220 |
| Excessive daytime sleepiness (ESS > 11) | 4 (22.2) | 0 (0) | 0.108 |
| Insomnia Severity Index | 21.7 ± 40.8 | 4.5 ± 4.0 | 0.002 * |
| Moderate to severe insomnia (ISI ≥ 15) | 8 (44.4) | 0 (0) | 0.004 * |
| Beck Anxiety Inventory score | 11.4 ± 12.7 | 3.3 ± 4.1 | 0.011 * |
| Moderate anxiety (BAI ≥ 22) | 3 (16.7) | 0 (0) | 0.233 |
| PHQ-9 Depression Scale score | 7.2 ± 7.2 | 2.9 ± 3.2 | 0.070 |
| Depressive mood (PHQ-9 ≥10) | 4 (22.2) | 1 (6.3) | 0.346 |
| Characteristics | Patient (N = 18) | Control (N = 15) | p-Value |
|---|---|---|---|
| Ferritin level (ng/mL) | 85.9 ± 61.7 | 112.4 ± 149.6 | 0.901 α |
| Hepcidin level (ng/mL) | 7.1 ± 2.4 | 7.0 ± 3.2 | 0.357 α |
| Hemoglobin (g/dL) | 13.2 ± 1.7 | 14.2 ± 1.5 | 0.120 |
| TIBC (μg/dL) | 315.4 ± 27.2 | 327.0 ± 41.4 | 0.344 |
| Iron (μg/dL) | 101.4 ± 44.1 | 82.0 ± 29.1 | 0.154 |
| Transferrin (mg/dL) | 253.5 ± 20.4 | 258.3 ± 30.3 | 0.602 |
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Decrease of hepcidin level (ng/mL) | 0.001 (0.00, 3.96) | 0.042 * | 0.001 (0.00, 0.00) | 0.025 * | 0.002 (0.00, 0.00) | 0.005 * |
| Difference of CRP level | 0.230 (−0.78, 1.24) | 0.617 | 0.041 (−1.01, 1.10) | 0.928 | −0.705 (−1.92, 0.51) | 0.130 |
| Age (years) | −0.172 (−0.49, 0.15) | 0.241 | −0.148 (−0.11, 0.31) | 0.057 | ||
| Male sex | 2.394 (−3.45,8.24) | 0.365 | 0.690 (−3.30, 4.68) | 0.534 | ||
| Sleep quality (PSQI score) | −0.379 (−1.37, 0.61) | 0.241 | ||||
| Insomnia severity (ISI score) | −1.192 (−1.92, 0.46) | 0.020 * | ||||
| Daytime sleepiness (ESS score) | −1.328 (−2.23, −0.43 | 0.014 * | ||||
| Depression severity (PHQ-9 score) | 4.872 (2.58, 7.17) | 0.012 * | ||||
| Anxiety severity (BAI score) | −1.328 (−2.23, −0.43) | 0.024 * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.-J.; Kim, J.H.; Yun, C.-H.; Kim, D.W.; Oh, J. Changes in Hepcidin Serum Levels Correlate with Clinical Improvement in Idiopathic Restless Legs Syndrome Patients. J. Clin. Med. 2020, 9, 4115. https://doi.org/10.3390/jcm9124115
Im H-J, Kim JH, Yun C-H, Kim DW, Oh J. Changes in Hepcidin Serum Levels Correlate with Clinical Improvement in Idiopathic Restless Legs Syndrome Patients. Journal of Clinical Medicine. 2020; 9(12):4115. https://doi.org/10.3390/jcm9124115
Chicago/Turabian StyleIm, Hee-Jin, Jee Hyun Kim, Chang-Ho Yun, Dong Wook Kim, and Jeeyoung Oh. 2020. "Changes in Hepcidin Serum Levels Correlate with Clinical Improvement in Idiopathic Restless Legs Syndrome Patients" Journal of Clinical Medicine 9, no. 12: 4115. https://doi.org/10.3390/jcm9124115
APA StyleIm, H.-J., Kim, J. H., Yun, C.-H., Kim, D. W., & Oh, J. (2020). Changes in Hepcidin Serum Levels Correlate with Clinical Improvement in Idiopathic Restless Legs Syndrome Patients. Journal of Clinical Medicine, 9(12), 4115. https://doi.org/10.3390/jcm9124115





