Fractal-Based Analysis of Bone Microstructure in Crohn’s Disease: A Pilot Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Study Design
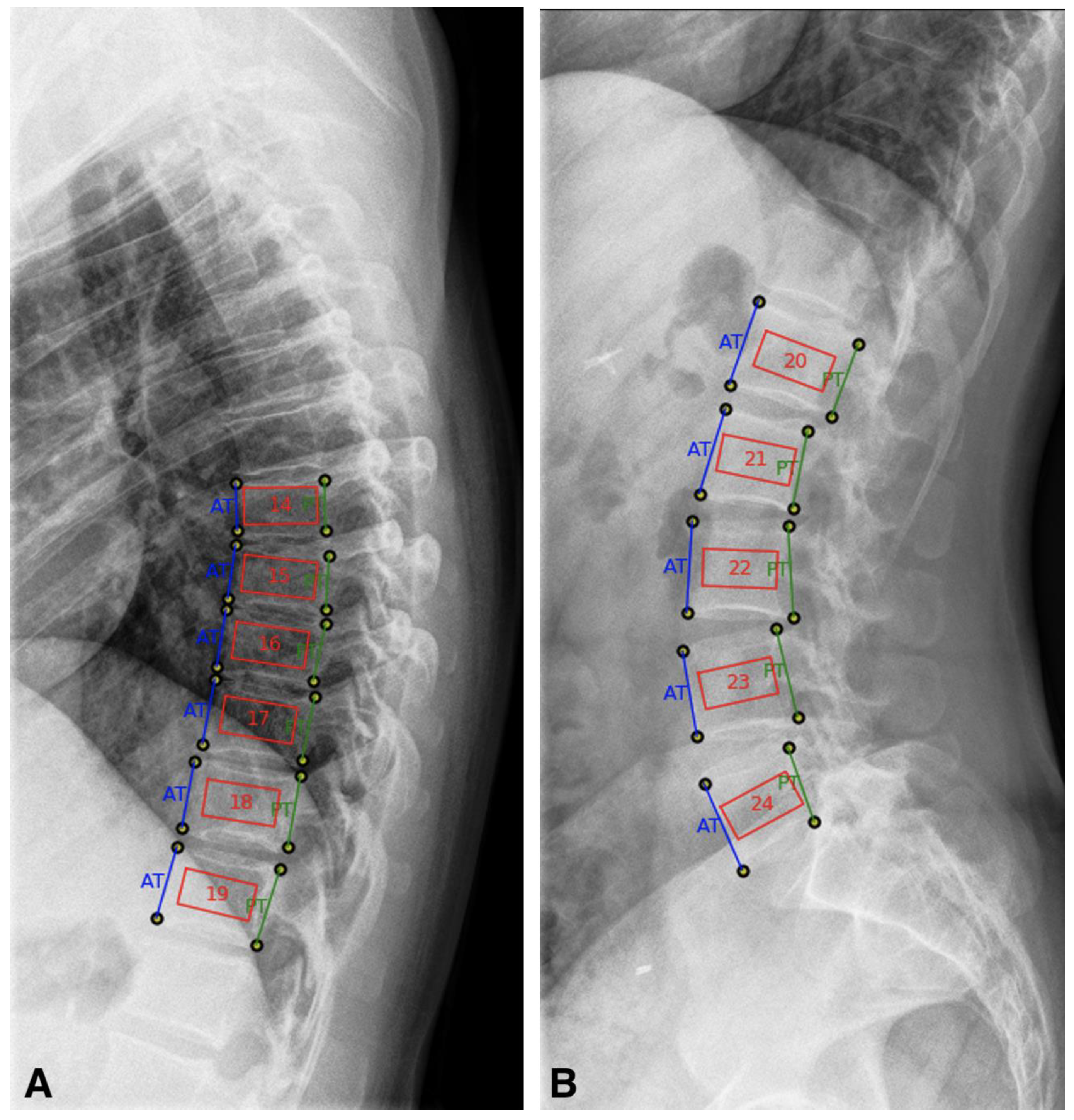
2.1.1. TX-Analyzer™
2.1.2. Dual X-ray Absorptiometry (DXA) and Trabecular Bone Score (TBS)
2.1.3. Statistics
3. Results
3.1. Patient Population and Demographic Characteristics
3.2. Bone Mineral Density and Bone Microarchitecture
3.3. Correlation of Imaging Parameters
3.4. Glucocorticoid Use in Crohn’s Disease Patients
3.5. Disease Duration in Crohn’s Disease Patients
3.6. History of Bowel Resection in Crohn’s Disease Patients
3.7. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Targownik, L.E.; Bernstein, C.N.; Nugent, Z.; Johansson, H.; Oden, A.; McCloskey, E.; Kanis, J.A.; Leslie, W.D. Inflammatory bowel disease and the risk of fracture after controlling for FRAX. J. Bone Miner. Res. 2013, 28, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- van Staa, T.P.; Cooper, C.; Brusse, L.S.; Leufkens, H.; Javaid, M.K.; Arden, N.K. Inflammatory bowel disease and the risk of fracture. Gastroenterology 2003, 125, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Bartko, J.; Reichardt, B.; Kocijan, R.; Klaushofer, K.; Zwerina, J.; Behanova, M. Inflammatory Bowel Disease: A Nationwide Study of Hip Fracture and Mortality Risk After Hip Fracture. J. Crohn’s Colitis 2020, 14, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Targownik, L.E.; Bernstein, C.N.; Nugent, Z.; Leslie, W.D. Inflammatory bowel disease has a small effect on bone mineral density and risk for osteoporosis. Clin. Gastroenterol. Hepatol. 2013, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Drinka, P.J.; DeSmet, A.A.; Bauwens, S.F.; Rogot, A. The effect of overlying calcification on lumbar bone densitometry. Calcif. Tissue Int. 1992, 50, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Oviatt, S.K.; Mann, T. The Impact of Osteophytic and Vascular Calcifications on Vertebral Mineral Density Measurements in Men*. J. Clin. Endocrinol. Metab. 1990, 70, 1202–1207. [Google Scholar] [CrossRef]
- Greenspan, S.L.; Maitland-Ramsey, L.; Myers, E. Classification of osteoporosis in the elderly is dependent on site-specific analysis. Calcif. Tissue Int. 1996, 58, 409–414. [Google Scholar] [CrossRef]
- Dufour, R.; Winzenrieth, R.; Heraud, A.; Hans, D.; Mehsen, N. Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2013, 24, 2837–2846. [Google Scholar] [CrossRef]
- Krajcovicova, A.; Kuzma, M.; Hlavaty, T.; Hans, D.; Koller, T.; Jackuliak, P.; Leskova, Z.; Sturdik, I.; Killinger, Z.; Payer, J. Decrease of trabecular bone score reflects severity of Crohn’s disease: Results of a case-control study. Eur. J. Gastroenterol. Hepatol. 2018, 30, 101–106. [Google Scholar] [CrossRef]
- Haschka, J.; Hirschmann, S.; Kleyer, A.; Englbrecht, M.; Faustini, F.; Simon, D.; Figueiredo, C.P.; Schuster, L.; Muschitz, C.; Kocijan, R.; et al. High-resolution Quantitative Computed Tomography Demonstrates Structural Defects in Cortical and Trabecular Bone in IBD Patients. J. Crohn’s Colitis 2016, 10, 532–540. [Google Scholar] [CrossRef]
- Mandelbrot, B.B.; Freeman, W.H. The Fractal Geometry of Nature; Henry Holt and Company: New York, NY, USA, 1983. [Google Scholar]
- Benhamou, C.L.; Poupon, S.; Lespessailles, E.; Loiseau, S.; Jennane, R.; Siroux, V.; Ohley, W.; Pothuaud, L. Fractal analysis of radiographic trabecular bone texture and bone mineral density: Two complementary parameters related to osteoporotic fractures. J. Bone Miner. Res. 2001, 16, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Pothuaud, L.; Lespessailles, E.; Harba, R.; Jennane, R.; Royant, V.; Eynard, E.; Benhamou, C.L. Fractal analysis of trabecular bone texture on radiographs: Discriminant value in postmenopausal osteoporosis. Osteoporos. Int. 1998, 8, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, P.; Giger, M.L.; Favus, M. Multifractal radiographic analysis of osteoporosis. Med. Phys. 1994, 21, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Dimai, H.P.; Ljuhar, R.; Ljuhar, D.; Norman, B.; Nehrer, S.; Kurth, A.; Fahrleitner-Pammer, A. Assessing the effects of long-term osteoporosis treatment by using conventional spine radiographs: Results from a pilot study in a sub-cohort of a large randomized controlled trial. Skeletal. Radiol. 2019, 48, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Pentland, A.P. Fractal-based description of natural scenes. IEEE Trans. Pattern Anal. Mach. Intell. 1984, 6, 661–674. [Google Scholar] [CrossRef]
- Nehrer, S.; Ljuhar, R.; Steindl, P.; Simon, R.; Maurer, D.; Ljuhar, D.; Bertalan, Z.; Dimai, H.P.; Goetz, C.; Paixao, T. Automated Knee Osteoarthritis Assessment Increases Physicians’ Agreement Rate and Accuracy: Data from the Osteoarthritis Initiative. Cartilage 2019. [Google Scholar] [CrossRef]
- Prouteau, S.; Ducher, G.; Nanyan, P.; Lemineur, G.; Benhamou, L.; Courteix, D. Fractal analysis of bone texture: A screening tool for stress fracture risk? Eur. J. Clin. Investig. 2004, 34, 137–142. [Google Scholar] [CrossRef]
- Lundahl, T.; Ohley, W.J.; Kay, S.M.; Siffert, R. Fractional brownian motion: A maximum likelihood estimator and its application to image texture. IEEE Trans. Med. Imaging 1986, 5, 152–161. [Google Scholar] [CrossRef]
- Shannon, C.E. The mathematical theory of communication. 1963. MD Comput. 1997, 14, 306–317. [Google Scholar]
- 2019 ISCD Official Positions—Adult. Available online: https://www.iscd.org/official-positions/2019-iscd-official-positions-adult/ (accessed on 19 December 2020).
- IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Hoffmann, P.; Krisam, J.; Kasperk, C.; Gauss, A. Prevalence, Risk Factors and Course of Osteoporosis in Patients with Crohn’s Disease at a Tertiary Referral Center. J. Clin. Med. 2019, 8, 2178. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ichikawa, Y.; Saito, E.; Homma, M. Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metab. Clin. Exp. 1983, 32, 151–156. [Google Scholar] [CrossRef]
- Hahn, T.J.; Halstead, L.R.; Baran, D.T. Effects off short term glucocorticoid administration on intestinal calcium absorption and circulating vitamin D metabolite concentrations in man. J. Clin. Endocrinol. Metab. 1981, 52, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kitazawa, R.; Yamaguchi, A.; Kitazawa, S. Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. J. Cell. Biochem. 2008, 103, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Saag, K.G. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos. Int. 2019, 30, 1145–1156. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Arlot, M.E.; Chavassieux, P.M.; Roux, J.P.; Portero, N.R.; Meunier, P.J. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 97–103. [Google Scholar] [CrossRef]
- Dempster, D.W. Bone histomorphometry in glucocorticoid-induced osteoporosis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1989, 4, 137–141. [Google Scholar] [CrossRef]
- Leib, E.S.; Winzenrieth, R. Bone status in glucocorticoid-treated men and women. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2016, 27, 39–48. [Google Scholar] [CrossRef]
- Oostlander, A.E.; Bravenboer, N.; Sohl, E.; Holzmann, P.J.; van der Woude, C.J.; Dijkstra, G.; Stokkers, P.C.; Oldenburg, B.; Netelenbos, J.C.; Hommes, D.W.; et al. Histomorphometric analysis reveals reduced bone mass and bone formation in patients with quiescent Crohn’s disease. Gastroenterology 2011, 140, 116–123. [Google Scholar] [CrossRef]
| Crohn’s Disease n = 39 | Controls n = 39 | p | |
|---|---|---|---|
| Demographics and Disease Duration | |||
| Sex [male/female] | 13/26 | 14/25 | 0.812 |
| Age [years] | 53.9 (40.4–60.7) | 44.8 (41.3–55.9) | 0.439 |
| Height [m] | 1.68 (1.6–1.74) | 1.67 (1.62–1.72) | 0.859 |
| Weight [kg] | 75 (59–94) | 78 (64.8–85.3) | 0.895 |
| BMI [kg/m2] | 27.4 (22.1–32.3) | 27.1 (23.4–30.2) | 0.924 |
| Laboratory Results | |||
| CRP [mg/L, 0.0–4.9] | 2.80 (1.40–7.30) | - | - |
| Ca [mmol/L, 2.20–2.65] | 2.37 (2.31–2.53) | - | - |
| Ph [mmol/L, 0.81–1.45] | 1.14 (1.00–1.27) | - | - |
| AP [U/L, 30–120] | 78 (65–95) | - | - |
| Beta-crosslaps [ng/mL, 0.03–0.37] | 0.390 (0.28–0.58) | - | - |
| PTH [pg/mL, 12–88] | 45.5 (31–73) | - | - |
| Osteocalcin [ng/mL, 4.6–65.4] | 16.5 (14.1–21.9) | - | - |
| 25(OH)-vitamin D [nmol/L, 75–250] | 64 (52–76) | - | - |
| Calprotectin [mg/kg, 0.0–50.0] | 88.9 (26.1–248) | - | - |
| Disease Characteristics | |||
| Disease duration [years] | 8 (2–20) | - | - |
| cDMARDs, n (%) | 21 (55.3) | - | - |
| bDMARDs, n (%) | 20 (52.6) | - | - |
| GC-Treatment > 3 mo, n (%) | 23 (59) | - | - |
| History of bowel resection due to CD, n (%) * | 14 (35.9) * | ||
| Ileocoecal Resection, n (%) | 13 (34.2) | - | - |
| Small bowel resection, n (%) | 4 (10.5) | - | - |
| Colonic resection, n (%) | 5 (13.2) | - | - |
| Crohn’s Disease n = 39 | Controls n = 39 | p | |
|---|---|---|---|
| DXA and TBS | |||
| Lumbar Spine (L1–L4) | |||
| BMD [g/cm2] | 1.030 (0.955–1.183) | - | - |
| T-Score [SD] | −1.4 (−2.1–0.1) | - | - |
| TBS [units] | 1.307 (1.218–1.402) | - | - |
| Total Hip | |||
| BMD [g/cm2] | 0.921 (0.847–0.978) | - | |
| T-Score [SD] | −0.9 (−1.4, −0.2) | - | |
| Femoral Neck | |||
| BMD [g/cm2] | 0.885 (0.787–0.938) | - | - |
| T-Score [SD] | −0.9 (−1.8, −0.5) | - | - |
| TX Analysis | |||
| Thoracic Spine (T7–T12) | |||
| BSV | 0.293 (0.268–0.309) | 0.270 (0.225–0.302) | 0.016 |
| BVV | 0.282 (0.260–0.302) | 0.251 (0.214–0.288) | 0.012 |
| BEV | 12.2 (12.1–12.3) | 12.2 (12.1–12.3) | 0.919 |
| Lumbar Spine (L1–L4) | |||
| BSV | 0.109 (0.093–0.128) | 0.110 (0.104–0.125) | 0.310 |
| BVV | 0.100 (0.092–0.118) | 0.103 (0.010–0.119) | 0.301 |
| BEV | 11.5 (11.4–11.6) | 11.5 (11.4–11.6) | 0.154 |
| Age | Weight | Height | BMI | BSV T7–T12 | BSV L1–L4 | BVV T7–T12 | BVV L1–L4 | BEV T7–T12 | BEV L1–L4 | BMD L1–L4 | BMD Fem.Neck | BMD Total Fem | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight | 0.220 | ||||||||||||
| Height | 0.151 | 0.498 ** | |||||||||||
| BMI | 0.159 | 0.876 ** | 0.025 | ||||||||||
| BSV T7–T12 | 0.029 | −0.046 | 0.032 | −0.078 | |||||||||
| BSV L1–L4 | −0.200 | −0.647 * | −0.359 ** | −0.568 ** | 0.109 | ||||||||
| BVV T7–T12 | 0.054 | −0.093 | 0.070 | −0.151 | 0.966 ** | 0.134 | |||||||
| BVV L1–L4 | −0.304 ** | −0.605 ** | −0.250 * | −0.594 ** | 0.152 | 0.800* * | 0.196 | ||||||
| BEV T7–T12 | −0.086 | −0.082 | −0.142 | −0.019 | 0.476 ** | 0.041 | 0.349 ** | −0.003 | |||||
| BEV L1–L4 | −0.154 | −0.476 ** | −0.259 * | −0.403 ** | 0.060 | 0.666 ** | 0.047 | 0.452 ** | 0.151 | ||||
| BMD L1–L4 | −0.095 | 0.407 * | 0.204 | 0.360 * | 0.014 | −0.250 | −0.121 | −0.134 | 0.417 ** | 0.115 | |||
| BMD fem.neck | −0.273 | 0.454 ** | 0.337 * | 0.353 * | 0.010 | −0.314 | −0.097 | −0.122 | 0.349 * | −0.029 | 0.785 ** | ||
| BMD total fem | −0.120 | 0.545 ** | 0.256 | 0.507 ** | −0.064 | −0.472 ** | −0.177 | −0.264 | 0.358 * | −0.238 | 0.771 ** | 0.881 ** | |
| TBS | −0.525 ** | 0.154 | −0.037 | 0.191 | −0.102 | −0.067 | −0.165 | 0.067 | 0.217 | 0.116 | 0.417 * | 0.466 ** | 0.451 ** |
| History of GC Treatment | p | Disease Duration | p | History for Surgery for CD | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| <3 months N = 16 | >3 months N = 23 | ≤15 years N = 25 | > 15 years N = 14 | No N = 25 | Yes N = 14 | ||||
| Demographics and Disease Duration | |||||||||
| Sex [male/female] | 5/11 | 8/15 | 0.818 | 6/19 | 7/7 | 0.098 | 4/21 | 9/5 | 0.002 |
| Age [years] | 44.4 (35.9–56.4) | 54.8 (41.8–61.4) | 0.228 | 54.3 (36.0–62.6) | 52.7 (42.1–56.9) | 0.883 | 53.9 (38.8–58.6) | 50.8 (39.9–62.6) | 0.972 |
| Height [m] | 1.65 (1.56–1.7) | 1.69 (1.63–1.74) | 0.579 | 1.66 (1.59–1.7) | 1.70 (1.62–1.78) | 0.268 | 1.67 (1.59–1.7) | 1.74 (1.65–1.79) | 0.016 |
| Weight [kg] | 70 (55-94.8) | 80 (61–90) | 0.346 | 75 (60.5–94.5) | 73 (49.8–89.3) | 0.519 | 73 (59–85) | 88.5 (57.8–105.5) | 0.111 |
| BMI [kg/m2] | 26.3 (21.7–32.9) | 28.3 (22.7–31.6) | 0.908 | 28.3 (23.1–32.6) | 26.7 (18.2–30.3) | 0.170 | 26.6 (22.3–32.0) | 29.2 (21.5–32.4) | 0.613 |
| Dis. duration [years] | 6.5 (2–13) | 12 (5–25) | 0.053 | 5 (1.5–8) | 22 (17.8–30.5) | < 0.001 | 5 (1.5–14) | 17 (8–22.8) | 0.013 |
| DXA and TBS | |||||||||
| Lumbar Spine (L1–L4) | |||||||||
| BMD [g/cm2] | 1.062 (0.994–1.282) | 0.976 (0.912-1.183) | 0.145 | 1.086 (0.972–1.223) | 0.963 (0.874–1.047) | 0.337 | 1.036 (0.952–1.223) | 1.029 (0.919–1.076) | 0.567 |
| T-Score [SD] | −1.2 (−1.6–0.8) | −1.8 (−2.2–0.1) | 0.146 | −1.1 (−1.8–0.3) | −1.9 (−2.6–1.4) | 0.412 | −1.2 (−2.0–0.3) | −1.5 (−2.3–1.2) | 0.357 |
| TBS [units] | 1.397 (1.299–1.459) | 1.257 (1.186–1.356) | 0.014 | 1.336 (1.222–1.403) | 1.274 (1.210–1.390) | 0.361 | 1.302 (1.202–1.399) | 1.323 (1.240–1.407) | 0.527 |
| Total Hip | |||||||||
| BMD [g/cm2] | 0.921 (0.895–1.040) | 0.922 (0.814–0.978) | 0.251 | 0.943 (0.886–1.024) | 0.855 (0.770–0.931) | 0.598 | 0.921 (0.849–0.990) | 0.926 (0.838–1.003) | 0.942 |
| T-Score [SD] | −0.8 (−1.5–0.3) | −1.1 (−1.7–0.2) | 0.159 | −0.6 (−1.1–0.2) | −1.4 (−2.3–0.6) | 0.425 | −0.8 (−1.3–0.1) | −1.2 (−1.6–0.3) | 0.443 |
| Femoral Neck | |||||||||
| BMD [g/cm2] | 0.900 (0.813–1.015) | 0.857 (0.766–0.938) | 0.224 | 0.900 (0.829–0.943) | 0.813 (0.757–0.947) | 0.724 | 0.885 (0.786–0.936) | 0.901 (0.781–1.015) | 0.536 |
| T-Score [SD] | −0.8 (−1.5–0.3) | −1.0 (−1.8–0.6) | 0.215 | -0.8 (−1.3–0.5) | -1.4(−2.3–0.6) | 0.373 | −0.8 (−1.7–0.5) | −1.1 (−2.1–0.3) | 0.844 |
| TX Analysis | |||||||||
| Thoracic Spine (T7–T12) | |||||||||
| BSV | 0.295 (0.270–0.305) | 0.290 (0.259–0.315) | 0.932 | 0.298 (0.269–0.308) | 0.284 (0.266–0.313) | 0.761 | 0.293 (0.269–0.308) | 0.284 (0.266–0.314) | 0.919 |
| BVV | 0.275 (0.260–0.294) | 0.286 (0.243–0.310) | 0.475 | 0.282 (0.251–0.303) | 0.278 (0.255–0.309) | 0.806 | 0.276 (0.251–0.303) | 0.286 (0.255–0.304) | 0.828 |
| BEV | 12.3 (12.2–12.4) | 12.2 (12.1–12.3) | 0.015 | 12.3 (12.2–12.4) | 12.1 (12.0–12.2) | 0.001 | 12.2 (12.1–12.3) | 12.2 (12.1–12.4) | 0.784 |
| Lumbar Spine (L1–L4) | |||||||||
| BSV | 0.115 (0.093–0.131) | 0.103 (0.096–0.126) | 0.797 | 0.109 (0.093–0.126) | 0.106 (0.093–0.176) | 0.478 | 0.115 (0.096–0.132) | 0.100 (0.090–0.117) | 0.228 |
| BVV | 0.107 (0.092–0.118) | 0.099 (0.086–0.122) | 0.549 | 0.099 (0.091–0.118) | 0.105 (0.091–0.146) | 0.515 | 0.099 (0.092–0.124) | 0.101 (0.086–0.115) | 0.675 |
| BEV | 11.6 (11.4–11.6) | 11.5 (11.3–11.6) | 0.138 | 11.5 (11.4–11.6) | 11.5 (11.3–11.6) | 0.303 | 11.6 (11.5–11.6) | 11.4 (11.3–11.5) | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haschka, J.; Kraus, D.A.; Behanova, M.; Huber, S.; Bartko, J.; Schanda, J.E.; Meier, P.; Bahrami, A.; Zandieh, S.; Zwerina, J.; et al. Fractal-Based Analysis of Bone Microstructure in Crohn’s Disease: A Pilot Study. J. Clin. Med. 2020, 9, 4116. https://doi.org/10.3390/jcm9124116
Haschka J, Kraus DA, Behanova M, Huber S, Bartko J, Schanda JE, Meier P, Bahrami A, Zandieh S, Zwerina J, et al. Fractal-Based Analysis of Bone Microstructure in Crohn’s Disease: A Pilot Study. Journal of Clinical Medicine. 2020; 9(12):4116. https://doi.org/10.3390/jcm9124116
Chicago/Turabian StyleHaschka, Judith, Daniel Arian Kraus, Martina Behanova, Stephanie Huber, Johann Bartko, Jakob E. Schanda, Philip Meier, Arian Bahrami, Shahin Zandieh, Jochen Zwerina, and et al. 2020. "Fractal-Based Analysis of Bone Microstructure in Crohn’s Disease: A Pilot Study" Journal of Clinical Medicine 9, no. 12: 4116. https://doi.org/10.3390/jcm9124116
APA StyleHaschka, J., Kraus, D. A., Behanova, M., Huber, S., Bartko, J., Schanda, J. E., Meier, P., Bahrami, A., Zandieh, S., Zwerina, J., & Kocijan, R. (2020). Fractal-Based Analysis of Bone Microstructure in Crohn’s Disease: A Pilot Study. Journal of Clinical Medicine, 9(12), 4116. https://doi.org/10.3390/jcm9124116






