Challenges Posed by Embryonic and Anatomical Factors in Systematic Lymphadenectomy for Endometrial Cancer
Abstract
1. Introduction
2. Anatomy
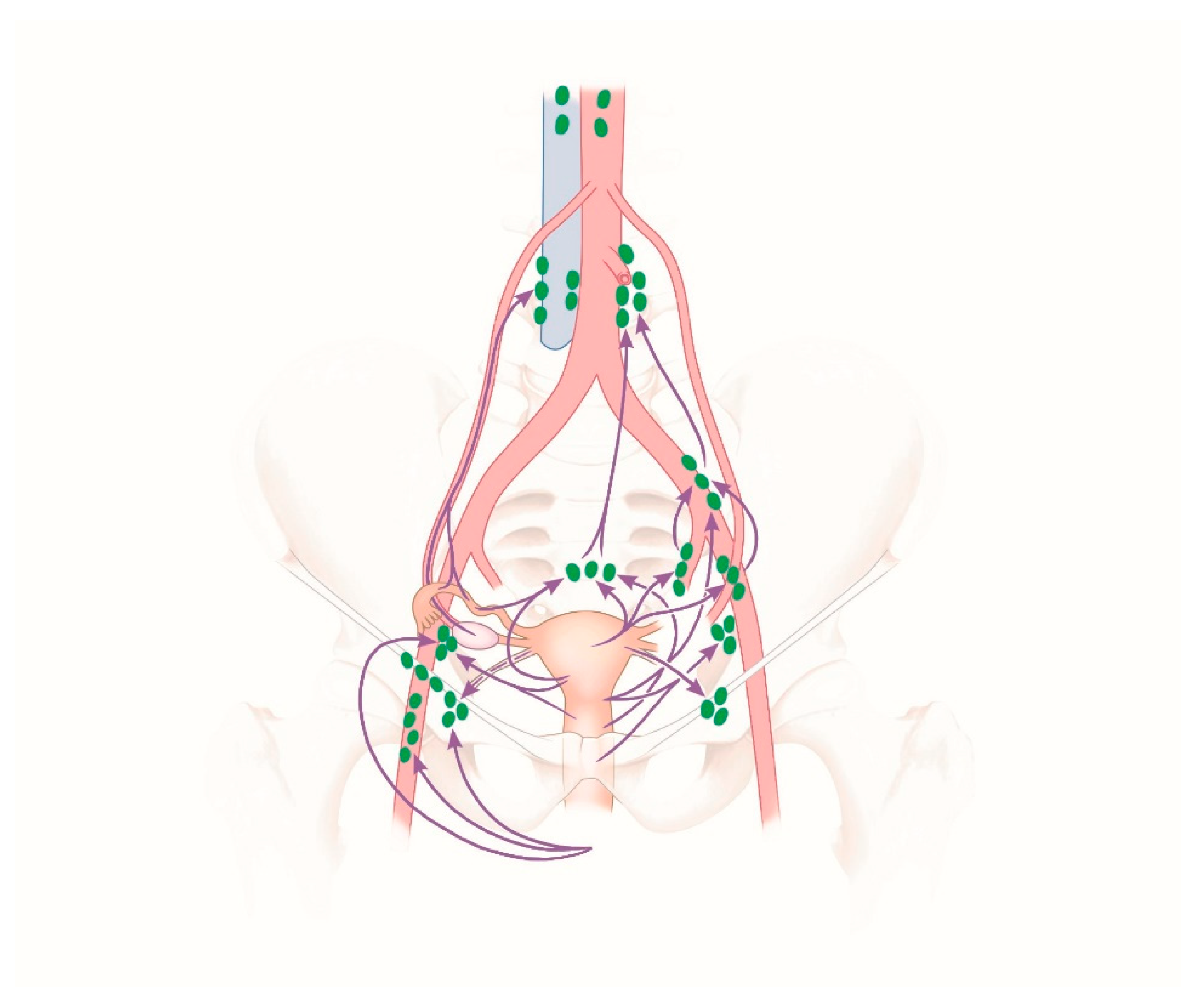
2.1. Lymphatic Drainage and Blood Supply to the Uterus and Uterine Adnexa
2.2. Blood Supply
2.3. Venous Blood Flow
2.4. Lymphatic Drainage
2.5. Embryologic Assessment of the Lymphatic Drainage in the Median Compartment
Embryonic Origins of Lymphatic Vessels
2.6. Anatomy-Based Methodology of Surgical Lymphadenectomy
2.7. Strategy of Lymphadenectomy
3. Surgical Staging in Endometrial Cancer
3.1. Systematic or Complete Pelvic and Para-Aortic Lymph Node Dissection
3.2. What Is the Role of Sentinel Lymph Nodes in Endometrial Cancer?
3.3. Therapeutic Pelvic and Para-Aortic Lymphadenectomy (tLNE)
4. Practical Aspects of Lymph Node Surgery
Technical Challenges in Endoscopic Surgery
- What is the most appropriate time to switch from the umbilical optical trocar to the suprasymphysiary trocar (perspective from above/below versus below/above)?
- What is the most suitable position for the working trocars with reference to the steps of surgery (perspective from above/below as well as below/above) so that the mutual angle of the instruments, as well as the mechanical actions of the surgeon, can be achieved smoothly and conventionally (i.e. not towards the surgeon but away from the surgeon)?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Althouse, A.D.; Freese, K.E.; Soisson, S.; Edwards, R.P.; Welburn, S.; Sukumvanich, P.; Comerci, J.; Kelley, J.; LaPorte, R.E.; et al. USA endometrial cancer projections to 2030: Should we be concerned? Future Oncol. 2014, 10, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Beller, U.; Benedet, J.L.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the corpus uteri. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), 105–143. [Google Scholar] [CrossRef]
- Benati, M.; Montagnana, M.; Danese, E.; Mazzon, M.; Paviati, E.; Garzon, S.; Laganà, A.S.; Casarin, J.; Giudici, S.; Raffaelli, R.; et al. Aberrant Telomere Length in Circulating Cell-Free DNA as Possible Blood Biomarker with High Diagnostic Performance in Endometrial Cancer. Pathol. Oncol. Res. 2020. [Google Scholar] [CrossRef]
- PDQ Adult Treatment Editorial Board. Endometrial cancer treatment (PDQ®): Health professional Version. 17 December 2019. In PDQ Cancer Information Summaries [Internet]; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Vitale, S.G.; Capriglione, S.; Zito, G.; Lopez, S.; Gulino, F.A.; Di Guardo, F.; Vitagliano, A.; Noventa, M.; La Rosa, V.L.; Sapia, F.; et al. Management of endometrial, ovarian and cervical cancer in the elderly: Current approach to a challenging condition. Arch. Gynecol. Obstet. 2019, 299, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Rossetti, D.; Tropea, A.; Biondi, A.; Laganà, A.S. Fertility sparing surgery for stage IA type I and G2 endometrial cancer in reproductive-aged patients: Evidence-based approach and future perspectives. Updates Surg. 2017, 69, 29–34. [Google Scholar] [CrossRef]
- Laganà, A.S.; La Rosa, V.L.; Rapisarda, A.M.; Vitale, S.G. Comment on: “Needs and priorities of women with endometrial and cervical cancer”. J. Psychosom. Obstet. Gynecol. 2017, 38, 85–86. [Google Scholar] [CrossRef]
- Papadia, A.; Garbade, A.; Gasparri, M.L.; Wang, J.; Radan, A.P.; Mueller, M.D. Minimally invasive surgery does not impair overall survival in stage IIIC endometrial cancer patients. Arch. Gynecol. Obstet. 2020, 301, 585–590. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Spiegel, G.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009, 27, 5331–5336. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO- ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Cignini, P.; Vitale, S.G.; Laganà, A.S.; Biondi, A.; La Rosa, V.L.; Cutillo, G. Preoperative work-up for definition of lymph node risk involvement in early stage endometrial cancer: 5-year follow-up. Updates Surg. 2017, 69, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Panici, P.B.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- ASTEC Study Group; Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Caserta, D.; Benedetti Panici, P.; Papadia, A.; Mueller, M.D. Surgical staging in endometrial cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 213–221. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Kraetschell, R.; Dowdy, S.; Fujiwara, K.; Yaegashi, N.; Larusso, D.; Casado, A.; Mahner, S.; Herzog, T.J.; Kehoe, S.; et al. Surgical and systemic management of endometrial cancer: An international survey. Arch. Gynecol. Obstet. 2015, 291, 897–905. [Google Scholar] [CrossRef]
- Obermair, A.; Abu-Rustum, N.R. Sentinel lymph node mapping in endometrial cancer—Areas where further research is needed. Int. J. Gynecol. Cancer 2020, 30, 283–284. [Google Scholar] [CrossRef]
- Wedel, T. Topographical Anatomy for Hysterectomy Procedures; Alkatout, I., Mettler, L., Eds.; Hysterectomy; Springer International Publishing: Cham, Switzerland, 2018; Volume 1, pp. 37–60. [Google Scholar]
- Schünke, K.; Schulte, E.; Schumacher, U. Prometheus Atlas of Anatomy; Thieme Publisher: Stuttgart, Germany, 2005; pp. 234, 262, 284, 290. [Google Scholar]
- Ribatti, D.; Crivellato, E. The embryonic origins of lymphatic vessels: An historical review. Br. J. Haematol. 2010, 149, 669–674. [Google Scholar] [CrossRef]
- Benninghoff, A.; Drenckhahn, D. Anatomy; Elsevier Publisher: München, Germany, 2004; Volume 16, pp. 168–184. [Google Scholar]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60, 2035–2041. [Google Scholar] [CrossRef]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009. [Google Scholar] [CrossRef]
- Franchi, M.; Garzon, S.; Zorzato, P.C.; Laganà, A.S.; Casarin, J.; Locantore, L.; Raffaelli, R.; Ghezzi, F. PET-CT scan in the preoperative workup of early stage intermediate- and high-risk endometrial cancer. Minim. Invasive Ther. Allied Technol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Selman, T.J.; Mann, C.H.; Zamora, J.; Khan, K.S. A systematic review of tests for lymph node status in primary endometrial cancer. BMC Womens Health 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G.; Gynecologic Oncology Group. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Todo, Y.; Kato, H.; Kaneuchi, M.; Watari, H.; Takeda, M.; Sakuragi, N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): A retrospective cohort analysis. Lancet 2010, 375, 1165–1172. [Google Scholar] [CrossRef]
- Bogani, G.; Dowdy, S.C.; Cliby, W.A.; Ghezzi, F.; Rossetti, D.; Mariani, A. Role of pelvic and para-aortic lymphadenectomy in endometrial cancer: Current evidence. J. Obstet. Gynaecol. Res. 2014, 40, 301–311. [Google Scholar] [CrossRef]
- Jung, U.S.; Choi, J.S.; Bae, J.; Lee, W.M.; Eom, J.M. Systemic Laparoscopic Para-Aortic Lymphadenectomy to the Left Renal Vein. JSLS 2019, 23, e2018.00110. [Google Scholar] [CrossRef]
- Mariani, A.; Dowdy, S.C.; Cliby, W.A.; Gostout, B.S.; Jones, M.B.; Wilson, T.O.; Podratz, K.C. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol. Oncol. 2008, 109, 11–18. [Google Scholar] [CrossRef]
- Pomel, C.; Naik, R.; Martinez, A. Systematic (complete) para-aortic lymphadenectomy: Description of a novel surgical classification with technical and anatomical considerations. BJOG 2012, 119, 249–253. [Google Scholar] [CrossRef]
- Lutman, C.V.; Havrilesky, L.J.; Cragun, J.M.; Secord, A.A.; Calingaert, B.; Berchuck, A.; Clarke-Pearson, D.L.; Soper, J.T. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol. Oncol. 2006, 102, 92–97. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Iasonos, A.; Zhou, Q.; Oke, E.; Soslow, R.A.; Alektiar, K.M.; Chi, D.S.; Barakat, R.R. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am. J. Obstet. Gynecol. 2008, 198. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Jeffrey Lowery, W.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.F.; Giuliano, A.E.; Veronesi, U.; Consensus Conference Committee. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast April 19 to 22, 2001, Philadelphia, Pennsylvania. Hum. Pathol. 2002, 33, 579–589. [Google Scholar] [CrossRef]
- Lecuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Daraï, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: Results of the SENTICOL study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef]
- How, J.; Boldeanu, I.; Lau, S.; Salvador, S.; How, E.; Gotlieb, R.; Abitbol, J.; Halder, A.; Amajoud, Z.; Probst, S.; et al. Unexpected locations of sentinel lymph nodes in endometrial cancer. Gynecol. Oncol. 2017, 147, 18–23. [Google Scholar] [CrossRef]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 170–199. [Google Scholar] [CrossRef]
- Geppert, B.; Lönnerfors, C.; Bollino, M.; Arechvo, A.; Persson, J. A study on uterine lymphatic anatomy for standardization of pelvic sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2017, 145, 256–261. [Google Scholar] [CrossRef]
- Holloway, R.W.; Gupta, S.; Stavitzski, N.M.; Zhu, X.; Takimoto, E.L.; Gubbi, A.; Bigsby, G.E.; Brudie, L.A.; Kendrick, J.E.; Ahmad, S. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar] [CrossRef]
- Bogani, G.; Murgia, F.; Ditto, A.; Raspagliesi, F. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2019, 153, 676–683. [Google Scholar] [CrossRef]
- Kimmig, R.; Aktas, B.; Buderath, P.; Rusch, P.; Heubner, M. Intraoperative navigation in robotically assisted compartmental surgery of uterine cancer by visualisation of embryologically derived lymphatic networks with indocyanine-green (ICG). J. Surg. Oncol. 2016, 113, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Freytag, D.; Mettler, L.; Maass, N.; Günther, V.; Alkatout, I. Uterine anomalies and endometriosis. Minerva Med. 2020, 111, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Quirke, P.; Durdey, P.; Dixon, M.F.; Williams, N.S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986, 996–999. [Google Scholar] [CrossRef]
- Höckel, M.; Horn, L.C.; Manthey, N.; Braumann, U.D.; Wolf, U.; Teichmann, G.; Frauenschläger, K.; Dornhöfer, N.; Einenkel, J. Resection of the embryologically defined uterovaginal (Müllerian) compartment and pelvic control in patients with cervical cancer: A prospective analysis. Lancet Oncol. 2009, 10, 683–692. [Google Scholar] [CrossRef]
- Höckel, M.; Schmidt, K.; Bornmann, K.; Horn, L.C.; Dornhöfer, N. Vulvar field resection: Novel approach to the surgical treatment of vulvar cancer based on ontogenetic anatomy. Gynecol. Oncol. 2010, 119, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Horn, L.C.; Illig, R.; Dornhöfer, N.; Fritsch, H. Ontogenetic anatomy of the distal vagina: Relevance for local tumor spread and implications for cancer surgery. Gynecol. Oncol. 2011, 122, 313–318. [Google Scholar] [CrossRef]
- Höckel, M.; Horn, L.C.; Tetsch, E.; Einenkel, J. Pattern analysis of regional spread and therapeutic lymph node dissection in cervical cancer based on ontogenetic anatomy. Gynecol. Oncol. 2012, 125, 168–174. [Google Scholar] [CrossRef]
- Kimmig, R.; Iannaccone, A.; Aktas, B.; Buderath, P.; Heubner, M. Embryologically based radical hysterectomy as peritoneal mesometrial resection (PMMR) with pelvic and para-aortic lymphadenectomy for loco-regional tumor control in endometrial cancer: First evidence for efficacy. Arch. Gynecol. Obstet. 2016, 294, 153–160. [Google Scholar] [CrossRef]
- Frumovitz, M.; Escobar, P.; Ramirez, P.T. Minimally invasive surgical approaches for patients with endometrial cancer. Clin. Obstet. Gynecol. 2011, 54, 226–234. [Google Scholar] [CrossRef]
- Alkatout, I. An atraumatic retractor for interdisciplinary use in conventional laparoscopy and robotic surgery. Minim. Invasive Ther. Allied Technol. 2018, 27, 265–271. [Google Scholar] [CrossRef]
- Alkatout, I.; Mettler, L. Hysterectomy A Comprehensive Surgical Approach. J. Turk. Ger. Gynecol. Assoc. 2017, 18, 221–223. [Google Scholar] [CrossRef] [PubMed]







| Radical/Complete or Systematic Lymphadenectomy | Excision of All Lymph Nodes with Surrounding Fatty Tissue along the Vascular Pathways Corresponding to Lymphatic Flow in the Targeted Anatomical Region |
|---|---|
| Sentinel lymph node biopsy | Excision of preoperatively marked sentinel lymph nodes as the primary filtering point of lymphatic flow to the organ and the tumor |
| Therapeutic lymphadenectomy | Radical lymphadenectomy within the limits of embryonic anatomical development (as established by Michael Höckel) |
| Lymph node debulking | Reduction of tumor burden by the excision of enlarged lymph nodes in an advanced stage of cancer |
| Lymph node sampling | Unsystematic excision of separate, clinically unusual lymph nodes |
| Tracer Characteristics | ICG | Blue Dyes | Tc-99m |
|---|---|---|---|
| Injection | intraoperative | intraoperative | preoperative, including lymphoscintigraphy/SPECT |
| Signal duration | persistent | 30 min | 24 h |
| Costs | Low | Low | high |
| Allergic reactions | 0.05% | 2% | 1–6/100,000 |
| Other toxicity | None | color change of skin and urine, skin necrosis | radioactivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freytag, D.; Pape, J.; Dhanawat, J.; Günther, V.; Maass, N.; Gitas, G.; Laganà, A.S.; Allahqoli, L.; Meinhold-Heerlein, I.; Moawad, G.N.; et al. Challenges Posed by Embryonic and Anatomical Factors in Systematic Lymphadenectomy for Endometrial Cancer. J. Clin. Med. 2020, 9, 4107. https://doi.org/10.3390/jcm9124107
Freytag D, Pape J, Dhanawat J, Günther V, Maass N, Gitas G, Laganà AS, Allahqoli L, Meinhold-Heerlein I, Moawad GN, et al. Challenges Posed by Embryonic and Anatomical Factors in Systematic Lymphadenectomy for Endometrial Cancer. Journal of Clinical Medicine. 2020; 9(12):4107. https://doi.org/10.3390/jcm9124107
Chicago/Turabian StyleFreytag, Damaris, Julian Pape, Juhi Dhanawat, Veronika Günther, Nicolai Maass, Georgios Gitas, Antonio Simone Laganà, Leila Allahqoli, Ivo Meinhold-Heerlein, Gaby N. Moawad, and et al. 2020. "Challenges Posed by Embryonic and Anatomical Factors in Systematic Lymphadenectomy for Endometrial Cancer" Journal of Clinical Medicine 9, no. 12: 4107. https://doi.org/10.3390/jcm9124107
APA StyleFreytag, D., Pape, J., Dhanawat, J., Günther, V., Maass, N., Gitas, G., Laganà, A. S., Allahqoli, L., Meinhold-Heerlein, I., Moawad, G. N., Biebl, M., Mettler, L., & Alkatout, I. (2020). Challenges Posed by Embryonic and Anatomical Factors in Systematic Lymphadenectomy for Endometrial Cancer. Journal of Clinical Medicine, 9(12), 4107. https://doi.org/10.3390/jcm9124107








