Comparisons of the Nonlinear Relationship of Cerebral Blood Flow Response and Cerebral Vasomotor Reactivity to Carbon Dioxide under Hyperventilation between Postural Orthostatic Tachycardia Syndrome Patients and Healthy Subjects
Abstract
:1. Introduction
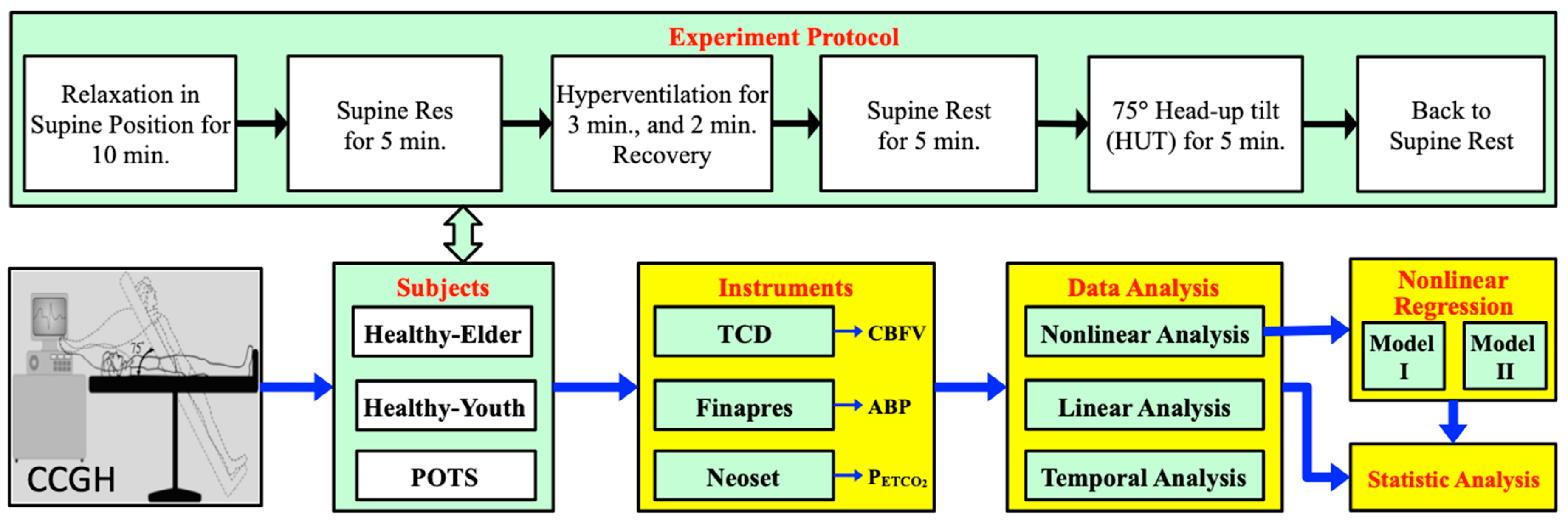
2. Materials and Methods
2.1. Subjects
2.2. Apparatus
2.3. Experiment Protocol
- First, baseline data of subjects at supine rest were recorded for 5 min after 10 min of relaxation.
- Then, the subject underwent voluntary hyperventilation in the supine position, with the breathing pattern of inhalation and exhalation for 1 s each.
- After 3 min of hyperventilation, the subject was allowed to breathe normally for 2 min.
- After 5 min of supine rest, the subject was tilted head-up by 75° for 10 min while breathing normally.
- At the end of the HUT, the subject was then returned to the supine resting position.
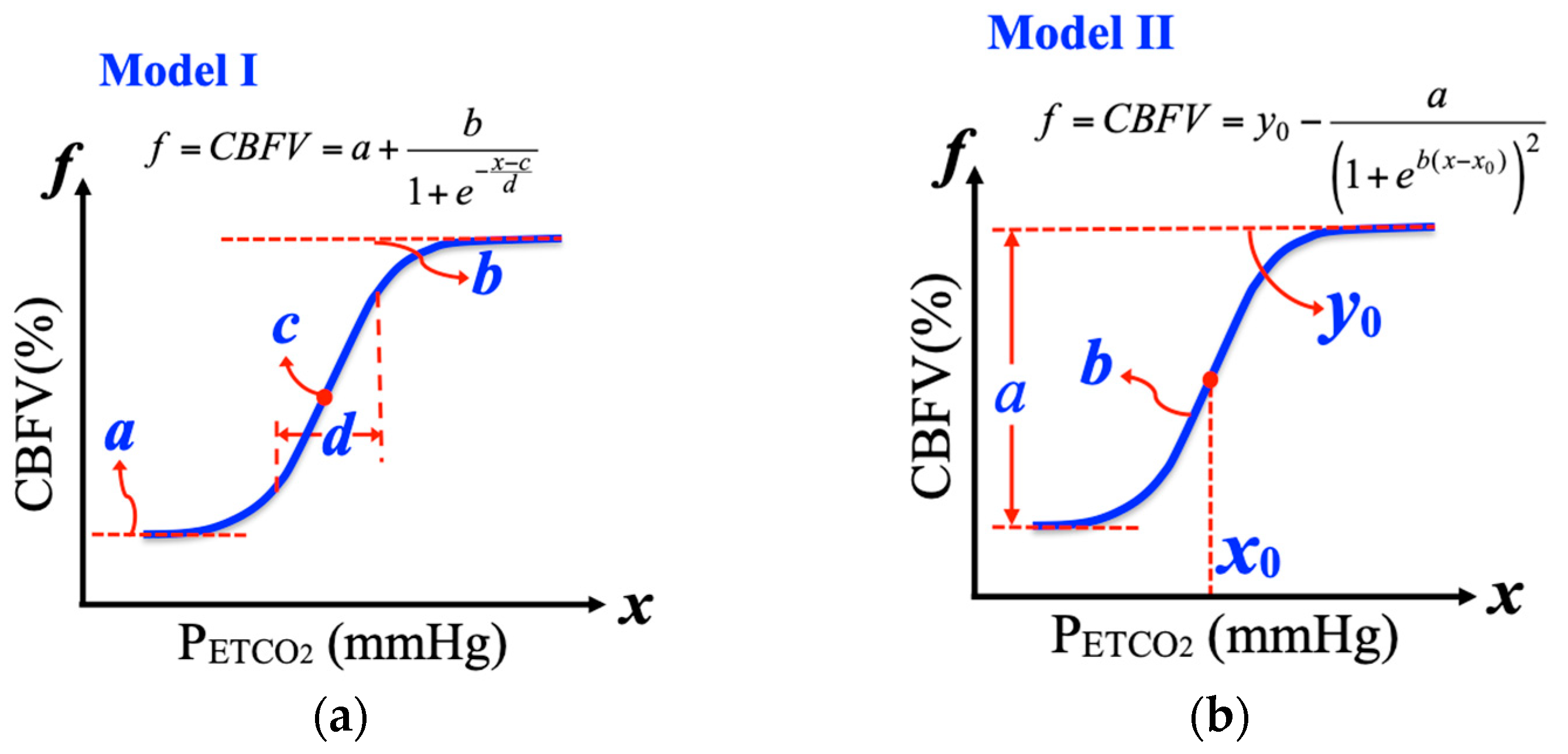
2.4. Nonlinear Regressing Models for Cerebrovascular Responses
2.4.1. Logistic Function Model I
2.4.2. Logistic Function Model II
2.5. Statistical Analysis
3. Results
- Compared with the Healthy-Youth group, the POTS group was significantly different in terms of:
- During supine (rest)—PETCO2, MHR, MSBP, MABP, and MBR.
- During hyperventilation —MSBP and MABP.
- During tilt-up—PETCO2, MHR, MSBP, and MABP.
- Compared with the Healthy-Elder group, the POTS group was significantly different in terms of:
- During supine (rest)—MHR, MSBP, MABP, and MCBFV.
- During hyperventilation—MHR, MSBP, MABP, MBR, and MCBFV.
- During tilt-up—MSBP.
- Compared with its own baseline data, the POTS group was significantly different in terms of:
- During hyperventilation —PETCO2, MBR, and MCBFV.
- During tilt-up—PETCO2, MHR, and MCBFV.
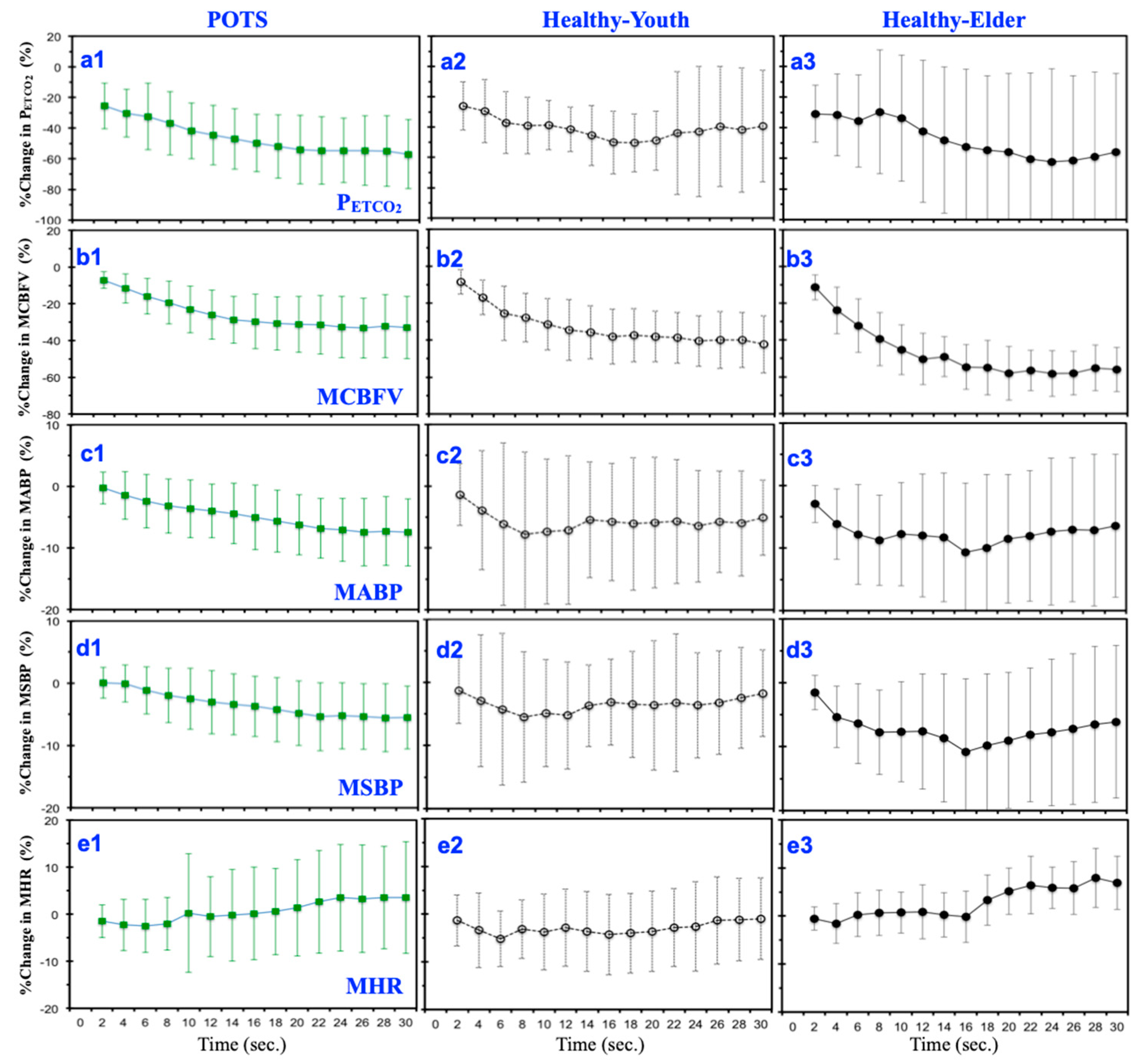
3.1. Mean Temporal Responses under Hyperventilation
3.2. Linear Responses of Cardio-Respiratory Signals to Carbon Dioxide under Hyperventilation
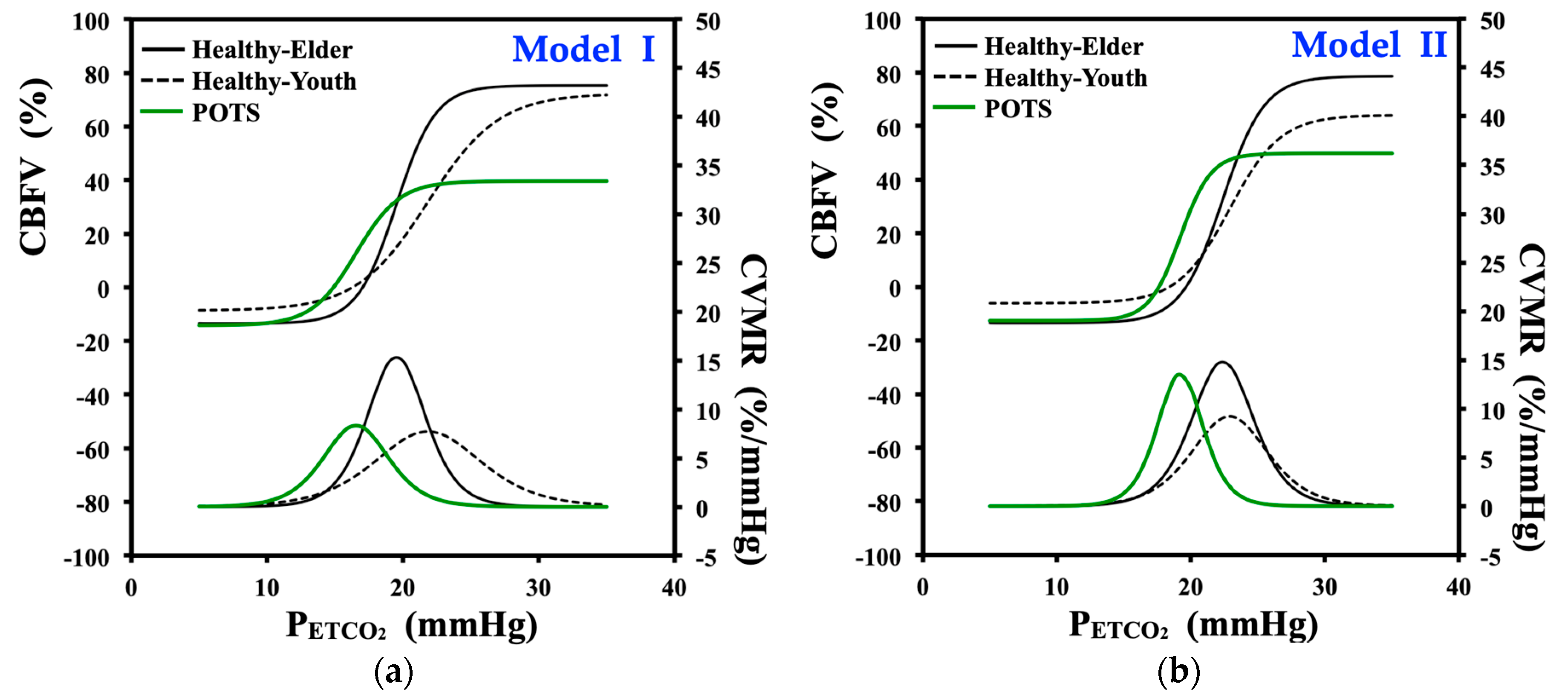
3.3. Nonlinear Regression of Cerebrovascular Response to Carbon Dioxide under Hyperventilation
3.3.1. Curve-Fitting and Model Parameters of Logistic Function Model I
3.3.2. Curve-Fitting and Model Parameters of Logistic Function Model II
3.3.3. Statistical Analysis of Fit Parameters for Models I and II
4. Discussion
4.1. Temporal Responses under Hyperventilation
4.2. Linear Responses of Cardiorespiratory Signals to CO2 under Hyperventilation
4.3. Nonlinear Curve-Fitting and Model Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Low, P.A.; Sandroni, P.; Joyner, M.; Shen, W.K. Postural Tachycardia Syndrome (POTS). J. Cardiovasc. Electrophysiol. 2009, 20, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Parkinson’s Disease Information Page. Available online: https://www.ninds.nih.gov/Disorders/All-Disorders/Parkinsons-Disease-Information-Page (accessed on 13 November 2020).
- Vinik, A.I.; Maser, R.E.; Mitchell, B.D.; Freeman, R. Diabetic autonomic neuropathy. Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [Green Version]
- Schondorf, R.; Low, P.A. Idiopathic postural orthostatic tachycardia syndrome: An attenuated form of acute pandysautonomia? Neurology 1993, 43, 132–137. [Google Scholar] [CrossRef]
- Novak, V.; Novak, P.; Opfer-Gehrking, T.L.; O’Brien, P.C.; Low, P.A. Clinical and laboratory indices that enhance the diagnosis of postural tachycardia syndrome. Mayo Clin. Proc. 1998, 73, 1141–1150. [Google Scholar] [CrossRef]
- Stewart, J.M.; Del Pozzi, A.T.; Pandey, A.; Messer, Z.R.; Terilli, C.; Medow, M.S. Oscillatory cerebral blood flow is associated with impaired neurocognition and functional hyperemia in postural tachycardia syndrome during graded tilt. Hypertension 2015, 65, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Ainslie, N.; Burgess, K.; Subedi, P.; Burgess, K.R. Alterations in cerebral dynamics at high altitude following partial acclimatization in humans: Wakefulness and sleep. J. Appl. Physiol. 2007, 102, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Ringelstein, E.B.; Sievers, C.; Ecker, S.; Schneider, P.A.; Otis, S.M. Non-invasive assessment of CO2-induced cerebral vasomotor reactivity in normal and patients with internal carotid artery occlusions. Stroke 1988, 19, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Claassen, J.A.H.R.; Zhang, R.; Fu, Q.; Witkowski, S.; Levine, B.D. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J. Appl. Physiol. 2007, 102, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Serrador, J.M.; Sorond, F.A.; Vyas, M.; Gagnon, M.; Iloputaife, I.D.; Lipsitz, L.A. Cerebral pressure-flow relations in hypertensive elderly humans: Transfer gain in different frequency domains. J. Appl. Physiol. 2005, 98, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Volpe, M. Hypertension, stroke, and endothelium. Curr. Hypertens. Rep. 2005, 7, 68–71. [Google Scholar] [CrossRef]
- Wijnhoud, A.D.; Koudstaal, P.J.; Dippel, D.W. Relationships of transcranial blood flow Doppler parameters with major vascular risk factors: TCD study in patients with a recent TIA or nondisabling ischemic stroke. J. Clin. Ultrasound 2006, 34, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Skatrud, J.B.; Khayat, R.; Dempsey, J.A.; Morgan, B.; Russell, D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am. J. Respir. Crit. Care Med. 2005, 172, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zuckerman, J.H.; Levine, B.D. Spontaneous fluctuations incerebral blood flow: Insights from extended-duration recordings in humans. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1848–H1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IDE, K.; Eliasziw, M.; Poulin, M.J. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range. J. Appl. Physiol. 2003, 95, 129–137. [Google Scholar] [CrossRef]
- Mitsis, G.D.; Harvey, A.K.; Dirckx, S.; Mayhew, S.D.; Rogers, R.; Tracey, I.; Wiseand, R.G.; Pattinson, K.T.S. Modeling of regional dynamic CO2 reactivity in respiratory related brain areas using BOLD fMRI. In Proceedings of the 8th IEEE International Conference on BioEngineering, Athens, Greece, 8–10 October 2008. [Google Scholar]
- Peebles, K.C.; Celi, L.; McGrattan, K.; Murrell, C.; Thomas, K.; Ainslie, P.N. Human cerebrovascular and ventilatory CO2 reactivity to end-tidal, arterial and internal jugular vein PCO2. J. Physiol. 2007, 584, 347–357. [Google Scholar] [CrossRef]
- Ogoh, S.; Ainslie, P.N.; Miyamoto, T. Onset responses of ventilation and cerebral blood flow to hypercapnia in humans: Rest and exercise. J. Appl. Physiol. 2009, 106, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Willie, C.K.; Macleod, D.B.; Shaw, A.D.; Smith, K.J.; Tzeng, Y.C.; Eves, N.D.; Ikeda, K.; Graham, J.; Lewis, N.C.; Day, T.A.; et al. Regional brain blood flow in man during acute changes in arterial blood gases. J. Physiol. 2012, 14, 3261–3275. [Google Scholar] [CrossRef] [Green Version]
- Battisti-Charbonney, A.; Fisher, J.; Duffin, J. The cerebrovascular response to carbon dioxide in humans. J. Physiol. 2011, 589, 3039–3048. [Google Scholar] [CrossRef]
- Panerai, R.B.; Evans, D.H.; Naylor, A.R. Influence of arterial blood pressure on cerebrovascular reactivity. Stroke 1999, 30, 1293–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panerai, R.B.; Simpson, D.M.; Deverson, S.T.; Mahony, P.; Hayes, P.; Evans, D.H. Multivariate dynamic analysis of cerebral blood flow regulation in humans. IEEE Trans. Biomed. Eng. 2000, 47, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Topor, Z.L.; Hughson, R.L. A new two-breath technique for extracting the cerebrovascular response to arterial carbon dioxide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R853–R859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsis, G.D.; Ainslie, P.N.; Poulin, M.J.; Robbins, P.A.; Marmarelis, V.Z. Nonlinear modeling of the dynamic effects of arterial pressure and blood gas variations on cerebral blood flow in healthy humans. Adv. Exp. Med. Biol. 2004, 551, 259–265. [Google Scholar]
- Zadourian, A.; Doherty, T.A.; Swiatkiewicz, I.; Taub, P.R. Postural Orthostatic Tachycardia Syndrome: Prevalence, Pathophysiology, and Management. Drugs 2018, 78, 983–994. [Google Scholar] [CrossRef]
- Olshanskya, B.; Cannomb, D.; Fedorowskic, A.; Stewartd, J.; Gibbonse, C.; Suttonf, R.; Sheng, W.K.; Muldowneyf, J.; Chungh, T.H.; Feigofskyi, S.; et al. Postural Orthostatic Tachycardia Syndrome (POTS): A critical assessment. Prog. Cardiovasc. Dis. 2020, 63, 263–270. [Google Scholar] [CrossRef]
- Sato, S.; Fisher, J.P.; Seifert, T.; Overgaard, M.; Secher, N.H.; Ogoh, S. Blood flowin internal carotid and vertebral arteriesduring orthostatic stress. Exp. Physiol. 2012, l97, 1272–1280. [Google Scholar] [CrossRef]
- Lin, S.L.; Liao, A.Y.C.; Yeh, S.J. The Cardiovascular and Respiratory Responses to CO2 under Hyperventilation and Posture Change in Parkinson’s Patients. J. Med. Bioeng. 2015, 4, 350–356. [Google Scholar] [CrossRef]
- Lin, S.L.; Liao, A.Y.C.; Yeh, S.J.; Lin, J.Y. The analysis of cardio-respiratory signals and cerebral autoregulation based on CO2 reactivity with healthy subjects and Parkinson’s patients. Tech. Health Care 2016, 24, S195–S203. [Google Scholar]
- Chiu, C.C.; Yeh, S.J. Assessment of cerebral autoregulation using time-domain cross-correlation analysis. Comput. Biol. Med. 2001, 31, 471–480. [Google Scholar] [CrossRef]
- Kent, B.B.; Drane, J.W.; Blumenstein, B.; Manning, J.W. A mathematical model to assess changes in the baroreceptor reflex. Cardiology 1972, 57, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Brugniaux, J.V.; Hodges, A.N.H.; Hanly, P.J.; Poulin, M.J. Cerebrovascular responses to altitude. Respir. Physiol. Neurobiol. 2007, 158, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.J.; Manktelow, B.N.; Panerai, R.B.; Potter, J.F. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 2001, 104, 898–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, B.J.; Panerai, R.B.; Potter, J.F. Effect of Aging on Dynamic Cerebral Autoregulation During Head-Up Tilt. Stroke 2003, 34, 1871–1875. [Google Scholar] [CrossRef]
- Dineen, N.E.; Panerai, R.B.; Brodie, F.; Robinson, T.G. Effects of ageing on cerebral haemodynamics assessed during respiratory manoeuvres. Age Aging 2011, 40, 199–204. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Tarumi, T.; Tseng, B.Y.; Palmer, D.M.; Levine, B.D.; Zhang, R. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J. Cereb. Blood Flow Metab. 2013, 33, 1190–1196. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.P.; Yezhuvath, U.S.; Tseng, B.Y.; Liu, P.; Levine, B.D.; Zhang, R.; Lu, H. Life-long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J. Magn. Reson. Imaging 2013, 38, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Xu, F.; Rodrigue, K.M.; Kennedy, K.M.; Cheng, Y.; Flicker, B.; Hebrank, A.C.; Uh, J.; Park, D.C. Alterations in Cerebral Metabolic Rate and Blood Supply across the Adult Lifespan. Cereb. Cortex 2011, 21, 1426–1434. [Google Scholar] [CrossRef] [Green Version]



| Groups | Subjects | Age | ||
|---|---|---|---|---|
| Gender | Number | Total | ||
| POTS | M | 27 | 60 | 32.31 ± 8.495 |
| F | 33 | |||
| Healthy-Youth | M | 4 | 13 | 29.3 ± 7.36 |
| F | 9 | |||
| Healthy-Elder | M | 8 | 10 | 56.5 ± 9.03 |
| F | 2 | |||
| Position | Subjects | PETCO2 (mmHg) | MHR (Beat/min) | MSBP (mmHg) | MABP (mmHg) | MBR (Breath/min) | MCBFV (cm/s) |
|---|---|---|---|---|---|---|---|
| Supine (rest) | POTS | 26.86 ± 3.50 † | 74.96 ± 11.38 †‡ | 104.37 ± 14.12 †‡ | 77.96 ± 9.76 †‡ | 15.39 ± 3.85 † | 55.65 ± 14.01 ‡ |
| Healthy-Youth | 30.84 ± 2.70 ‡ | 65.80 ± 8.56 | 123.75 ± 11.44 | 84.57 ± 8.97 | 17.84 ± 2.44 ‡ | 49.67 ± 15.28 ‡ | |
| Healthy-Elder | 28.03 ± 3.55 † | 64.31 ± 9.30 | 121.25 ± 8.22 | 88.45 ± 8.88 | 16.14 ± 2.26 † | 39.00 ± 11.39 † | |
| Supine (hyperventilation) | POTS | 11.38 ± 2.70 ✶ | 71.77 ± 14.47 | 105.39 ± 15.80 †‡ | 79.20 ± 10.94 †‡ | 33.26 ± 4.10 ‡✶ | 40.98 ± 13.46 ‡✶ |
| Healthy-Youth | 13.31 ± 3.51 ‡✶ | 68.43 ± 8.27 | 125.24 ± 13.35 | 83.88 ± 8.97 ‡ | 30.10 ± 4.18 ✶ | 36.13 ± 13.88 ‡✶ | |
| Healthy-Elder | 9.81 ± 4.07 † | 73.49 ± 15.2 †✶ | 125.41 ± 13.33 | 91.32 ± 10.29 †✶ | 29.63 ± 4.40 | 24.37 ± 9.64 † | |
| Tilt up | POTS | 24.67 ± 4.11 †✶ | 85.13 ± 18.27 †‡✶ | 102.99 ± 17.88 †‡ | 79.14 ± 12.45 † | 15.68 ± 5.60 | 46.96 ± 13.68 ✶ |
| Healthy-Youth | 28.20 ± 3.30 ✶ | 71.94 ± 9.57 ✶ | 133.93 ± 16.56 ✶ | 96.95 ± 14.63 | 16.93 ± 2.41 ✶ | 45.51 ± 13.37 ✶ | |
| Healthy-Elder | 25.03 ± 4.71 ✶ | 68.20 ± 7.58 ✶ | 130.39 ± 19.17 | 95.67 ± 10.93 ✶ | 17.44 ± 3.15 | 38.68 ± 10.16 ✶ |
| Parameters | POTS | Healthy-Youth | Healthy-Elder | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | CV% | Mean | (SD) | CV% | Mean | (SD) | CV% | |
| a, CBFVmin (%) | −14.02 | ±14.11 | −100.65 | −8.66 | ±18.13 | −209.4 | −13.48 | ±13.75 | −101.97 |
| b, CBFVmax (%) | 52.53 | ±31.09 | 59.18 | 80.95 | ±28.80 | 35.58 | 88.77 | ±16.51 | 18.60 |
| c, mid-PETCO2 (mmHg) | 16.38 | ±3.48 | 21.24 | 21.83 | ±5.84 | 26.76 | 19.52 | ±7.58 | 38.05 |
| d, PETCO2 range (mmHg) | 1.47 | ±1.18 | 80.24 | 2.62 | ±1.92 | 73.36 | 1.443 | ±1.447 | 100.27 |
| CVMRmax (%·mmHg−1) | 12.65 | ±8.10 | 64.08 | 10.92 | ±6.92 | 63.39 | 31.00 | ±22.74 | 73.33 |
| Parameters | POTS | Healthy-Youth | Healthy-Elder | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | CV% | Mean | (SD) | CV% | Mean | (SD) | CV% | |
| a, range of change (%) | 62.42 | ±31.94 | 31.35 | 69.89 | ±39.38 | 36.84 | 92.05 | ±32.67 | 30.25 |
| y0, CBFVmax (%) | 49.8 | ±32.93 | 32.31 | 62.13 | ±38.66 | 36.16 | 78.52 | ±28.65 | 26.52 |
| x0, PETCO2 level (mmHg) | 19.2 | ±4.83 | 4.74 | 23.27 | ±4.58 | 4.28 | 22.37 | ±5.29 | 4.9 |
| b, curvilinear (mmHg−1) | 0.87 | ±0.54 | 0.53 | 0.47 | ±0.16 | 0.15 | 0.64 | ±0.59 | 0.55 |
| CVMRmax (%·mmHg−1) | 12.49 | ±9.18 | 9.01 | 7.67 | ±3.67 | 3.43 | 12.41 | ±8.47 | 7.84 |
| Regression Model | Group Comparison | Fit Parameters | ||||
|---|---|---|---|---|---|---|
| a | b | c | d | CVMRmax | ||
| CBFVmin(%) | CBFVmax (%) | mid-PETCO2 | PETCO2 Range | |||
| I | POTS vs. Healthy-Youth | p = 0.057 | p = 0.011 ✶ | p = 0.013 ✶ | p = 0.023 ✶ | p = 0.697 |
| POTS vs. Healthy-Elder | p = 0.716 | p = 0.003 ✶ | p = 0.741 | p = 0.530 | p = 0.038 ✶ | |
| Regression Model | Group Comparison | Fit Parameters | ||||
|---|---|---|---|---|---|---|
| a | b | x0 | y0 | CVMRmax | ||
| Range of Change | Curvilinear | PETCO2 Level | CBFVmax | |||
| II | POTS vs. Healthy-Youth | p = 0.695 | p = 0.036 ✶ | p = 0.033 ✶ | p = 0.466 | p = 0.251 |
| POTS vs. Healthy-Elder | p = 0.042 ✶ | p = 0.053 | p = 0.033 ✶ | p = 0.033 ✶ | p = 0.676 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-L.; Yeh, S.-J.; Chen, C.-K.; Hsu, Y.-L.; Kuo, C.-E.; Chen, W.-Y.; Hsieh, C.-P. Comparisons of the Nonlinear Relationship of Cerebral Blood Flow Response and Cerebral Vasomotor Reactivity to Carbon Dioxide under Hyperventilation between Postural Orthostatic Tachycardia Syndrome Patients and Healthy Subjects. J. Clin. Med. 2020, 9, 4088. https://doi.org/10.3390/jcm9124088
Lin S-L, Yeh S-J, Chen C-K, Hsu Y-L, Kuo C-E, Chen W-Y, Hsieh C-P. Comparisons of the Nonlinear Relationship of Cerebral Blood Flow Response and Cerebral Vasomotor Reactivity to Carbon Dioxide under Hyperventilation between Postural Orthostatic Tachycardia Syndrome Patients and Healthy Subjects. Journal of Clinical Medicine. 2020; 9(12):4088. https://doi.org/10.3390/jcm9124088
Chicago/Turabian StyleLin, Shyan-Lung, Shoou-Jeng Yeh, Ching-Kun Chen, Yu-Liang Hsu, Chih-En Kuo, Wei-Yu Chen, and Cheng-Pu Hsieh. 2020. "Comparisons of the Nonlinear Relationship of Cerebral Blood Flow Response and Cerebral Vasomotor Reactivity to Carbon Dioxide under Hyperventilation between Postural Orthostatic Tachycardia Syndrome Patients and Healthy Subjects" Journal of Clinical Medicine 9, no. 12: 4088. https://doi.org/10.3390/jcm9124088
APA StyleLin, S.-L., Yeh, S.-J., Chen, C.-K., Hsu, Y.-L., Kuo, C.-E., Chen, W.-Y., & Hsieh, C.-P. (2020). Comparisons of the Nonlinear Relationship of Cerebral Blood Flow Response and Cerebral Vasomotor Reactivity to Carbon Dioxide under Hyperventilation between Postural Orthostatic Tachycardia Syndrome Patients and Healthy Subjects. Journal of Clinical Medicine, 9(12), 4088. https://doi.org/10.3390/jcm9124088






