The Retinal Vessel Density as a New Vascular Biomarker in Multisystem Involvement in Fabry Disease: An Optical Coherence Tomography Angiography Study
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Optical Coherence Tomography Angiography
2.3. Echocardiography
2.4. Statistical Analysis
3. Results
Univariate and Multivariate Associations
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Brady, R.O.; Gal, A.E.; Bradley, R.M.; Martensson, E.; Warshaw, A.L.; Laster, L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N. Engl. J. Med. 1967, 276, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Visciano, B.; Imbriaco, M.; Di Nuzzi, A.; Mancini, A.; Marchetiello, C.; Riccio, E. The kidney in Fabry’s disease. Clin. Genet. 2014, 86, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Linhart, A.; Lubanda, J.C.; Palecek, T.; Bultas, J.; Karetová, D.; Ledvinová, J.; Elleder, M.; Aschermann, M. Cardiac manifestations in Fabry disease. J. Inherit. Metab. Dis. 2001, 24 (Suppl. 2), 75–83. [Google Scholar] [CrossRef] [PubMed]
- Sivley, M.D. Fabry disease: A review of ophthalmic and systemic manifestations. Optom. Vis. Sci. 2013, 90, e63–e78. [Google Scholar] [CrossRef]
- Sodi, A.; Ioannidis, A.S.; Mehta, A.; Davey, C.; Beck, M.; Pitz, S. Ocular manifestations of Fabry’s disease: Data from the Fabry Outcome Survey. Br. J. Ophthalmol. 2007, 91, 210–214. [Google Scholar] [CrossRef]
- Acampa, W.; D’Antonio, A.; Imbriaco, M.; Pisani, A.; Cuocolo, A. Multimodality imaging approach to Fabry cardiomyopathy: Any role for nuclear cardiology? J. Nucl. Cardiol. 2020. [Google Scholar] [CrossRef]
- Capuano, I.; Garofalo, C.; Buonanno, P.; Pinelli, M.; Di Risi, T.; Feriozzi, S.; Riccio, E.; Pisani, A. Identifying Fabry patients in dialysis population: Prevalence of GLA mutations by renal clinic screening, 1995–2019. J. Nephrol. 2019, 33, 569–581. [Google Scholar] [CrossRef]
- Zarate, Y.A.; Hopkin, R.J. Fabry’s disease. Lancet 2008, 372, 1427–1435. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Gin, T.; Nicholls, K.; Low, M.; Galanos, J.; Crawford, A. Ophthalmological manifestations of Fabry disease: A survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin. Exp. Ophthalmol. 2005, 33, 164–168. [Google Scholar] [CrossRef]
- Libert, J.; Toussaint, D. Tortuosities of retinal and conjunctival vessels in lysosomial storage diseases. Birth Defects Orig. Artic. Ser. 1982, 18, 347–358. [Google Scholar]
- San Roman, I.; Rodrıguez, M.-E.; Caporossi, O.; Zoppetti, C.; Sodi, A.; Mecocci, A.; López, D.; Rodríguez, B.; Gimeno, J.R. Computer assisted retinal vessel tortuosity evaluation in novel mutation Fabry disease: Towards new prognostic markers. Retina 2017, 37, 592–603. [Google Scholar] [CrossRef]
- Michaud, L. Longitudinal study on ocular manifestations in a cohort of patients with Fabry disease. PLoS ONE 2019, 14, e0213329. [Google Scholar] [CrossRef] [PubMed]
- Lavia, C.; Bonnin, S.; Maule, M.; Erginay, A.; Tadayoni, R.; Gaudric, A. Vessel density of superficial, ıntermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 2019, 39, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Garrity, S.T.; Iafe, N.A.; Phasukkijwatana, N.; Chen, X.; Sarraf, D. Quantitative Analysis of Three Distinct Retinal Capillary Plexuses in Healthy Eyes Using Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5548–5555. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Di Maio, L.G.; Montorio, D.; Tranfa, F.; Russo, C.; Pontillo, G.; Cocozza, S.; Esposito, R.; Di Risi, T.; Imbriaco, M.; et al. Optical Coherence Tomography Angiography Findings in Fabry Disease. J. Clin. Med. 2019, 17, 528. [Google Scholar] [CrossRef]
- Minnella, A.M.; Barbano, L.; Verrecchia, E.; Martelli, F.; Pagliei, V.; Gambini, G.; Placidi, G.; Falsini, B.; Caporossi, A.; Manna, R. Macular Impairment in Fabry Disease: A Morpho-functional Assessment by Swept-Source OCT Angiography and Focal Electroretinography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2667–2675. [Google Scholar] [CrossRef]
- Cakmak, A.I.; Atalay, E.; Cankurtaran, V.; Yaşar, E.; Turgut, F.H. Optical coherence tomography angiography analysis of fabry disease. Int. Ophthalmol. 2020, 40, 3023–3032. [Google Scholar] [CrossRef]
- Esposito, R.; Galderisi, M.; Santoro, C.; Imbriaco, M.; Riccio, E.; Pellegrino, A.M.; Sorrentino, R.; Lembo, M.; Citro, R.; Losi, M.A.; et al. Prominent longitudinal strain reduction of left ventricular basal segments in treatment-naïve Anderson-Fabry disease patients. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 438–445. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.J.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef]
- Huang, D.; Jia, Y.; Gao, S.S.; Lumbroso, B.; Rispoli, M. Optical Coherence Tomography Angiography Using the Optovue Device. Dev. Ophthalmol. 2016, 56, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Esposito, R.; Santoro, C.; Sorrentino, R.; Riccio, E.; Citro, R.; Buonauro, A.; Di Risi, T.; Imbriaco, M.; Trimarco, B.; Pisani, A.; et al. Layer-specific longitudinal strain in Anderson-Fabry disease at diagnosis: A speckle tracking echocardiography analysis. Echocardiography 2019, 36, 1273–1281. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- Konz, K.H.; Haap, M.; Walsh, R.A.; Burk, R.F.; Seipel, L. Diastolic dysfunction as a precursor of myocardial damage by reoxygenation. Eur. Heart J. 1991, 12, 182–186. [Google Scholar] [CrossRef]
- Galderisi, M.; Rapacciuolo, A.; Esposito, R.; Versiero, M.; Schiano-Lomoriello, V.; Santoro, C.; Piscione, F.; de Simone, G. Site-dependency of the E/e’ ratio in predicting invasive left ventricular filling pressure in patients with suspected or ascertained coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 555–561. [Google Scholar] [CrossRef]
- Elleder, M.; Bradova, V.; Smid, F.; Budesinsky, M.; Harzer, K.; Kustermann-Kuhn, B.; Ledvinová, J.; Bĕlohlávek; Král, V.; Dorazilová, V. Cardiocyte storage and hypertrophy as a sole manifestation of Fabry’s disease: Report on a case simulating hypertrophic non-obstructive cardiomyopathy. Virchows Arch. A Pathol. Anat. Histopathol. 1990, 417, 449–455. [Google Scholar] [CrossRef]
- Linhart, A.; Palecek, T.; Bultas, J.; Ferguson, J.J.; Hrudová, J.; Karetová, D.; Zeman, J.; Ledvinová, J.; Poupetová, H.; Elleder, M.; et al. New insights in cardiac structural changes in patients with Fabry’s disease. Am. Heart J. 2000, 139, 1101–1108. [Google Scholar] [CrossRef]
- Liu, D.; Oder, D.; Salinger, T.; Hu, K.; Müntze, J.; Weidemann, F.; Herrmann, S.; Ertl, G.; Wanner, C.; Frantz, S.; et al. Association and diagnostic utility of diastolic dysfunction and myocardial fibrosis in patients with Fabry disease. Open Heart 2018, 5, e000803. [Google Scholar] [CrossRef]
- Elliott, P.M.; Kindler, H.; Shah, J.S.; Sachdev, B.; Rimoldi, O.E.; Thaman, R.; Tome, M.T.; McKenna, W.J.; Lee, P.; Camici, P.G. Coronary microvascular dysfunction in male patients with Anderson–Fabry disease and the effect of treatment with alpha galactosidase A. Heart 2006, 92, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Tomberli, B.; Cecchi, F.; Sciagrà, R.; Berti, V.; Lisi, F.; Torricelli, F.; Morrone, A.; Castelli, G.; Yacoub, M.H.; Olivotto, I. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson-Fabry disease. Eur. J. Heart Fail. 2013, 15, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Tøndel, C.; Bostad, L.; Larsen, K.; Hirth, A.; Vikse, B.E.; Houge, G.; Svarstad, E. Agalsidase benefits renal histology in young patients with Fabry disease. J. Am. Soc. Nephrol. 2013, 24, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Cocozza, S.; Russo, C.; Pontillo, G.; Pisani, A.; Brunetti, A. Neuroimaging in Fabry disease: Current knowledge and future directions. Insights Imaging 2018, 9, 1077–1088. [Google Scholar] [CrossRef]
- Cocozza, S.; Pontillo, G.; Quarantelli, M.; Saccà, F.; Riccio, E.; Costabile, T.; Olivo, G.; Brescia Morra, V.; Pisani, A.; Brunetti, A.; et al. Default mode network modifications in Fabry disease: A resting-state fMRI study with structural correlations. Hum. Brain Mapp. 2018, 39, 1755–1764. [Google Scholar] [CrossRef]
- Cocozza, S.; Schiavi, S.; Pontillo, G.; Battocchio, M.; Riccio, E.; Caccavallo, S.; Russo, C.; Di Risi, T.; Pisani, A.; Daducci, A.; et al. Microstructural damage of the cortico-striatal and thalamo-cortical fibers in Fabry disease: A diffusion MRI tractometry study. Neuroradiology 2020, 62, 1459–1466. [Google Scholar] [CrossRef]
- DeGraba, T.; Azhar, S.; Dignat-George, F.; Brown, E.; Boutière, B.; Altarescu, G.; McCarron, R.; Schimann, R. Profile of endothelial and leukocyte activation in Fabry patients. Ann. Neurol. 2000, 47, 229–233. [Google Scholar] [CrossRef]
- Ersoz, M.G.; Ture, G. Cilioretinal artery occlusion and anterior ischemic optic neuropathy as the initial presentation in a child female carrier of Fabry disease. Int. Ophthalmol. 2018, 38, 771–773. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wiseman, S.; Valdés-Hernández, M.C.; Doubal, F.N.; Dhillon, B.; Wu, Y.C.; Wardlaw, J.M. The Application of Optical Coherence Tomography Angiography in Cerebral Small Vessel Disease, Ischemic Stroke, and Dementia: A Systematic Review. Front. Neurol. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Hanff, T.C.; Richey Sharrett, A.; Mosley, T.H.; Shibata, D.; Knopman, D.S.; Klein, R.; Klein, B.E.K.; Gottesman, R.F. Retinal Microvascular Abnormalities Predict Progression of Brain Microvascular Disease. Stroke 2014, 45, 1012–1017. [Google Scholar] [CrossRef]
- Mitchell, P.; Wang, J.J.; Wong, T.Y.; Smith, W.; Klein, R.; Leeder, S.R. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology 2005, 65, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mosley, T.; Islam, A.; Kawasaki, R.; Sharrett, A.R.; Klein, R.; Coker, L.H.; Knopman, D.S.; Shibata, D.K.; Catellier, D.; et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: A prospective study. Brain 2010, 133, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, O.M.; Demaerschalk, B.M.; Sanchez, C.V.; Almader-Douglas, D.; O’Carroll, C.B.; Aguilar, M.I.; Lyden, P.D.; Kumar, G. Retinal Microvascular Abnormalities as Surrogate Markers of Cerebrovascular Ischemic Disease: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 1960–1968. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Carotenuto, A.; Montorio, D.; Petracca, M.; Moccia, M.; Melenzane, A.; Tranfa, F.; Lamberti, A.; Spiezia, A.L.; Servillo, G.; et al. Peripapillary Vessel Density as Early Biomarker in Multiple Sclerosis. Front. Neurol. 2020, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, C.; Cennamo, G.; Montorio, D.; Carotenuto, A.; Strianese, A.; Salvatore, E.; Tranfa, F.; Cennamo, G.; Lanzillo, R.; Morra, B.V. Assessment of retinal vascular network in amnestic mild cognitive impairment by optical coherence tomography angiography. PLoS ONE. 2020, 15, e0233975. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef]
| Variable | FD (n = 50) Mean ± SD (Range) |
|---|---|
| Gender (F/M) | 36/14 |
| Age (years) | 41.5 ± 15.3 (14–79) |
| BMI (kg/m2) | 25.8 ± 4.8 (16.8–38.9) |
| Systolic BP (mmHg) | 120.4 ± 17.5 (90–160) |
| Diastolic BP (mmHg) | 74.5 ± 10.3 (60–100) |
| Heart rate (bpm) Proteinuria (mg/24 h) GFR (mL/min/1.73 m2) | 67.5 ± 9.4 (47–92) 162.6 ± 144.7 (0–600) 101.3 ± 17.9 (55.0 ± 140) |
| Variable | aADF (n = 50) Mean ± SD (Range) |
|---|---|
| IVS thickness (cm) | 1.00 ± 0.33 (0.6–2.3) |
| LV mass index (g/h 2.7) | 43.9 ± 21.5 (23.1–124.9) |
| RWT | 0.42 ± 0.18 (0.22–1.24) |
| E/a ratio | 1.26 ± 0.50 (0.19–2.6) |
| E/e’ ratio | 8.3 ± 3.7 (1.21–16.8) |
| PAPs (mmHg) | 28.7 ± 6.4 (16–48) |
| LAVi (mL/m2) | 32.1 ± 9.7 (15–60) |
| LV EF (%) | 61.7 ± 5.5 (44–73) |
| LV GLS (%) | 19.4 ± 3.73 (11–28.2) |
| Dependent Variable | Covariate | Β Coefficient | p |
|---|---|---|---|
| SCP | E/e’ ratio | −0.32 | <0.03 |
| LAVi (mL/m2) | −0.48 | <0.001 | |
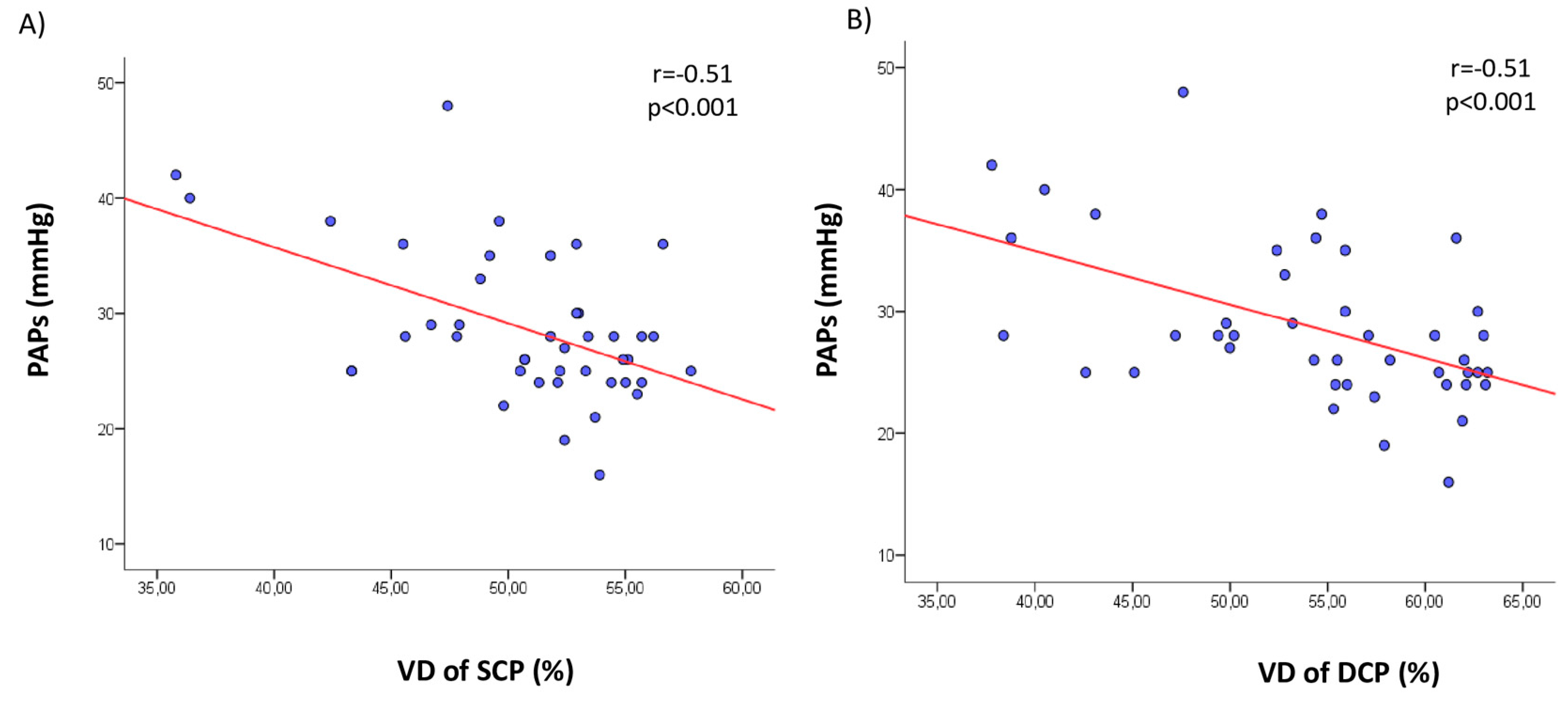
| PAPs (mmHg) | −0.51 | <0.0001 | |
| IVS thickness (cm) | −0.32 | <0.04 | |
| GLS (%) | 0.31 | <0.04 | |
| DCP | E/e’ ratio | −0.29 | <0.05 |
| LAVi (mL/m2) | −0.45 | <0.002 | |
| PAPs (mmHg) | −0.51 | <0.0001 | |
| IVS thickness (cm) | −0.34 | <0.03 | |
| GLS (%) | 0.34 | <0.02 |
| Dependent Variable | Covariate | Β Coefficient | p |
|---|---|---|---|
| SCP | Age (years) | −0.027 | 0.858 |
| IVS thickness (cm) | −0.301 | 0.213 | |
| GLS (%) | −0.151 | 0.535 | |
| PAPs (mmHg) | −0.553 | <0.001 | |
| DCP | Age (years) | −0.059 | 0.709 |
| IVS thickness (cm) | −0.164 | 0.514 | |
| GLS (%) | 0.018 | 0.945 | |
| PAPs (mmHg) | −0.474 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cennamo, G.; Montorio, D.; Santoro, C.; Cocozza, S.; Spinelli, L.; Di Risi, T.; Riccio, E.; Russo, C.; Pontillo, G.; Esposito, R.; et al. The Retinal Vessel Density as a New Vascular Biomarker in Multisystem Involvement in Fabry Disease: An Optical Coherence Tomography Angiography Study. J. Clin. Med. 2020, 9, 4087. https://doi.org/10.3390/jcm9124087
Cennamo G, Montorio D, Santoro C, Cocozza S, Spinelli L, Di Risi T, Riccio E, Russo C, Pontillo G, Esposito R, et al. The Retinal Vessel Density as a New Vascular Biomarker in Multisystem Involvement in Fabry Disease: An Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine. 2020; 9(12):4087. https://doi.org/10.3390/jcm9124087
Chicago/Turabian StyleCennamo, Gilda, Daniela Montorio, Ciro Santoro, Sirio Cocozza, Letizia Spinelli, Teodolinda Di Risi, Eleonora Riccio, Camilla Russo, Giuseppe Pontillo, Roberta Esposito, and et al. 2020. "The Retinal Vessel Density as a New Vascular Biomarker in Multisystem Involvement in Fabry Disease: An Optical Coherence Tomography Angiography Study" Journal of Clinical Medicine 9, no. 12: 4087. https://doi.org/10.3390/jcm9124087
APA StyleCennamo, G., Montorio, D., Santoro, C., Cocozza, S., Spinelli, L., Di Risi, T., Riccio, E., Russo, C., Pontillo, G., Esposito, R., Imbriaco, M., & Pisani, A. (2020). The Retinal Vessel Density as a New Vascular Biomarker in Multisystem Involvement in Fabry Disease: An Optical Coherence Tomography Angiography Study. Journal of Clinical Medicine, 9(12), 4087. https://doi.org/10.3390/jcm9124087







