1. Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a disease entity of unknown cause. The disease is characterized by the formation of mass lesions resembling tumor, thickening of various tissues, infiltration of immunoglobulin G4 (IgG4)-positive cells, and high serum IgG4 [
1,
2]. Although the concept of IgG4-RD as a systemic disease has been recognized relatively recently, the diseases that were clinically diagnosed as autoimmune pancreatitis in gastroenterology and Mikulicz disease in ophthalmology in the past have been revealed to be lgG4-RD [
1,
2]. Thereafter, the presence of similar masses or thickened lesions in various organs such as the hepatobiliary system, lung, kidney, prostate, and thyroid in addition to the pancreas, lacrimal gland (LG), and salivary glands (SG) was revealed, and comprehensive diagnostic criteria for IgG4-RD were reported in 2012 [
3,
4]. Moreover, other than LG and SG, some patients with IgG4-RD have enlargement of ocular adnexal tissues such as extraocular muscles, trigeminal nerve, and orbital fat. Ocular involvement is one of the most common factors of disability caused by IgG4-RD, and has been reported to affect nearly 12–65% of patients with IgG4-RD [
5,
6,
7,
8]. All the ocular manifestations are collectively called IgG4-related ophthalmic disease (IgG4-ROD), and the diagnostic criteria for IgG4-ROD were published in 2015 [
9]. Although the pathogenesis of IgG4-RD and IgG4-ROD is not completely understood, an infectious or an autoimmune or autoinflammatory etiology has been proposed [
10,
11,
12,
13]. Ophthalmologists have been aware that the clinical presentation and symptoms of IgG4-ROD vary. For example, some patients improve upon treatment with systemic steroids with no recurrence, while others do not respond to systemic steroids; some patients have more severe disease and systemic involvement, whereas others do not. Therefore, the fact that IgG4-ROD has diverse manifestations has driven interest in using unbiased approaches to better phenotype these patients in terms of essential clinical and laboratory features. Recently, elevations of serum immunoglobulin G2 and soluble interleukin-2 receptor (sIL-2R) in IgG4-RD have been reported [
14,
15]. However, biomarkers were identified based on the clinical features of IgG4-ROD.
Cluster analysis refers to statistical methods that attempt to discriminate relatively homogeneous groups of individuals based on selected characteristics. Recently, cluster analysis has been used to identify phenotypic groups within a disease for various diseases [
6,
16,
17]. To date, only one study of IgG4-RD including IgG4-ROD using cluster analysis has been reported [
6]. This study showed that the total dose of systemic steroids used in maintenance therapy was significantly greater in the cluster of patients with hypergammaglobulinemia, elevated serum IgG4 levels, and hypocomplementemia than in the cluster of patients with older onset and relatively low peripheral eosinophil count. Moreover, the cluster of patients with elder onset and relatively low peripheral eosinophil count had a low rate of relapse and often were able to discontinue steroids [
6]. To more comprehensively analyze IgG4-ROD, we used an unsupervised modeling method to study a relatively large number of conventional laboratory blood test data, aiming to identify clusters of individuals with IgG4-ROD and to evaluate the degree of phenotypic heterogeneity.
As IgG4-ROD is a heterogeneous disease with diverse clinical symptoms, the goal of IgG4-ROD classification is to improve prediction of the phenotype of an individual patient, so as to aid ophthalmologists in making a decision on treatment strategy (such as whether to prescribe systemic steroid therapy), ultimately achieving personalized or stratified care for IgG4-ROD.
3. Results
3.1. Patient Demographics and Number of Patients Having Abnormal Peripheral Blood Test
Patient demographics are shown in
Table 1. Frequencies of patients with abnormal peripheral blood tests are shown in
Table 2. The male/female ratio was also the same, and almost all patients with IgG4-ROD had abnormal serum IgG4 levels.
3.2. Unbiased Clustering Analysis Identifies Distinct Groups in IgG4-ROD
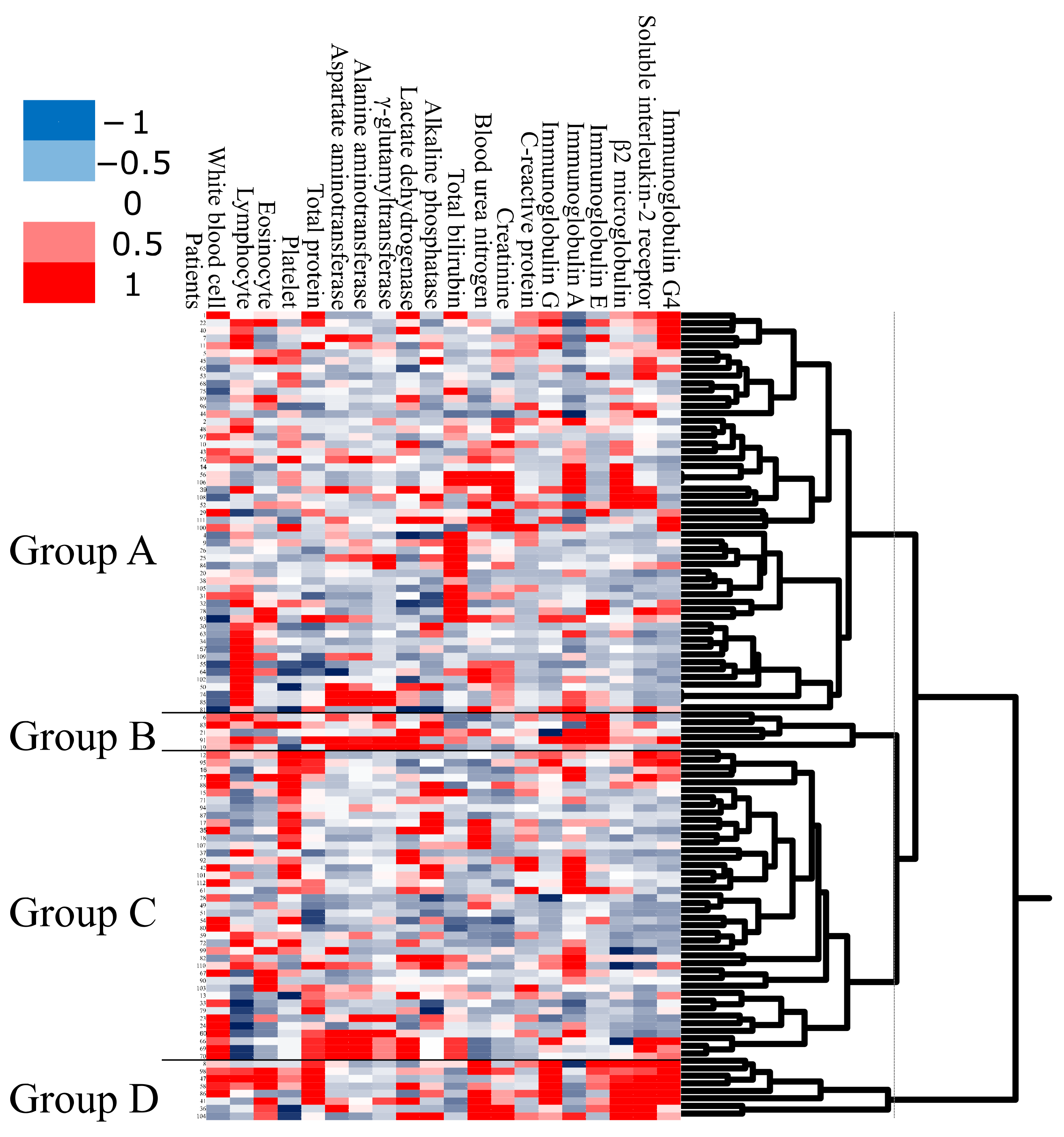
As the first step in the analysis, we applied unbiased clustering analysis to obtain a global overview of laboratory test data (
Figure 1). Using unbiased clustering analysis, patients were divided into four groups. Group A was the largest cluster (
n = 53; 50% of subjects), consisting of patients who had a low WBC count. Group B was the smallest cluster (
n = 5; 5% of subjects), consisting of patients with high serum IgA and high serum IgE. Group C consisted of 41 subjects (38%) with high platelet count. Group D consisted of 8 subjects (7%) with older onset, high serum IgG, high β2MG, high serum sIL-2R, and high serum IgG4.
We then compared the clinical features between groups A, B, C, and D. The clinical features in the four groups are shown in
Table 3. Group D had significantly older onset, higher serum IgG4, and higher frequency of worsened BCVA than the other groups (
p < 0.001,
p < 0.001, and
p = 0.002, respectively). Group B had significantly higher frequency of extraocular muscle enlargement than the other groups (
p < 0.001). Groups B and D had significantly higher frequency of lesion above neck excluding LG and SG than the other groups (
p = 0.03). No significant differences were observed among the four groups in other clinical features. These results indicated that peripheral blood test data may be useful for the prediction of the clinical course of IgG4-ROD. However, there were several issues of using 20 peripheral blood tests for such analysis, including cost and whether all the tests are necessary for all patients with IgG4-ROD. Therefore, we performed another clustering analysis to examine whether similar results can be obtained even when the peripheral blood test data were reduced to the minimum.
3.3. Condensed Unbiased Clustering Analysis with Reduced Data Identifies Four Distinct Groups in IgG4-ROD
We performed a condensed unbiased clustering analysis using a smaller number of selected peripheral blood test data. We excluded tests that were deemed unnecessary, costly, or had problems of complexity and reproducibility in clinical use. Peripheral blood tests that showed high frequencies of abnormality in patients with IgG4-ROD were selected for the condensed clustering analysis, as shown in
Table 2. The condensed unbiased clustering analysis divided patients into four groups: E, F, G, and H (
Figure S1). We then compared the clinical features between these four groups. Group E was the largest cluster (
n = 41; 38% of subjects), consisting of patients who had a high percent of Eo, high serum IgE, and high serum sIL-2R. Group F was the smallest cluster (
n = 5; 5% of subjects), consisting of patients who had a high percent of Eo, high serum IgG, high serum IgE, and high serum IgG4. Group G (
n = 32; 30% of subjects) consisted of patients who had a low WBC count. Group H (
n = 29; 27% of subjects) consisted of patients who had a low percent of Eo. Clinical features in the four groups divided by the condensed unbiased clustering analysis are shown in
Table S1.
Group F had a significantly longer follow-up period, higher serum IgG4, and higher frequency of worsened BCVA than the other groups (p = 0.04, p < 0.001, and p = 0.001, respectively). Group F tended to have higher frequencies of orbital mass, orbital diffuse lesion, optic neuropathy, and visual field defect compared with the other groups (p = 0.05, p = 0.05, p = 0.05, and p = 0.05, respectively). On the other hand, the Steel–Dwass test showed that group F had significantly high frequencies of orbital mass, orbital diffuse lesion, optic neuropathy, and visual field defect compared with group G (p = 0.02, p = 0.02, p = 0.02, and p = 0.02, respectively). Group E had a significantly higher frequency of extraocular muscle enlargement (p = 0.02). Other parameters were not significantly different.
This result also indicated that data of peripheral blood tests may be useful for the prediction of clinical course, especially worsened BCVA and extraocular muscle enlargement, in IgG4-ROD.
3.4. Comparison of Clinical Features and Laboratory Findings in Patients Divided into Two Groups Based on Specific Clinical Features
First, we compared the clinical features and laboratory findings between subjects divided into two groups based on extraocular muscle enlargement (
Table 4). Patients with extraocular muscle enlargement had significantly higher WBC count, Eo count, CRP, and serum IgE; higher frequencies of lesion excluding LG and SG and lesion above the neck (excluding LG and SG); high frequencies of enlargement of trigeminal nerve and extraocular muscle; a higher frequency of worsened BCVA; and a higher frequency of diplopia (
p = 0.01,
p = 0.01,
p = 0.04,
p = 0.01,
p = 0.01,
p = 0.001,
p = 0.007,
p < 0.001,
p = 0.01, and
p = 0.007, respectively). Other clinical features and laboratory findings were not significantly difference between the two groups. These results showed that patients with extraocular muscle enlargement had abnormal peripheral blood findings of elevated WBC count, Eo count, CRP, and serum IgE.
We then compared the clinical features and laboratory findings between subjects divided into two groups based on worsened BCVA (
Table 5). Patients with worsened BCVA had significantly older onset; higher serum IgG4, WBC count, serum TP, CRP, serum IgG, serum IgA, serum IgE, and β2MG; and higher frequencies of lesion above the neck excluding LG and SG, trigeminal nerve enlargement, extraocular muscle enlargement, orbital mass, orbital diffuse lesion, optic neuropathy, and visual field defect (
p = 0.01,
p < 0.001,
p = 0.003,
p = 0.004,
p = 0.004,
p = 0.02,
p = 0.03,
p = 0.04,
p = 0.002,
p = 0.02,
p = 0.01,
p < 0.001,
p = 0.006,
p = 0.006,
p < 0.001, and
p < 0.001, respectively).
Other clinical features and laboratory findings were not significantly different between the group with and that without worsened BCVA. These results showed that patients with worsened BCVA had abnormal peripheral blood findings of increased serum IgG4, WBC count, serum TP, CRP, serum IgG, serum IgA, serum IgE, and β2MG.
Next, we compared the clinical features and laboratory findings between subjects divided into two groups based on lesion above the neck excluding LG and SG (
Table 6). Patients with lesion above the neck excluding LG and SG had a significantly higher WBC count and higher frequencies of lesion excluding LG and SG, lesion above the neck (excluding LG and SG), lesion below the neck, enlargement of trigeminal nerve, extraocular muscle enlargement, orbital mass, orbital diffuse lesion, worsened BCVA, optic neuropathy, and visual field defect (
p = 0.01,
p < 0.001,
p < 0.001,
p < 0.001,
p < 0.001,
p < 0.001,
p = 0.001,
p = 0.001,
p < 0.001,
p < 0.001, and
p < 0.001, respectively).
Other clinical features and laboratory findings were not significantly different between the two groups. These results showed that patients with lesion above neck excluding LG and SG had abnormal peripheral blood finding of an elevated WBC count.
The results of this analysis indicate that routine laboratory findings may be useful for predicting extraocular muscle enlargement, worsened BCVA, and lesion above neck excluding LG and SG in IgG4-ROD.
3.5. ROC Curves for Optimal Peripheral Blood Data Predicting Clinical Features
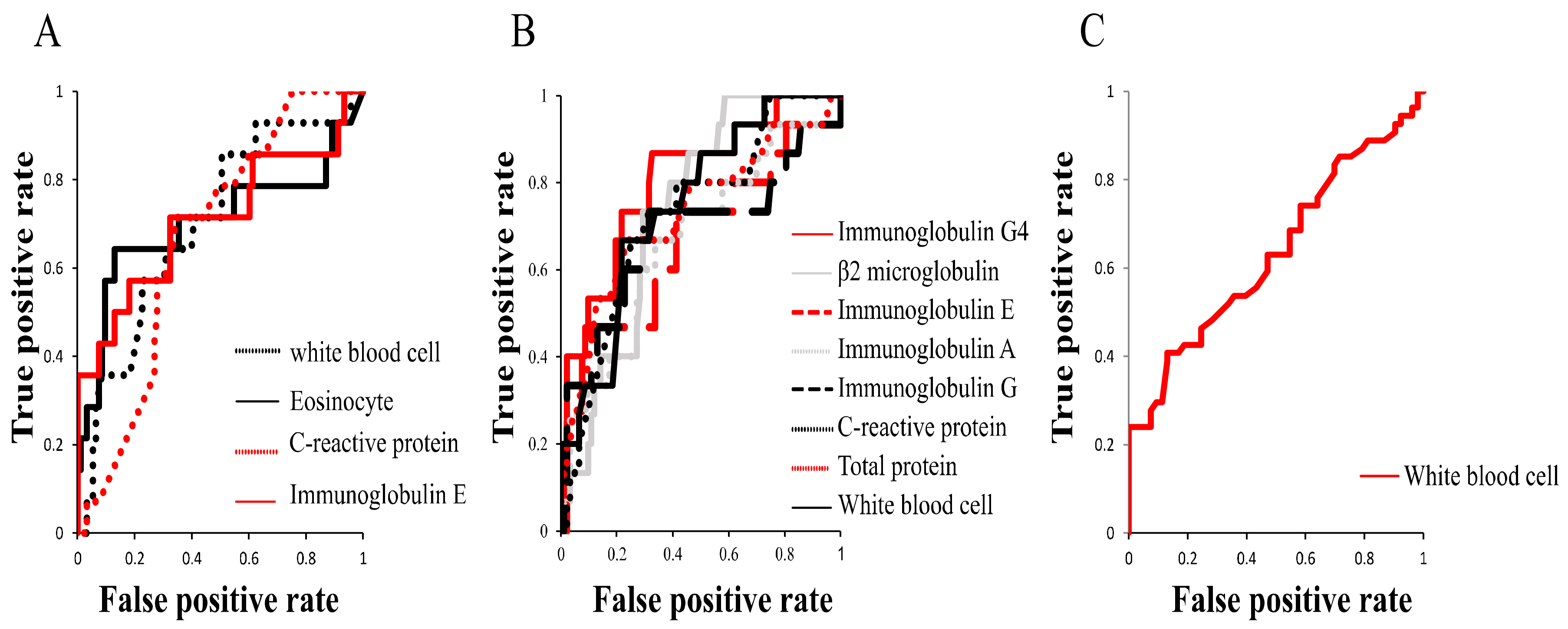
Having identified the peripheral blood findings that were significantly different between patients divided based on extraocular muscle enlargement, worsened BCVA, or lesion above the neck excluding LG and SG, we plotted ROC curves to investigate which of the laboratory test findings is most suitable for predicting each of the three clinical features (
Figure 2 and
Table 7). ROC curves for extraocular muscle enlargement using WBC count, Eo count, CRP, and IgE yielded AUCs (95% confidence interval (CI)) of 0.70 (0.55–0.85), 0.70 (0.51–0.90), 0.67 (0.54–0.80), and 0.71 (0.53–0.89), respectively. ROC curve analysis for worsened BCVA using serum IgG4, WBC, TP, CRP, IgG, IgA, IgE, and β2MG yielded AUCs (95% CI) of 0.80 (0.68–0.93), 0.75 (0.62–0.88), 0.73 (0.57–0.89), 0.73 (0.59–0.86), 0.68 (0.50–0.86), 0.67 (0.51–0.83), 0.67 (0.49–0.84), and 0.74 (0.63–0.85), respectively. ROC curves for lesion above the neck excluding LG and SG using WBC count yielded an AUC (95% CI) of 0.63 (0.53–0.74), which was small.
These results indicate that serum IgE is most suitable for the prediction of extraocular muscle enlargement and serum IgG4 is most suitable for the prediction of worsened BCVA, while prediction of lesion above the neck excluding LG and SG using peripheral blood tests may be difficult.
3.6. Rank Plot of Feature Importance Using Machine Learning and ROC Curve Using Panel of Tests Selected by Machine Learning
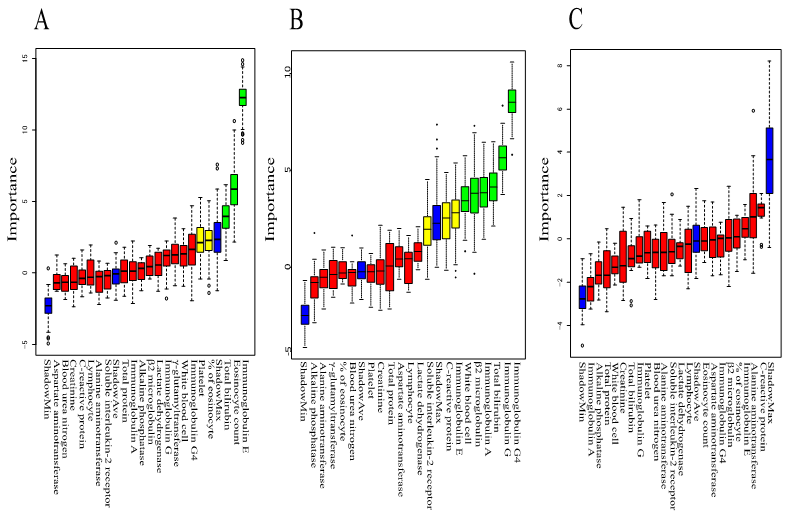
We performed machine learning using Boruta to analyze which peripheral blood findings are more important for predicting clinical features such as extraocular muscle enlargement, worsened BCVA, and lesion above the neck excluding LG and SG. The rank plot of feature importance is shown in
Figure 3. Serum IgE, Eo count, and T-Bil were more important for extraocular muscle enlargement than other peripheral blood findings. Serum IgG4, serum IgG, T-Bil, serum IgA, β2MG, and WBC count were more important for worsened BCVA than other peripheral blood findings. All peripheral blood findings were not important for lesion above the neck excluding LG and SG. These results indicate that peripheral blood tests are useful for predicting extraocular muscle enlargement and worsened BCVA. On the other hand, the peripheral blood test is not useful for predicting lesion above the neck excluding LG and SG.
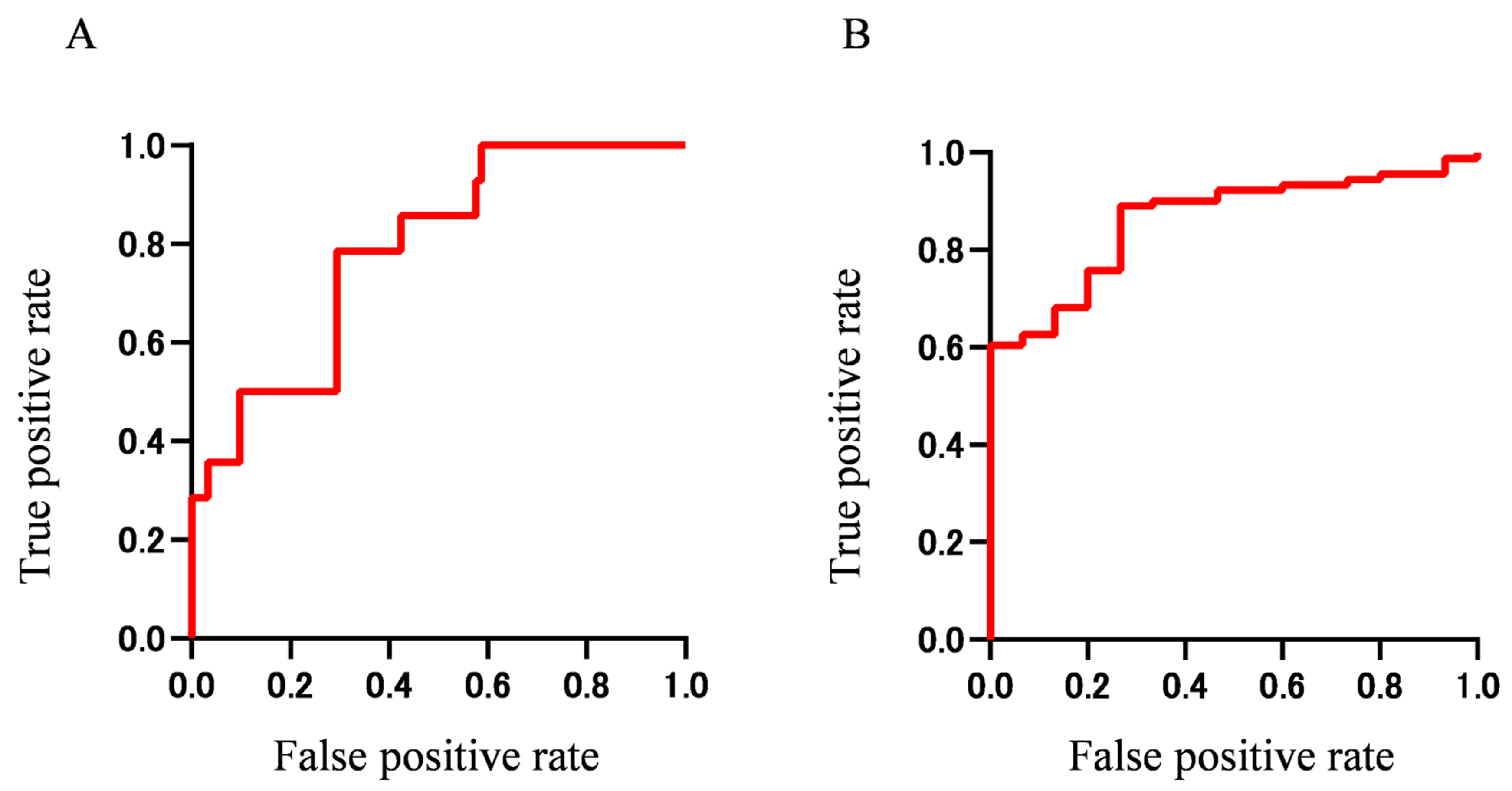
We then generated ROC curves using combinations of the important findings obtained from the Boruta analysis. ROC curves using panels of important findings for extraocular muscle enlargement (IgE, Eo count, and T-Bil) and worsened BCVA (WBC, T-BIL, serum IgG, serum IgA, β2-MG, and serum IgG4) are shown in
Figure 4. The AUC was 0.78 (95% CI 0.668–0.904) for extraocular muscle enlargement and 0.86 (95% CI 0.781–0.942) for worsened BCVA, indicting more accurate prediction when using multiple important findings than when using a single finding. These results indicate that extraocular muscle enlargement and worsened BCVA in IgG4-ROD may be predicted with high accuracy using a panel of biomarkers that can be tested routinely in a hospital laboratory.
3.7. Difference of Clinical Features in Patients Divided by Concentration of Serum IgG4 or IgE
Finally, we compared clinical features in patients divided by the cut-off serum IgE concentration of 425 IU/mL or cutoff serum IgG4 concentration of 713 mg/dL obtained from ROC analysis (
Table 8 and
Table 9). Patients with IgE above 425 IU/mL had high serum IgG4 and extraocular muscle enlargement (
p = 0.01 and
p = 0.004, respectively). Patients with IgG4 above 713 mg/dL had a high male ratio; older onset; and high frequencies of bilateral LG swelling, lesion excluding LG and SG, lesion above the neck excluding LG and SG, trigeminal nerve enlargement, worsened BCVA, optic neuropathy, visual field defect, and dry eye symptoms (
p = 0.04,
p = 0.02,
p = 0.02,
p = 0.009,
p = 0.03,
p = 0.001,
p < 0.001,
p = 0.005,
p = 0.005, and
p = 0.04, respectively).
These results indicate that patients with serum IgE above 425 mg/dL are more likely to have extraocular muscle enlargement, while patients with serum IgG4 above 713 mg/dL have a higher probability of lesions involving ocular adnexal tissues and other tissues above the neck, higher frequency of worsened BCVA due to optic neuropathy, and higher frequency of dry eye symptoms.
4. Discussion
The Japanese study group of IgG4-ROD reported that the prevalence of IgG4-ROD ranked second among ocular adnexal lymphoproliferative disorders [
19]. In that study, among 1014 cases of orbital lymphoproliferative disorders, 404 cases (39.8%) were extranodal mucosa-associated lymphoid tissue (MALT) lymphoma and 219 (21.6%) were IgG4-ROD [
19]. Despite this relatively high prevalence, the clinical features of IgG4-ROD remain unclear because only a few studies have analyzed the clinical features of IgG4-ROD in a relatively large sample of over 50 cases [
20,
21,
22]. IgG4-ROD has been reported to cause not only enlargement of ocular adnexal tissues, but also worsening of BCVA due to optic neuropathy [
23,
24]. Moreover, although systemic steroids are efficacious in all patients with IgG4-RD, some patients cannot be weaned from systemic steroids and require maintenance treatment [
22,
25,
26]. IgG4-RD is a heterogeneous group with diverse clinical phenotypes. Therefore, unbiased clustering analysis is a useful approach to evaluate patient characteristics.
In the first unbiased clustering analysis, groups B and D had a significantly higher frequency of lesion above the neck excluding LG and SG than other groups. Patients with serum IgG4 above 713 mg/dL also had a higher frequency of lesion excluding LG and SG, especially lesion above the neck, than patients with serum IgG4 below 713 mg/dL. In another study of 493 patients with IgG4-RD divided by phenotype, serum IgG4 levels were 316 and 178 mg/dL in pancreato-hepato-biliary disease and aortitis, respectively, while the levels were 445 and 1170 mg/dL in head and neck-limited disease and classic Mikulicz syndrome, respectively.5 These results show that IgG4-RD patients with lesion above the neck have higher serum IgG4 than those without above the neck lesion. Moreover, we found that patients with serum IgG4 above 713 mg/dL had higher frequencies of bilateral LG enlargement and dry eye symptoms than patients with serum IgG4 below 713 mg/dL. These findings thus suggest that high serum IgG4 may be a risk factor of multi-organ lesion, particularly lesion above the neck, and dry eye symptoms. On the other hand, patients with lesion above the neck excluding LG and SG had a significantly lower frequency of lesion below the neck in this study. However, Boruta analysis found that none of the peripheral blood test findings including serum IgG4 were important for lesion above neck excluding LG and SG. Even when ROC analysis was conducted for WBC count, the only abnormal laboratory data found in lesion above the neck, the AUC was low (0.63). These results indicate that peripheral blood tests including serum IgG4 are not useful for predicting above the neck lesions excluding LG and SG.
Interestingly, patients with serum IgG4 above 713 mg/dL had a significantly higher frequency of enlargement of trigeminal nerve, but not enlargement of extraocular muscles. Moreover, patients with a high frequency of enlargement of extraocular muscles were not classified into group D or F with high serum IgG4 (above 1000 mg/dL), but into groups B and E with moderate elevation of serum IgG4. Groups B and E consisted of patients who had a high percentage of Eo and high serum IgE. A previous report also indicated that eosinophilia and relatively elevated serum IgG4 were associated with enlargement of extraocular muscles [
22]. Moreover, other studies reported that serum IgE was significantly elevated in IgG4-RD [
27,
28]. These findings indicate the potential importance of Eo and serum IgE in determining severity. IgG4-RD including IgG4-ROD have been shown to be associated with allergy [
27]. Analysis using machine learning also revealed that serum IgE, Eo count, and T-BIL were more important for the prediction of extraocular muscle enlargement than other peripheral blood findings in this study. ROC analysis using a combination of three laboratory tests (IgE, Eo count, and T-BIL) selected by machine learning using Boruta had a greater AUC and higher significance compared with that using serum IgG4 alone (AUC 0.80;
p < 0.001 versus AUC 0.71;
p = 0.01).
The mechanisms behind the diverse inflammatory markers such as eosinophilia and serum IgE are likely to be complex, but may signify a Th2-driven autoimmune mechanism [
29,
30]. Therefore, percentage of Eo and serum IgE may be better markers than serum IgG4 for the prediction of enlargement of extraocular muscles. In other words, the presence of variable types of IgG4-ROD may be explained by heterogenous phenotypes including high serum IgG4 without a high percentage of Eo and moderately elevated serum IgG4 with a high percentage of Eo.
Although a case of IgG4-ROD with optic neuropathy was reported [
23], optic neuropathy caused by IgG4-ROD is occasionally misdiagnosed as thyroid-associated orbitopathy (TAO) at disease onset, because enlargements of extraocular muscles and LG are also observed in TAO [
23,
24]. Patients with TAO also show red conjunctiva, eyelid retraction, extraocular muscle enlargement, and increased orbital tissue. Although TAO most frequently affects the inferior rectus muscle, with preferential swelling of the muscle belly, both IgG4-ROD and TAO show similar orbital imaging findings and some clinical features including bilateral disease with a chronic onset, enlargement of extraocular muscles, increased orbital fat, and enlargement of LG [
23]. The relationship between TAO and IgG4-ROD has been reported [
31,
32]. Eight percent of patients with TAO had high serum IgG4 above 135 mg/dL [
31]. In IgG4-RD patients, the presence of lesion above the neck may be associated with high serum IgG4 [
5]. A case report showed serum IgG4 of 1020 mg/dL in a IgG4-ROD patient with optic neuropathy [
23], which is in good agreement with our results. Thus, previous results suggest that IgG4-RD patients with ocular lesion have higher serum IgG4 compared with those without ocular lesion. Our study also found that group D with high serum IgG4 had a significantly higher frequency of orbital lesion than the other groups. Although the ROC curve showed high performance of serum IgG4 alone with AUC of 0.80, a combination of six laboratory tests (WBC, T-BIL, serum IgG, serum IgA, β2-MG, and serum IgG4) obtained from machine learning using Boruta showed a greater AUC (0.86) and higher significance than serum IgG4 alone. Patients with IgG4 above 713 mg/dL, the cutoff level obtained from ROC, had a significantly higher frequency of worsened BCVA. Therefore, these results suggest that serum IgG4 is associated with optic neuropathy owing to orbital lesion, and high serum IgG4 is a risk factor of worsened BCVA. The AUC of serum IgG4 for worsened BCVA was 0.80 in this study (
Table 7). Analysis of a large sample of patients with IgG4-ROD may improve the AUC and facilitate interpretation of the model. Therefore, accumulation of more cases in future studies may yield a better AUC and cutoff values.
In this study, WBC, CRP, TP, and β2MG were significantly higher in patients with worsened BCVA than in those with preserved BCVA. One previous study reported higher serum CRP in IgG4-related aortic aneurysms and periaortitis compared with IgG4-related retroperitoneal fibrosis [
33]. CRP is a biomarker of inflammatory activity in some rheumatic diseases [
34]. Elevated CRP may cause an increase in WBC count. While these results suggest CRP to possibly be a biomarker of IgG4-ROD activity, other reports concluded that serum CRP is not necessarily elevated in IgG4-RD patients with involvement of different organs [
35,
36]. Therefore, there is controversy over WBC count and CRP as biomarkers of IgG4-ROD. Serum TP is elevated with an increase of serum immunoglobulins, because serum TP consists of serum albumin and immunoglobulins. Hence, serum TP would not be a better biomarker for IgG4-ROD activity than serum IgG4. Serum β2MG increases in other inflammatory diseases [
37]. Therefore, IgG4-ROD activity likely causes elevation of serum β2MG. However, Xie et al. [
38]. reported that β2MG plays a role in inflammatory diseases as a potential initiator of inflammatory responses. Further study should examine whether β2MG is a useful marker. These inflammatory biomarkers are increasingly being used to elucidate the underlying pathophysiological mechanisms of individual phenotypes, with the hypothesis that each phenotype has a different underlying pathophysiological mechanism.
This study has several strengths and weaknesses. This is the first study to use unsupervised hierarchical cluster analysis to identify a panel of biomarkers in a relatively large sample of patients with IgG4-ROD, despite the rarity of the disease. This study demonstrates the potential of using a panel of commonly available laboratory test data in predicting the prognosis of IgG4-ROD, which will have wide applicability once validated. On the other hand, there are several limitations. First, the retrospective nature of the study may cause selection bias and confounding bias. Thus, the results should be considered exploratory. Second, because all cases were collected from a single tertiary care hospital, there is potential referral bias. Future studies should prospectively validate these laboratory data in independent cohorts and evaluate whether the addition of other markers improves the accuracy of predicting the clinical course such as enlargement of extraocular muscles, worsening of BCVA, and development of extraocular lesions. Future studies are needed to assess the feasibility of using this unbiased cluster analysis in a prospective manner to predict the visual outcome and development of extraocular lesions.
In conclusion, using unbiased clustering analysis, we were able to identify four groups among IgG4-ROD patients, which are associated with different clinical courses, and discover the biomarkers for predicting enlargement of extraocular muscles and worsened BCVA in IgG4-ROD. These phenotypes within IgG4-ROD may in part explain the heterogeneity of this disease. Unbiased clustering analysis reveals that patients with high serum IgE have a high frequency of extraocular muscle enlargement and patients with high serum IgG4 have a high frequency of multi-organ lesion especially lesion above the neck, dry eye symptoms, and worsened BCVA. Surprisingly, although high serum IgG4 alone predicts worsened BCVA among IgG4-ROD patients, using a panel of tests comprising IgG4, IgG, IgA, WBC count, T-BIL, and β2MG improves the accuracy of predicting high risk of worsened BCVA, suggesting that a panel approach may be more effective than serum IgG4 alone, and provides additional evidence for the prevention and management of worsened BCVA in IgG4-ROD. We also noted that these parameters were highly variable among patients. Indeed, for IgG4-ROD patients, data based on serum IgG4 and other markers that can be tested routinely in the hospital laboratory may impact clinical decisions and potentially contribute to the understanding of the mechanisms behind why some patient with IgG4-ROD develop visual disturbance, whereas others do not, and why some patients have extraocular lesions, whereas others do not. A better understanding of this heterogeneity and the link to visual function and extraocular lesions may provide opportunities to conduct personalized or stratified care for IgG4-ROD.