Helicobacter pylori Infection and the Patterns of Gastric Mucin Expression in Children
Abstract
1. Introduction
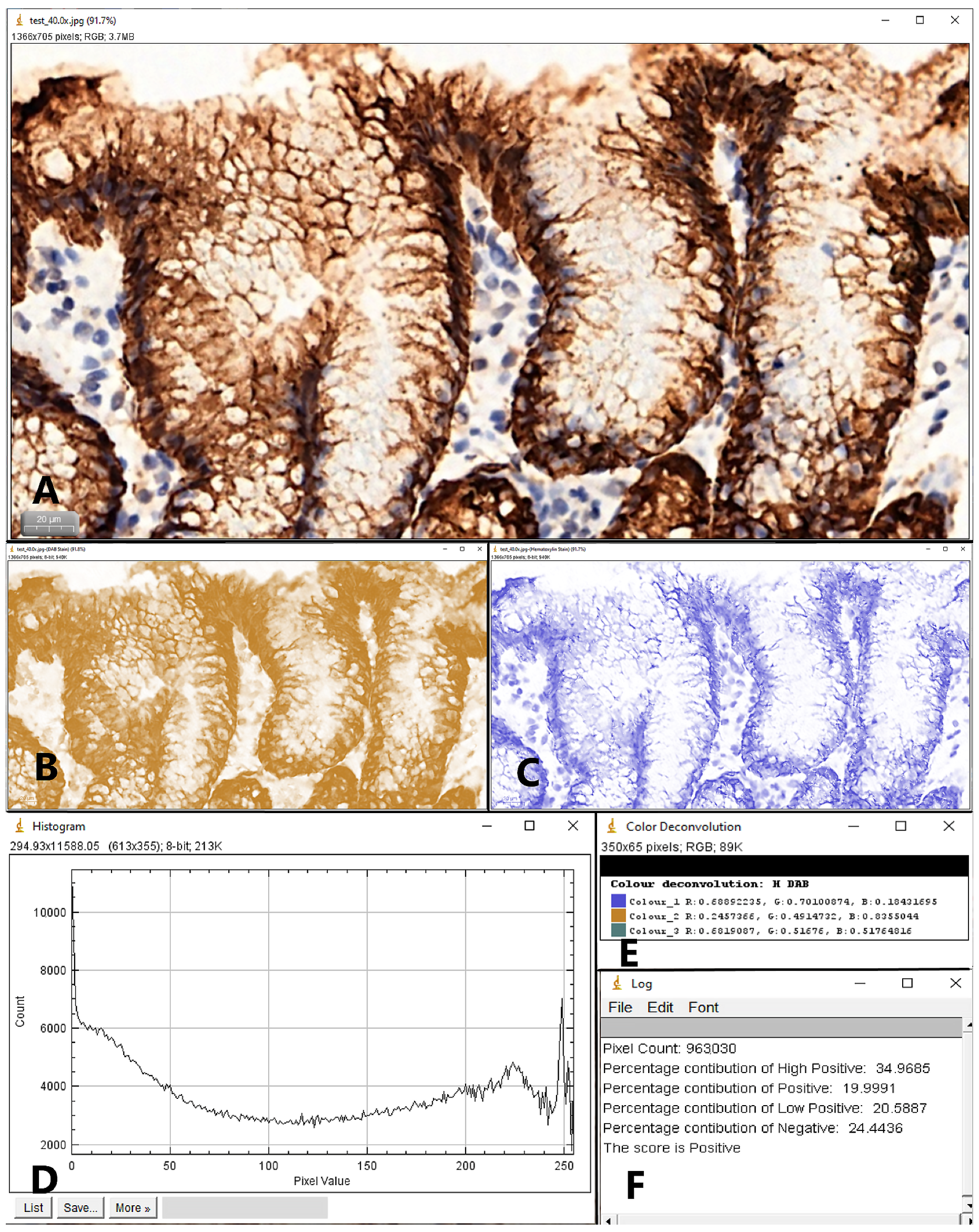
2. Materials and Methods
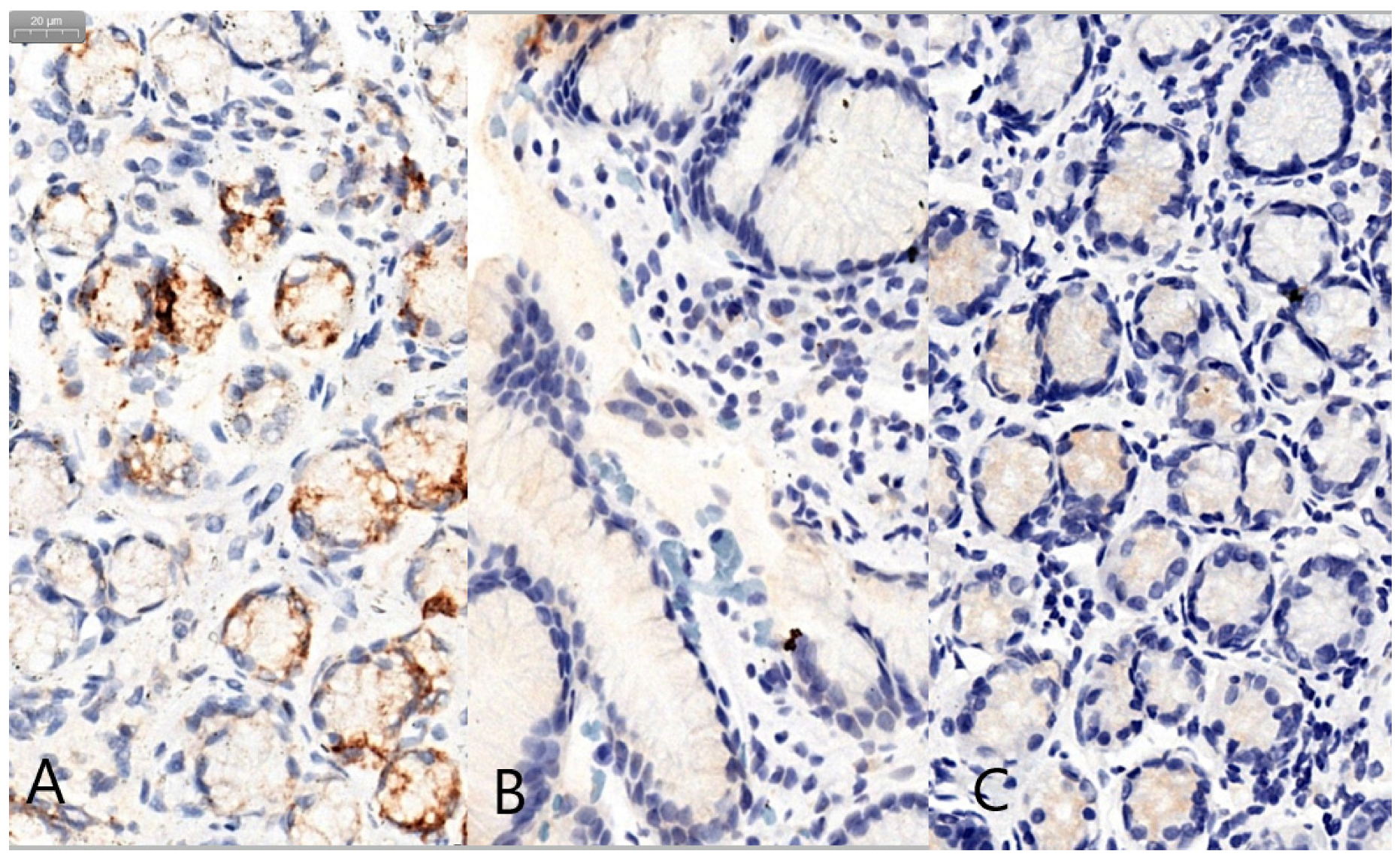
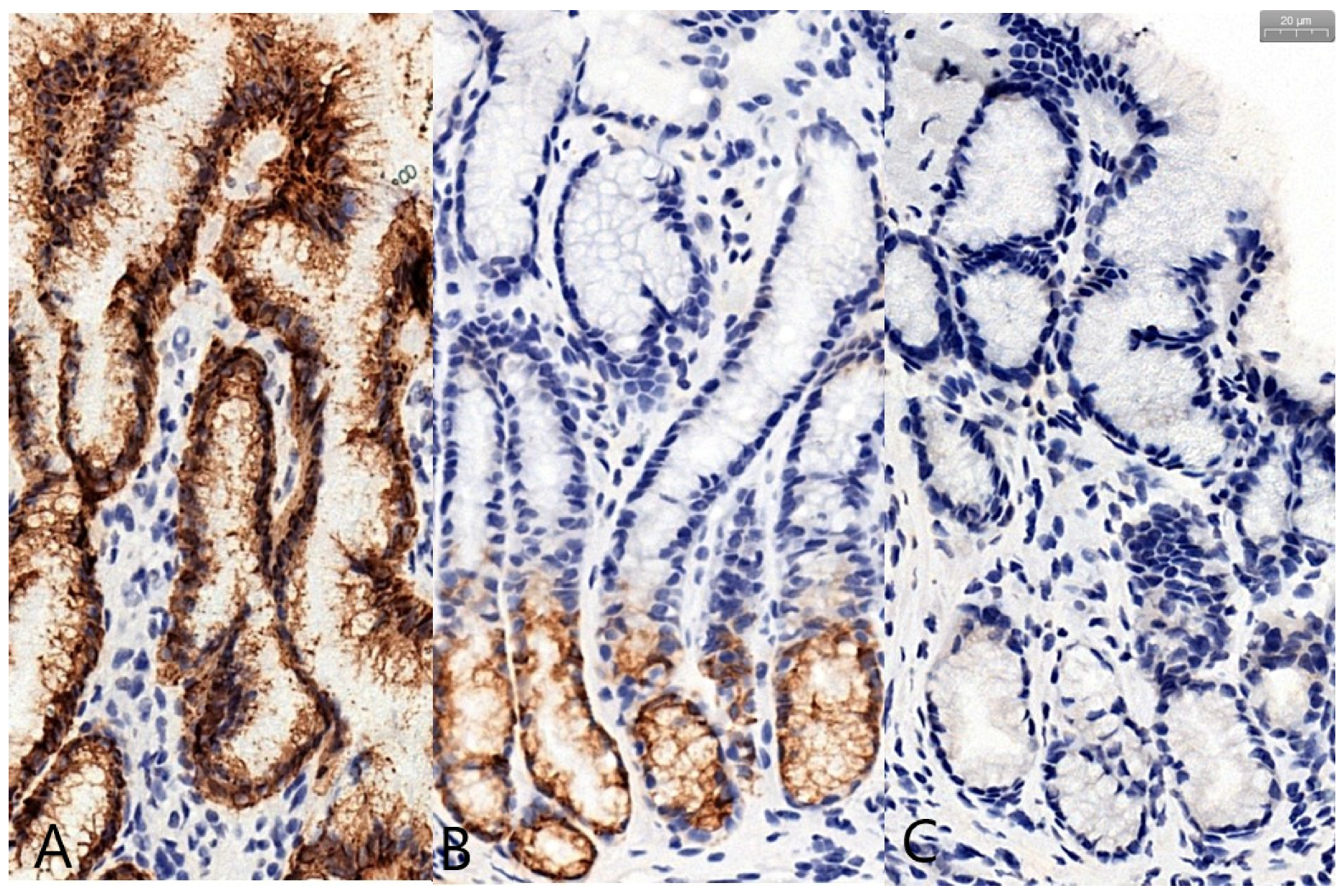
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Axon, A.T.R. Helicobacter pyloriand Public Health. Helicobacter 2014, 19, 68–73. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 61, 1–241. [Google Scholar]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y. Helicobacter pyloriand gastric mucin expression: A systematic review and meta-analysis. World J. Gastroenterol. 2015, 21, 9430–9436. [Google Scholar] [CrossRef]
- Ho, S.B.; Takamura, K.; Anway, R.; Shekels, L.L.; Toribara, N.W.; Ota, H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig. Dis. Sci. 2004, 49, 1598–1606. [Google Scholar] [CrossRef]
- Buisine, M.P.; Devisme, L.; Savidge, T.C.; Gespach, C.; Gosselin, B.; Porchet, N.; Aubert, J.P. Mucin gene expression in human embryonic and fetal intestine. Gut 1998, 43, 519–524. [Google Scholar] [CrossRef]
- Wang, R.-Q.; Fang, D.-C. Effects of Helicobacter pylori infection on mucin expression in gastric carcinoma and pericancerous tissues. J. Gastroenterol. Hepatol. 2006, 21, 425–431. [Google Scholar] [CrossRef]
- Lindén, S.K.; Wickström, C.; Lindell, G.; Gilshenan, K.; Carlstedt, I. Four Modes of Adhesion are Used During Helicobacter pylori Binding to Human Mucins in the Oral and Gastric Niches. Helicobacter 2008, 13, 81–93. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Yoon, H.; Surh, Y.-J. Diagnosis and Management of High Risk Group for Gastric Cancer. Gut Liver 2015, 9, 5–17. [Google Scholar] [CrossRef]
- Kim, N.; Park, Y.S.; Cho, S.-I.; Lee, H.S.; Choe, G.; Kim, I.W.; Won, Y.-D.; Park, J.H.; Kim, J.S.; Jung, H.C.; et al. Prevalence and Risk Factors of Atrophic Gastritis and Intestinal Metaplasia in a Korean Population Without Significant Gastroduodenal Disease. Helicobacter 2008, 13, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. Chronic gastritis: A clinico-pathological classification. Am. J. Gastroenterol. 1988, 83, 504–509. [Google Scholar] [PubMed]
- De Vries, A.C.; Kuipers, E.J. Epidemiology of Premalignant Gastric Lesions: Implications for the Development of Screening and Surveillance Strategies. Helicobacter 2007, 12, 22–31. [Google Scholar] [CrossRef]
- Jass, J.R.; Filipe, M.I. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants and gastric carcinoma. J. Mol. Histol. 1981, 13, 931–939. [Google Scholar] [CrossRef]
- Kang, H.M.; Kim, N.; Park, Y.S.; Hwang, J.-H.; Kim, J.-W.; Jeong, S.-H.; Lee, D.H.; Lee, H.S.; Jung, H.C.; Song, I.S. Effects of Helicobacter pylori Infection on Gastric Mucin Expression. J. Clin. Gastroenterol. 2008, 42, 29–35. [Google Scholar] [CrossRef]
- Kang, K.P.; Lee, H.S.; Surh, Y.-J.; Kang, H.M.; Park, Y.S.; Lee, D.H.; Choe, G.; Kim, J.S.; Jung, H.C.; Song, I.S. Role of intestinal metaplasia subtyping in the risk of gastric cancer in Korea. J. Gastroenterol. Hepatol. 2009, 24, 140–148. [Google Scholar] [CrossRef]
- Rokkas, T.; Pistiolas, D.; Sechopoulos, P.; Robotis, I.; Margantinis, G. The Long-term Impact of Helicobacter pylori Eradication on Gastric Histology: A Systematic Review and Meta-analysis. Helicobacter 2007, 12, 32–38. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Shi, R.; Huang, X.; Li, S.W.H.; Huang, Z.; Zhang, G. Gastric Atrophy and Intestinal Metaplasia before and after Helicobacter pylori Eradication: A Meta-Analysis. Digestion 2011, 83, 253–260. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Romiti, A.; Giusto, M.; Guerriero, C.; Lorenzetti, R.; Campo, S.M.; Tomao, S. Follow-up of intestinal metaplasia in the stomach: When, how and why. World J. Gastrointest. Oncol. 2012, 4, 30–36. [Google Scholar] [CrossRef]
- Cohen, M.; Drut, R.; Cueto Rua, E. SIALYL-Tn antigen distribution in Helicobacter pylori chronic gastritis in children: An immunohistochemical study. Pediatr. Pathol. Mol. Med. 2003, 22, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lindén, S.; Semino-Mora, C.; Liu, H.; Rick, J.; Dubois, A. Role of Mucin Lewis Status in Resistance to Helicobacter pylori Infection in Pediatric Patients. Helicobacter 2010, 15, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Der Pathol. 1987, 8, 138–140. [Google Scholar]
- Jafari, S.M.S.; Hunger, R.E. IHC Optical Density Score. Appl. Immunohistochem. Mol. Morphol. 2017, 25, e12–e13. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Piazuelo, M.B.; Correa, P. Gastric cancer: Overview. Colomb. Med. 2013, 44, 192–201. [Google Scholar]
- Harris, P.R.; Smythies, L.E.; Smith, P.D.; Perez-Perez, G.I. Role of childhood infection in the sequelae of H. pylori disease. Gut Microbes 2013, 4, 426–438. [Google Scholar] [CrossRef]
- Skoog, E.C.; Sjöling, Å.; Navabi, N.; Holgersson, J.; Lundin, S.B.; Lindén, S.K. Human Gastric Mucins Differently Regulate Helicobacter pylori Proliferation, Gene Expression and Interactions with Host Cells. PLoS ONE 2012, 7, e36378. [Google Scholar] [CrossRef]
- Dunne, C.; Naughton, J.; Duggan, G.; Loughrey, C.; Kilcoyne, M.; Joshi, L.; Carrington, S.D.; Earley, H.; Backert, S.; Robbe-Masselot, C.; et al. Binding of Helicobacter pylori to Human Gastric Mucins Correlates with Binding of TFF1. Microorganisms 2018, 6, 44. [Google Scholar] [CrossRef]
- Ho, S.B.; Roberton, A.M.; Shekels, L.L.; Lyftogt, C.T.; Niehans, G.A.; Toribara, N.W. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology 1995, 109, 735–747. [Google Scholar] [CrossRef]
- Park, J.S.; Yeom, J.-S.; Seo, J.-H.; Lim, J.-Y.; Park, C.-H.; Woo, H.-O.; Youn, H.-S.; Jun, J.-S.; Park, J.-H.; Ko, G.-H.; et al. Immunohistochemical Expressions of MUC2, MUC5AC, and MUC6 in Normal, Helicobacter pylori Infected and Metaplastic Gastric Mucosa of Children and Adolescents. Helicobacter 2015, 20, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Boltin, R.; Halpern, M.; Levi, Z.; Vilkin, A.; Morgenstern, S.; Ho, S.B.; Niv, Y. Gastric mucin expression in Helicobacter pylori-related, nonsteroidal anti-inflammatory drug-related and idiopathic ulcers. World J. Gastroenterol. 2012, 18, 4597–4603. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Yan, P.; Sternberg, L.; Yunker, C.K.; Scheiman, J.M.; Bresalier, R.S. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology 1997, 113, 455–464. [Google Scholar] [CrossRef]
- Byrd, J.C.; Yunker, C.K.; Xu, Q.; Sternberg, L.R.; Bresalier, R.S. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology 2000, 118, 1072–1079. [Google Scholar] [CrossRef]
- Niv, Y.H. pylori/NSAID-Negative peptic ulcer-The mucin theory. Med. Hypotheses 2010, 75, 433–435. [Google Scholar] [CrossRef]
- Brink, G.R.V.D.; Tytgat, K.M.A.J.; Van Der Hulst, R.W.M.; Van Der Loos, C.M.; Einerhand, A.W.C.; Büller, H.A.; Dekker, J. H pylori colocalises with MUC5AC in the human stomach. Gut 2000, 46, 601–607. [Google Scholar] [CrossRef]
- Lindén, S.; Nordman, H.; Hedenbro, J.; Hurtig, M.; Borén, T.; Carlstedt, I. Strain- and blood group–dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology 2002, 123, 1923–1930. [Google Scholar] [CrossRef]
- Sheu, B.-S.; Yang, H.-B.; Yeh, Y.-C.; Wu, J.-J. Helicobacter pyloricolonization of the human gastric epithelium: A bug’s first step is a novel target for us. J. Gastroenterol. Hepatol. 2010, 25, 26–32. [Google Scholar] [CrossRef]
- Navabi, N.; Johansson, M.E.V.; Raghavan, S.; Lindén, S. Helicobacter pylori Infection Impairs the Mucin Production Rate and Turnover in the Murine Gastric Mucosa. Infect. Immun. 2012, 81, 829–837. [Google Scholar] [CrossRef]
- Gonciarz, W.; Walencka, M.; Moran, A.P.; Hinc, K.; Nagórska, K.; Chmiela, M. Upregulation of MUC5AC production and deposition of LEWIS determinants by Helicobacter Pylori facilitate gastric tissue colonization and the maintenance of infection. J. Biomed. Sci. 2019, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, M. Natural Antibiotic Function of a Human Gastric Mucin Against Helicobacter pylori Infection. Science 2004, 305, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.; Yamauchi, K.; Sugano, M.; Kawasaki, K.; Sugiyama, A.; Matsuzawa, K.; Akamatsu, T.; Ohmoto, Y.; Ota, H. Pathophysiological Investigation of the Gastric Surface Mucous Gel Layer of Patients with Helicobacter pylori Infection by Using Immunoassays for Trefoil Factor Family 2 and Gastric Gland Mucous Cell-Type Mucin in Gastric Juice. Dig. Dis. Sci. 2011, 56, 3498–3506. [Google Scholar] [CrossRef] [PubMed]
- Van De Bovenkamp, J.H.B.; Male, A.M.K.-V.; Büller, H.A.; Einerhand, A.W.C.; Dekker, J.; Van Bovenkamp, J.H.B. Infection with Helicobacter pylori Affects All Major Secretory Cell Populations in the Human Antrum. Dig. Dis. Sci. 2005, 50, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.; Mahdavi, J.; Semino-Mora, C.; Olsen, C.; Carlstedt, I.; Borén, T.; Dubois, A. Role of ABO Secretor Status in Mucosal Innate Immunity and H. pylori Infection. PLoS Pathog. 2008, 4, e2. [Google Scholar] [CrossRef]
- Cam, S. Risk of gastric cancer in children with Helicobacter pylori infection. Asian Pac. J. Cancer Prev. 2014, 15, 9905–9908. [Google Scholar] [CrossRef]
- Yu, Y.; Su, L.; Wang, X.; Wang, X.; Xu, C. Association between Helicobacter pylori Infection and Pathological Changes in the Gastric Mucosa in Chinese Children. Intern. Med. 2014, 53, 83–88. [Google Scholar] [CrossRef][Green Version]
- Reis, C.A.; David, L.; Correa, P.; Carneiro, F.; De Bolós, C.; Garcia, E.; Mandel, U.; Clausen, H.; Sobrinho-Simões, M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999, 59, 1003–1007. [Google Scholar]
- Babu, S.D.; Jayanthi, V.; Devaraj, N.; Reis, C.A.; Devaraj, H. Expression profile of mucins (MUC2, MUC5AC and MUC6) in Helicobacter pylori infected pre-neoplastic and neoplastic human gastric epithelium. Mol. Cancer 2006, 5, 10. [Google Scholar] [CrossRef]
- Song, J.-Y.; Kim, B.-W.; Lee, A.-W.; Lee, K.-Y.; Chung, I.-S.; Lee, B.-I.; Choi, H.; Ji, J.-S.; Chae, H.-S.; Choi, K.-Y. Expression of MUC5AC and Trefoil Peptide 1 (TFF1) in the Subtypes of Intestinal Metaplasia. Clin. Endosc. 2012, 45, 151–154. [Google Scholar] [CrossRef]
- Shi, D.; Qiu, X.-M.; Yan, X.-J. The changes in MUC5AC expression in gastric cancer before and after Helicobacter pylori eradication. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.-S.; Lee, H.-J.; Jung, E.-J.; Kim, M.-A.; Nam, K.T.; Goldenring, J.R.; Yang, H.-K.; Kim, W.H. The Combined Expression of Metaplasia Biomarkers Predicts the Prognosis of Gastric Cancer. Ann. Surg. Oncol. 2011, 19, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Gomceli, I.; Demiriz, B.; Tez, M. Gastric carcinogenesis. World J. Gastroenterol. 2012, 18, 5164–5170. [Google Scholar] [PubMed]
- Ilhan, O.; Han, U.; Onal, B.; Celik, S.Y. Prognostic significance of MUC1, MUC2 and MUC5AC expressions in gastric carcinoma. Turk. J. Gastroenterol. 2010, 21, 345–352. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, H.; Xie, D.; Xiao, Q. Differentially expressed genes ASPN, COL1A1, FN1, VCAN and MUC5AC are potential prognostic biomarkers for gastric cancer. Oncol. Lett. 2019, 17, 3191–3202. [Google Scholar] [CrossRef]
- Jung, S.H.; Chung, W.C.; Lee, K.-M.; Paik, C.N.; Jung, J.H.; Lee, M.K.; Lee, Y.K.; Chung, I.-S. Risk factors in malignant transformation of gastric epithelial neoplasia categorized by the revised Vienna classification: Endoscopic, pathological, and immunophenotypic features. Gastric Cancer 2010, 13, 123–130. [Google Scholar] [CrossRef]
- Marín, F.; Bonet, C.; Muñoz, X.; García, N.; Pardo, M.L.; Ruiz-Liso, J.M.; Alonso, P.; Capellá, G.; Sanz-Anquela, J.M.; González, C.A.; et al. Genetic variation in MUC1, MUC2 and MUC6 genes and evolution of gastric cancer precursor lesions in a long-term follow-up in a high-risk area in Spain. Carcinogenesis 2012, 33, 1072–1080. [Google Scholar] [CrossRef]
| Type of Mucin | Determined Score | H. pylori+ | H. pylori− | p-Value |
|---|---|---|---|---|
| MUC5AC | IRS | 11.21 ± 1.55 | 10.81 ± 1.90 | 0.41 |
| MUC6 | IRS | 8.55 ± 3.11 | 9.21 ± 3.01 | 0.37 |
| MUC2 | IRS | 0.31 ± 0.24 | 0.21 ± 0.71 | 0.72 |
| MUC5AC | ODS in superficial/foveolar region | 2.28 ± 0.23 | 2.33 ± 0.24 | 0.04 |
| MUC5AC | ODS in glandular region | 1.65 ± 0.23 | 1.64 ± 0.17 | 0.88 |
| MUC6 | ODS in superficial/foveolar region | 1.63 ± 0.24 | 1.76 ± 0.22 | 0.04 |
| MUC6 | ODS in glandular region | 2.05 ±0.08 | 2.09 ± 0.23 | 0.58 |
| MUC2 | ODS in superficial/foveolar region | 1.73 ± 0.20 | 1.66 ± 0.24 | 0.26 |
| MUC2 | ODS in glandular region | 1.68 ± 0.21 | 1.61 ± 0.11 | 0.23 |
| Type of Mucin | Determined Score | Inflammation+ | Inflammation− | p-Value |
|---|---|---|---|---|
| MUC5AC | IRS | 11.18 ± 1.57 | 10.81 ± 2.01 | 0.43 |
| MUC6 | IRS | 8.86 ± 3.11 | 9.00 ± 3.35 | 0.87 |
| MUC2 | IRS | 0.32 ± 0.17 | 0.13 ± 0.63 | 0.49 |
| MUC5AC | ODS in superficial/foveolar region | 2.31 ± 0.22 | 2.30 ± 0.28 | 0.86 |
| MUC5AC | ODS in glandular region | 1.65 ± 0.21 | 1.63 ± 0.17 | 0.71 |
| MUC6 | ODS in superficial/foveolar region | 1.78 ± 0.18 | 1.67 ± 0.27 | 0.03 |
| MUC6 | ODS in glandular region | 2.13 ± 0.24 | 1.97 ± 0.27 | 0.02 |
| MUC2 | ODS in superficial/foveolar region | 1.73 ± 0.24 | 1.64 ± 0.19 | 0.13 |
| MUC2 | ODS in glandular region | 1.70 ± 0.25 | 1.55 ± 0.19 | 0.01 |
| Type of Mucin | Determined Score | Atrophy+ | Atrophy− | p-Value |
|---|---|---|---|---|
| MUC5AC | IRS | 11.46 ± 1.40 | 10.90 ± 1.84 | 0.28 |
| MUC6 | IRS | 10.46 ± 2.06 | 8.38 ± 3.22 | 0.02 |
| MUC2 | IRS | 0.60 ± 0.68 | 0.13 ± 0.63 | 0.12 |
| MUC5AC | ODS in superficial/foveolar region | 2.30 ± 0.24 | 2.30 ± 0.25 | 0.95 |
| MUC5AC | ODS in glandular region | 1.60 ± 0.15 | 1.66 ± 0.21 | 0.32 |
| MUC6 | ODS in superficial/foveolar region | 1.78 ± 0.12 | 1.67 ± 0.27 | 0.13 |
| MUC6 | ODS in glandular region | 2.22 ±0.21 | 2.02 ± 0.26 | 0.007 |
| MUC2 | ODS in superficial/foveolar region | 1.74 ± 0.23 | 1.68 ± 0.22 | 0.37 |
| MUC2 | ODS in glandular region | 1.72 ± 0.19 | 1.62 ± 0.25 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domșa, A.-M.T.; Lupușoru, R.; Gheban, D.; Buruiană-Simic, A.; Gheban, B.A.; Lazăr, C.; Borzan, C.M. Helicobacter pylori Infection and the Patterns of Gastric Mucin Expression in Children. J. Clin. Med. 2020, 9, 4030. https://doi.org/10.3390/jcm9124030
Domșa A-MT, Lupușoru R, Gheban D, Buruiană-Simic A, Gheban BA, Lazăr C, Borzan CM. Helicobacter pylori Infection and the Patterns of Gastric Mucin Expression in Children. Journal of Clinical Medicine. 2020; 9(12):4030. https://doi.org/10.3390/jcm9124030
Chicago/Turabian StyleDomșa, Ana-Maria Teodora, Raluca Lupușoru, Dan Gheban, Alexandra Buruiană-Simic, Bogdan Alexandru Gheban, Camelia Lazăr, and Cristina Maria Borzan. 2020. "Helicobacter pylori Infection and the Patterns of Gastric Mucin Expression in Children" Journal of Clinical Medicine 9, no. 12: 4030. https://doi.org/10.3390/jcm9124030
APA StyleDomșa, A.-M. T., Lupușoru, R., Gheban, D., Buruiană-Simic, A., Gheban, B. A., Lazăr, C., & Borzan, C. M. (2020). Helicobacter pylori Infection and the Patterns of Gastric Mucin Expression in Children. Journal of Clinical Medicine, 9(12), 4030. https://doi.org/10.3390/jcm9124030





