Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review
Abstract
1. Introduction
2. The Different 3D Printing Techniques
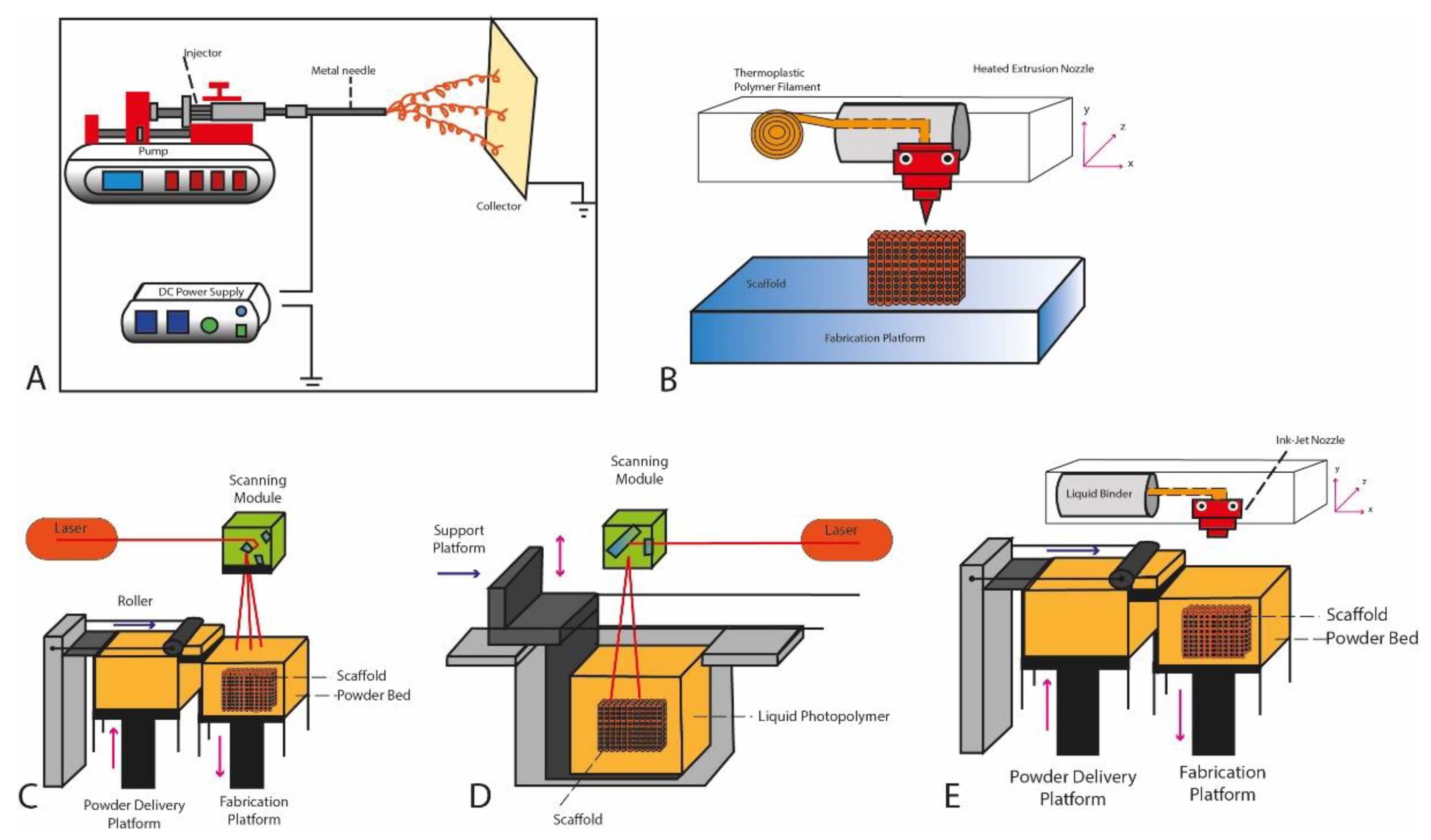
2.1. Electrospinning
2.2. Material Extrusion
2.3. Powder Bed Fusion
2.4. Stereolithography (SLA)
2.5. Inkjet Printing
3. The Different Materials Used for 3D Printing
3.1. Natural Polymers
3.2. Synthetic Polymers
3.3. Bioceramics
4. Scaffold Structure Necessary for Bone Regeneration
5. Computer Assisted Design and Manufacturing
6. Applications of Tissue Engineering and 3D Printing for Periodontal Regeneration
6.1. Tissue Engineering in Periodontal Regeneration
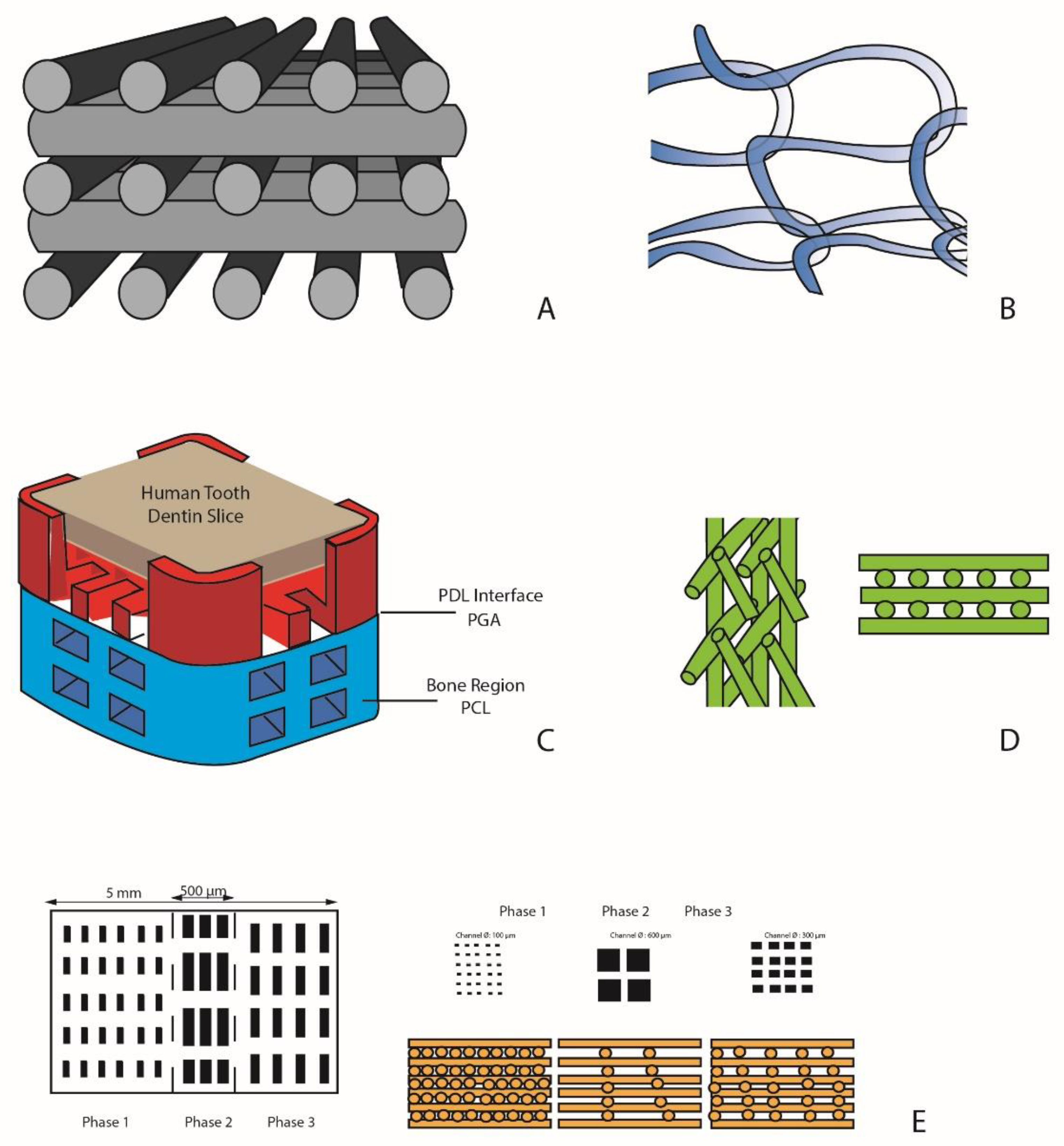
6.2. Monophasic Scaffolds
6.2.1. Simple Monophasic Scaffolds
6.2.2. Single-Phase Scaffold for Cell Delivery
6.2.3. Monophasic Scaffolds for the Release of Growth Factors
6.3. Biphasic Scaffolds
6.4. Triphasic Scaffolds
7. Limitations and Future Perspective
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Caton, J.; Nyman, S.; Zander, H. Histometric evaluation of periodontal surgery: II. Connective tissue attachment levels after four regenerative procedures. J. Clin. Periodontol. 1980, 7, 224–231. [Google Scholar] [CrossRef]
- Bartold, P.M.; McCulloch, C.A.; Narayanan, A.S.; Pitaru, S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology 2000 2000, 24, 253–269. [Google Scholar] [CrossRef]
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014, 59, 117–130. [Google Scholar] [CrossRef]
- Karring, T.; Nyman, S.; Gottlow, J.; Laurell, L. Development of the biological concept of guided tissue regeneration: Animal and human studies. Periodontology 2000 1993, 1, 26–35. [Google Scholar] [CrossRef]
- Siciliano, V.I.; Andreuccetti, G.; Siciliano, I.A.; Blasi, A.; Sculean, A.; Salvi, G.E. Clinical outcomes after treatment of non-contained intrabony defects with enamel matrix derivative or guided tissue regeneration: A 12-months randomized controlled clinical trial. J. Periodontol. 2011, 82, 62–71. [Google Scholar] [CrossRef]
- Needleman, I.G.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R.J. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2006, 2, CD001724. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Blasi, A.; Nuzzolo, P.; Matarasso, M.; Isola, G.; Ramaglia, L. Treatment of periodontal intrabony defects using enamel matrix derivative: Surgical re-entry after an observation period of at least 5 years. Int. J. Periodontics Restor. Dent. 2019, 39, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Blasi, A.; Stratul, S.I.; Ramaglia, L.; Vela, O.; Salvi, G.E.; Sculean, A. Healing of periodontal suprabony defects following treatment with open flap debridement with or without an enamel matrix derivative: A randomized controlled clinical study. Clin. Oral Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain(R)) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst. Rev. 2009, 4, CD003875. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.B.; Morris, K.H. A systematic review of the use of growth factors in human periodontal regeneration. J. Periodontol. 2013, 84, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, M.; Iorio-Siciliano, V.; Blasi, A.; Ramaglia, L.; Salvi, G.E.; Sculean, A. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta-analysis. Clin. Oral Investig. 2015, 19, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Andreuccetti, G.; Blasi, A.; Matarasso, M.; Sculean, A.; Salvi, G.E. Clinical outcomes following regenerative therapy of non-contained intrabony defects using a deproteinized bovine bone mineral combined with either Enamel Matrix derivative or Collagen Membrane. J. Periodontol. 2014, 85, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Arif, A.; Ghafoor, R. Role of three-dimensional printing in periodontal regeneration and repair: Literature review. J. Indian Soc. Periodontol. 2019, 23, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Luan, X.; Liua, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ding, B.; Yu, J. Direct fabrication of highly nanoporous polystyrene fibers via electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 521–528. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Bottino, M.C.; Kamocki, K.; Yassen, G.H.; Platt, J.A.; Vail, M.M.; Ehrlich, Y.; Spolnik, K.J.; Gregory, R.L. Bioactive nanofibrous scaffolds for regenerative endodontics. J. Dent. Res. 2013, 92, 963–969. [Google Scholar] [CrossRef]
- Bottino, M.C.; Arthur, R.A.; Waeiss, R.A. Biodegradable nanofibrous drug delivery systems: Effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin. Oral Investig. 2014, 18, 2151–2158. [Google Scholar] [CrossRef]
- Bottino, M.C.; Yassen, G.H.; Platt, J.A. A novel three-dimensional scaffold for regenerative endodontics: Materials and biological characterizations. J. Tissue Eng. Regen. Med. 2015, 9, E116–E123. [Google Scholar] [CrossRef]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2015, 21, 250–261. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Claessens, T.; Van Der Smissen, B.; Van Holsbeke, C.S.; De Backer, J.W.; Van Ransbeeck, P.; Verdonck, P. Manufacturing of patient-specific optically accessible airway models by fused deposition modeling. Rapid Prototyp. J. 2013, 19, 312–318. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomaki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-based 3D printing for bone tissue engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Bertand, P.; Bayle, F.; Combe, C.; Goeuriot, P.; Smurov, I. Ceramic components manufacturing by selective laser sintering. Appl. Surf. Sci. 2007, 254, 989–992. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Phys. Rev. Appl. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Shirazi, S.F.S.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.C.; Kadri, N.A.; Abu Osman, N.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar] [CrossRef]
- Bártolo, P.J. Stereolithography: Materials, Processes and Applications; Springer: Berlin, Germany, 2011. [Google Scholar]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D Printing of Polymer Nanocomposites via Stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Kebede, M.A.; Asiku, K.S.; Imae, T.; Kawakami, M.; Furukawa, H.; Wud, C.M. Stereolithographic and molding fabrications of hydroxyapatite-polymer gels applicable to bone regeneration materials. J. Taiwan Inst. Chem. Eng. 2018, 92, 91–96. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing ceramics: From drops to solid. J. Eur. Ceram. Soc. 2011, 31, 2543–2550. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Ann. Rev. Mater. Res. 2015, 40, 395–414. [Google Scholar] [CrossRef]
- Zocca, A.; Colombo, P.; Gomes, C.M.; Gunster, J. Additive manufacturing of ceramics: Issues, potentialities, and opportunities. J. Am. Ceram. Soc. 2015, 98, 1983–2001. [Google Scholar] [CrossRef]
- Alamán, J.; Alicante, R.; Peña, J.I.; Sánchez-Somolinos, C. Inkjet Printing of Functional Materials for Optical and Photonic Applications. Materials 2016, 9, 910. [Google Scholar] [CrossRef]
- Lenz, R. Biodegradable polymers. Biopolymers. Adv. Polym. Sci. 1993, 107, 1–40. [Google Scholar]
- Piskin, E. Biodegradable polymers as biomaterials. J. Biomater. Sci. Polym. Ed. 1995, 6, 775–795. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nip, J.; Harmon, M.D.; James, R.; Deng, M.; Laurencin, C.T.; Yu, X.; Kumbar, S.G. Cellulose and collagen derived micro-nano structured scaffolds for bone tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 719–731. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering—A mini review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Liu, B. Recent Advances in Biodegradable Conducting Polymers and Their Biomedical Applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Limongelli, L.; Moreo, G.; Favia, G.; Carinci, F. Nanomaterials for Periodontal Tissue Engineering: Chitosan-Based Scaffolds. A Systematic Review. Nanomaterials 2020, 10, 605. [Google Scholar] [CrossRef]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef]
- Mitsak, A.G.; Kemppainen, J.M.; Harris, M.T.; Hollister, S.J. Effect of polycaprolactone scaffold permeability on bone regeneration in vivo. Tissue Eng. Part A 2011, 17, 1831–1839. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najee, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef]
- Lee, J.W.; Serna, F.; Nickels, J.; Schmidt, C.E. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules 2006, 7, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, W.; Noga, D.; Hardcastle, K.; García, A.; Collard, D.; Weck, M. Functional lactide monomers: Methodology and polymerization. Biomacromolecules 2006, 7, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Hird, B.; Eisenberg, A. p-Carboxylation of linear high molecular-mass polystyrene. J. Polym. Sci. 1993, 31, 1377–1381. [Google Scholar] [CrossRef]
- Barradas, A.M.; Yuan, H.; van Blitterswijk, C.A.; Habibovic, P. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur. Cell Mater. 2011, 21, 407–429. [Google Scholar] [CrossRef]
- Blokhuis, T.J.; Arts, J.J. Bioactive and osteoinductive bone graft substitutes: Definitions, facts and myths. Injury 2011, 42, S26–S29. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J. Biomed. Mater. Res. Part A 2003, 64, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J. Fabrication and characterization of bioactive wollastonite/PHBV composite scaffolds. Biomaterials 2004, 25, 5473–5480. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Hoffmann, W.; Bormann, T.; Rossi, A.; Müller, B.; Schumacher, R.; Martin, I.; de Wild, M.; Wendt, D. Rapid prototyped porous nickel-titanium scaffolds as bone substitutes. J. Tissue Eng. 2014, 5, 2041731414540674. [Google Scholar] [CrossRef]
- Cheng, K.; Kisaalita, W.S. Exploring cellular adhesion and differentiation in a micro-/nano-hybrid polymer scaffold. Biotechnol. Prog. 2010, 26, 838–846. [Google Scholar] [CrossRef]
- Dave, K.; Gomes, V.G. Interactions at scaffold interfaces: Effect of surface chemistry, structural attributes and bioaffinity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110078. [Google Scholar] [CrossRef]
- Hollister, S.J.; Maddox, R.D.; Taboas, J.M. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials 2012, 23, 4095–4103. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electro-spinning applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 96, 566–574. [Google Scholar] [CrossRef]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.L.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar] [CrossRef]
- Lee, B.L.-P.; Jeon, H.; Wang, A.; Yan, Z.; Yu, J.; Grigoropoulos, C.; Li, S. Femtosecond laser ablation enhances cell infiltration into three-dimensional electrospun scaffolds. Acta Biomater. 2012, 8, 2648–2658. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Chung, B.G.; Ortmann, D.; Hattori, N.; Moeller, H.-C.; Khademhosseini, A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl. Acad. Sci. USA 2009, 106, 16978–16983. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Chen, L.; Li, H.; Yin, T.; Wang, B.; Lee, J.C.-M.; Yu, Q. Electrospun nanofiber meshes with tailored architectures and patterns as potential tissue-engineering scaffolds. Biofabrication 2009, 1, 015001. [Google Scholar] [CrossRef] [PubMed]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.-J.; Dean, D.R.; Jun, H.-W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Cooper-White, J.J. Increasing electrospun scaffold pore size with tailored collectors for improved cell penetration. Acta Biomater. 2011, 7, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ouyang, G.; McCann, J.T.; Xia, Y. Collecting electrospun nanofibers with patterned electrodes. Nano Lett. 2005, 5, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chang, J. Patterning of Electrospun Fibers Using Electroconductive Templates. Adv. Mater. 2007, 19, 3664–3667. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, J. Electrospinning of three-dimensional nanofibrous tubes with controllable architectures. Nano Lett. 2008, 8, 3283–3287. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Salim, A.; Ziaie, B. Selective nanofiber deposition through field-enhanced electrospinning. Langmuir 2009, 25, 9648–9652. [Google Scholar] [CrossRef]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef]
- Wissing, T.B.; Bonito, V.; Bouten, C.V.C.; Smits, A.I.P.M. Biomaterial-driven in situ cardiovascular tissue engineering—A multi-disciplinary perspective. NPJ Regen. Med. 2017, 2, 18. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Generali, M.; Dijkman, P.E.; Hoerstrup, S.P. Bioresorbable scaffolds for cardiovascular tissue engineering. EMJ Int. Cardiol. 2014, 1, 91–99. [Google Scholar]
- Pastorino, L.; Pioli, F.; Zilli, M.; Converti, A.; Nicolini, C. Lipase- catalyzed degradation of poly(ε-caprolactone). Enzym. Microbiol. Technol. 2004, 35, 321–326. [Google Scholar] [CrossRef]
- McBane, J.E.; Santerre, J.P.; Labow, R.S. The interaction between hydrolytic and oxidative pathways in macrophage-mediated polyurethane degradation. J. Biomed. Mater. Res. A 2007, 82, 984–994. [Google Scholar] [CrossRef]
- Martins, A.M.; Pham, Q.P.; Malafaya, P.B.; Sousa, R.A.; Gomes, M.E.; Raphael, R.M. The role of lipase and alpha-amylase in the degradation of starch/poly(epsilon-caprolactone) fiber meshes and the osteogenic differentiation of cultured marrow stromal cells. Tissue Eng. Part A 2009, 15, 295–305. [Google Scholar] [CrossRef]
- Peng, H.; Ling, J.; Liu, J.; Zhu, N.; Ni, X.; Shen, Z. Controlled enzymatic degradation of poly(ε-caprolactone)-based copolymers in the presence of porcine pancreatic lipase. Polym. Degrad. Stab. 2010, 95, 643–650. [Google Scholar] [CrossRef]
- Brugmans, M.C.P.; Söntjens, S.H.M.; Cox, M.A.J.; Nandakumar, A.; Bosman, A.W.; Mes, T. Hydrolytic and oxidative degradation of electrospun supramolecular biomaterials: In vitro degradation pathways. Acta Biomater. 2015, 27, 21–31. [Google Scholar] [CrossRef]
- Anderson, J.M. Mechanisms of inflammation and infection with implanted devices. Cardiovasc. Pathol. 1993, 2, 33–41. [Google Scholar] [CrossRef]
- Labow, R.S.; Meek, E.; Santerre, J.P. Model systems to assess the destructive potential of human neutrophils and monocyte-derived macrophages during the acute and chronic phases of inflammation. J. Biomed. Mater. Res. 2001, 54, 189–197. [Google Scholar] [CrossRef]
- Balguid, A.; Mol, A.; van Marion, M.H.; Bank, R.A.; Bouten, C.V.; Baaijens, F.P. Tailoring fiber diameter in electrospun Poly(epsilon-Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng. Part A 2009, 15, 437–444. [Google Scholar] [CrossRef]
- Kurpinski, K.T.; Stephenson, J.T.; Janairo, R.R.; Lee, H.; Li, S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds. Biomaterials 2010, 31, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Saino, E.; Focarete, M.L.; Gualandi, C.; Emanuele, E.; Cornaglia, A.I.; Imbriani, M. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules 2011, 12, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015, 344, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K. The effect of thick fibers and large pores of electrospun poly(ε- caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; McHugh, K.; Chew, S.Y.; Anderson, J.M. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J. Biomed. Mater. Res. A 2010, 93, 1151–1159. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Zhang, Z.; Jiang, X. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv. Healthc. Mater. 2019, 8, e1801433. [Google Scholar] [CrossRef]
- Chang, H.I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; In Tech: Shanghai, China, 2011. [Google Scholar] [CrossRef]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic bone scaffold design parameters: Osteogenic differentiation and signal expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef]
- Yeo, A.; Rai, B.; Sju, E.; Cheong, J.J.; Teoh, S.H. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: An in vitro and in vivo study. J. Biomed. Mater. Res. A 2008, 84, 208–218. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and challenges in biomaterials used for bone tissue engineering: Bioactive glasses and elastomeric composites. Prog. Biomater. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. A 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Taut, A.D.; Padial-Molina, M.; Flanagan, C.L.; Pilipchuk, S.P.; Hollister, S.J.; Giannobile, W.V. Image-based, fiber guiding scaffolds: A platform for regenerating tissue interfaces. Tissue Eng. Part C Methods 2014, 20, 533–542. [Google Scholar] [CrossRef]
- Murakami, S. Periodontal tissue regeneration by signaling molecule(s): What role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol. 2000 2011, 56, 188–208. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S. Periodontal regeneration. Aust. Dent. J. 2009, 54, S118–S128. [Google Scholar] [CrossRef]
- Bartold, P.M.; Xiao, Y.; Lyngstaadas, S.P.; Paine, M.L.; Snead, M.L. Principles and applications of cell delivery systems for periodontal regeneration. Periodontology 2000 2006, 41, 123–135. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Clinical utility of stem cells for periodontal regeneration. Periodontology 2000 2012, 59, 203–227. [Google Scholar] [CrossRef]
- Ishikawa, I.; Iwata, T.; Washio, K.; Okano, T.; Nagasawa, T.; Iwasaki, K.; Ando, T. Cell sheet engineering and other novel cell-based approaches to periodontal regeneration. Periodontology 2000 2009, 51, 220–238. [Google Scholar] [CrossRef]
- Hirose, M.; Kwon, O.H.; Yamato, M.; Kikuchi, A.; Okano, T. Creation of designed shape cell sheets that are non-invasively harvested and moved onto another surface. Biomacromolecules 2000, 1, 377–381. [Google Scholar] [CrossRef]
- Akizuki, T.; Oda, S.; Komaki, M.; Tsuchioka, H.; Kawakatsu, N.; Kikuchi, A.; Yamato, M.; Okano, T.; Ishikawa, I. Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. J. Periodontal Res. 2005, 40, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005, 11, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.G.; Hasegawa, M.; Yamato, M.; Takagi, R.; Okano, T.; Ishikawa, I. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J. Periodontal Res. 2008, 43, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.G.; Yashiro, R.; Washio, K.; Yamato, M.; Okano, T.; Ishikawa, I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J. Clin. Periodontol. 2008, 35, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C.H.; Kim, B.K.; Mao, J.J. Anatomically shaped tooth and periodontal regeneration by cell homing. J. Dent. Res. 2010, 89, 842–847. [Google Scholar] [CrossRef]
- Mangano, C.; Barboni, B.; Valbonetti, L.; Berardinelli, P.; Martelli, A.; Muttini, A.; Bedini, R.; Tetè, S.; Piattelli, A.; Mattioli, M. In Vivo Behavior of a custom-made 3D synthetic bone substitute in sinus augmentation procedures in sheep. J. Oral Implantol. 2015, 41, 240–250. [Google Scholar] [CrossRef]
- Baba, S.; Hashimoto, Y.; Inoue, T.; Kimura, D.; Sumikura, S.; Sonoda, Y.; Yamada, Y.; Ito, K.; Hojo, M.; Adachi, T. Evaluation of a 3-D, Woven-fabric, Composite Scaffold Using Experimental Canine Models of Bone Defects in Mandibles. J. Oral Tissue Eng. 2011, 8, 212–221. [Google Scholar]
- Baba, S.; Yamada, Y.; Komuro, A.; Yotsui, Y.; Umeda, M.; Shimuzutani, K.; Nakamura, S. Phase I/II trial of autologous bone marrow stem cell transplantation with a three-dimensional woven-fabric scaffold for periodontitis. Stem Cells Int. 2016, 2016, 6205910. [Google Scholar] [CrossRef]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implant. Res. 2016, 27, 55–62. [Google Scholar] [CrossRef]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Migone, C.; Grassi, L.; Pirosa, A.; Maisetta, G.; Batoni, G.; Chiellini, F. Integrated three-dimensional fiber/hydrogel biphasic scaffolds for periodontal bone tissue engineering. Polym. Int. 2016, 65, 631–640. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Jin, Q.; Bland, M.E.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 2010, 31, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, C.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef]
- Vaquette, C.; Ivanovski, S.; Hamlet, S.M.; Hutmacher, D.W. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 2013, 34, 5538–5551. [Google Scholar] [CrossRef]
- Dan, H.; Vaquette, C.; Fisher, A.G.; Hamlet, S.M.; Xiao, Y.; Hutmacher, D.W.; Ivanovski, S. The influence of cellular source on periodontal regeneration using calcium phosphate coated polycaprolactone scaffold supported cell sheets. Biomaterials 2014, 35, 113–122. [Google Scholar] [CrossRef]
- Mathew, A.; Vaquette, C.; Hashimi, S.; Rathnayake, I.; Huygens, F.; Hutmacher, D.W.; Ivanovski, S. Antimicrobial and immunomodulatory surface-functionalized electrospun membranes for bone regeneration. Adv. Healthc. Mater. 2017, 6, 1601345. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed biorésorbable scaffold for periodontal repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, M.S.; Eltohamy, M.; Kim, T.H.; Kim, H.W. Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering. PLoS ONE 2016, 11, e0149967. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D printed and micropatterned polycaprolactone scaffolds for guidance of oriented collagenous tissue formation in vivo. Adv. Healthc. Mater. 2016, 5, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Staples, R.J.; Ivanovski, S.; Vaquette, C. Fibre guiding scaffolds for periodontal tissue engineering. J. Periodontal Res. 2020, 55, 331–341. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Arkudas, A.; Euler, S.; Beier, J.P.; Horch, R.E.; Kneser, U. Prevascularisation strategies in tissue engineering. Handchir. Mikrochir. Plast. Chir. 2006, 38, 217–223. [Google Scholar] [CrossRef]
- Will, J.; Melcher, R.; Treul, C.; Travitzky, N.; Kneser, U.; Polykandriotis, E.; Horch, R.; Greil, P. Porous ceramic bone scaffolds for vascularized bone tissue regeneration. J. Mater. Sci. Mater. Med. 2008, 19, 2781–2790. [Google Scholar] [CrossRef]
- Gbureck, U.; Holzel, T.; Doillon, C.; Muller, F.A.; Barralet, J.E. Direct printing of bioceramic implants with spatially localized angiogenic factors. Adv. Mater. 2007, 19, 795–800. [Google Scholar] [CrossRef]
- Barralet, J.; Gbureck, U.; Habibovic, P.; Vorndran, E.; Gerard, C.; Doillon, C.J. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. Part A 2009, 15, 1601–1609. [Google Scholar] [CrossRef]
- Liu, H.; Maekawa, T.; Patrikalakis, N.M.; Sachs, E.M.; Cho, W. Methods for feature-based design of heterogeneous solids. Comput. -Aided Des. 2004, 36, 1141–1159. [Google Scholar] [CrossRef]
- Spillmann, A. Flowability modification of lactose powder by plasma enhanced chemical vapor deposition. Plasma Process Polym. 2007, 4, S16–S20. [Google Scholar] [CrossRef]
| Materials | Advantages | Disadvantages |
|---|---|---|
| Natural materials | Good biocompatibility and cellular affinity | Significant degradation rate Weak mechanical properties |
| Synthetic materials | Good physicochemical and mechanical properties High variability in degradation rate and resorption kinetics | Low bioactivity |
| Ceramics | Composition similar to bone tissue Osteoconductivity Stimulates bone healing | Not compatible with cell encapsulation Fragility Variety of cellular reactions according to their surface properties |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raveau, S.; Jordana, F. Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review. J. Clin. Med. 2020, 9, 4008. https://doi.org/10.3390/jcm9124008
Raveau S, Jordana F. Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review. Journal of Clinical Medicine. 2020; 9(12):4008. https://doi.org/10.3390/jcm9124008
Chicago/Turabian StyleRaveau, Simon, and Fabienne Jordana. 2020. "Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review" Journal of Clinical Medicine 9, no. 12: 4008. https://doi.org/10.3390/jcm9124008
APA StyleRaveau, S., & Jordana, F. (2020). Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review. Journal of Clinical Medicine, 9(12), 4008. https://doi.org/10.3390/jcm9124008





