Repeated Pancreatectomy for Isolated Local Recurrence in the Remnant Pancreas Following Radical Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Pooled Analysis
Abstract
1. Introduction
2. Materials and Methods
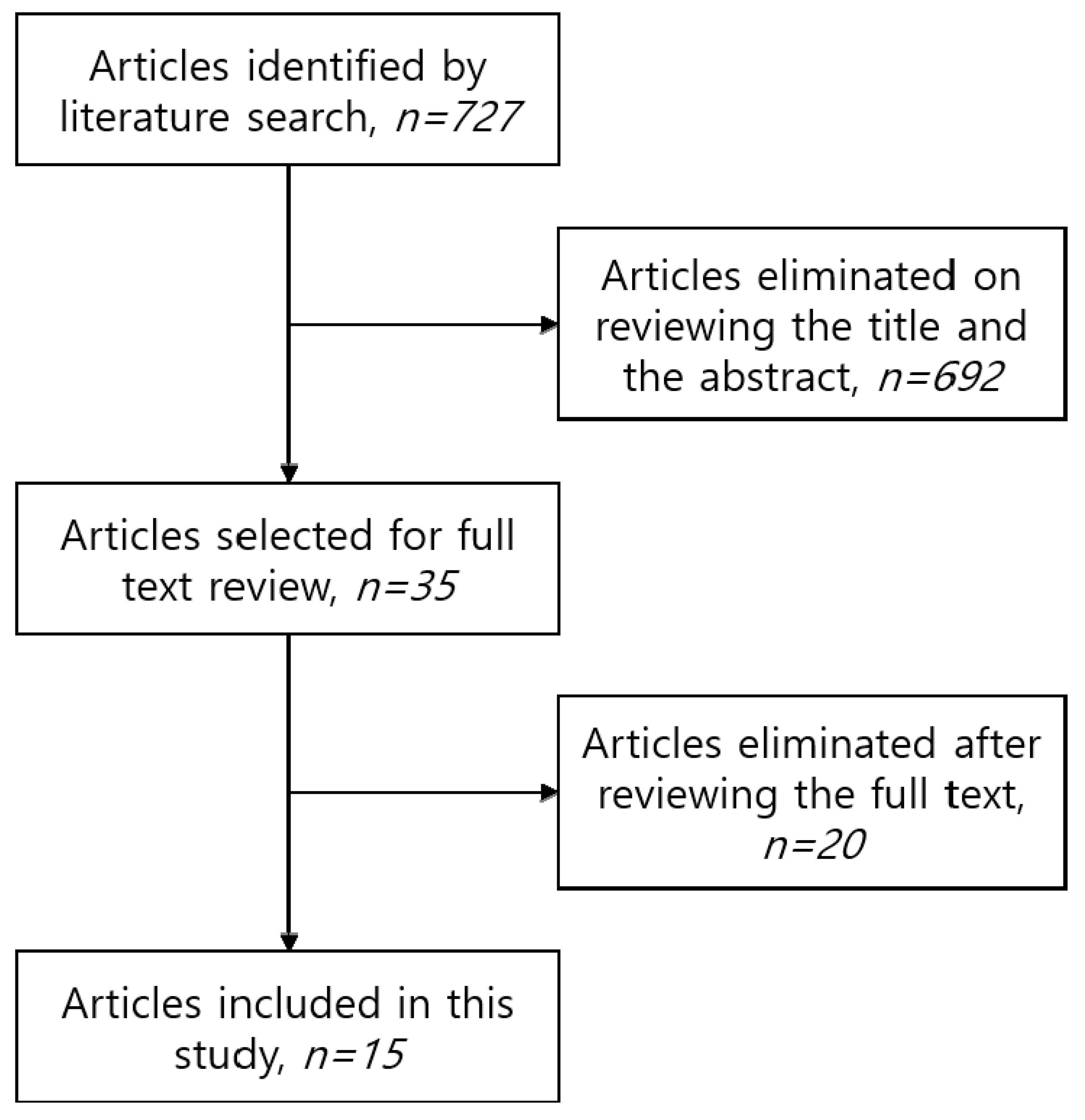
2.1. Search Strategy and Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Patients
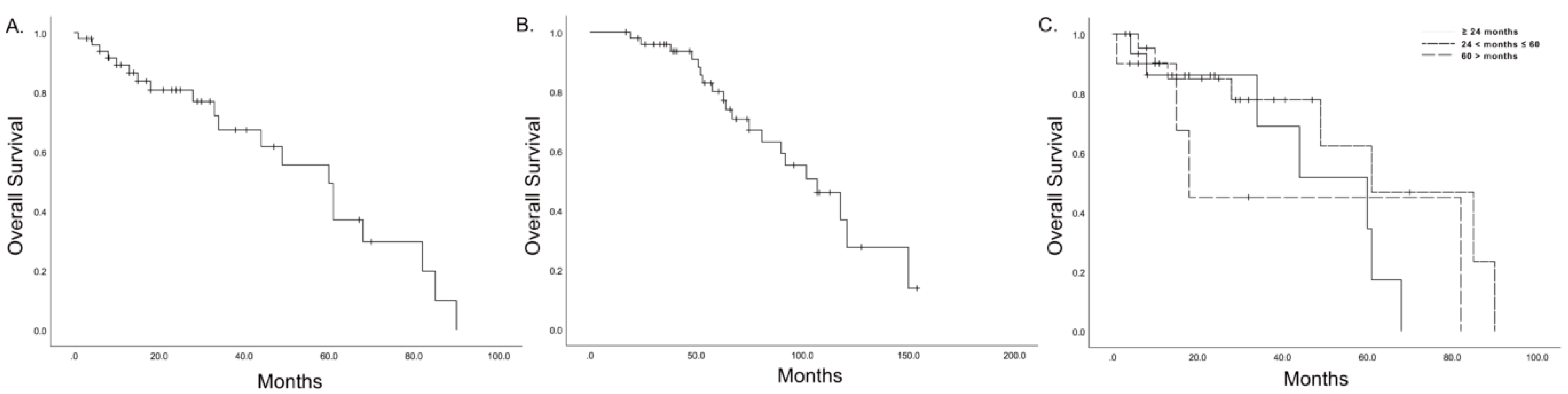
3.2. Long-Term Oncologic Outcomes
3.3. Short-Term Operative Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garcea, G.; Dennison, A.R.; Pattenden, C.J.; Neal, C.P.; Sutton, C.D.; Berry, D.P. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. Jop 2008, 9, 99–132. [Google Scholar]
- Van den Broeck, A.; Sergeant, G.; Ectors, N.; Van Steenbergen, W.; Aerts, R.; Topal, B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 2009, 35, 600–604. [Google Scholar] [CrossRef]
- Hishinuma, S.; Ogata, Y.; Tomikawa, M.; Ozawa, I.; Hirabayashi, K.; Igarashi, S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J. Gastrointest. Surg. 2006, 10, 511–518. [Google Scholar] [CrossRef]
- Hashimoto, D.; Arima, K.; Nakagawa, S.; Negoro, Y.; Hirata, T.; Hirota, M.; Inomata, M.; Fukuzawa, K.; Ohga, T.; Saeki, H.; et al. Pancreatic cancer arising from the remnant pancreas after pancreatectomy: A multicenter retrospective study by the kyushu study group of clinical cancer. J. Gastroenterol. 2019, 54, 437–448. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Yamada, S.; Kobayashi, A.; Nakamori, S.; Baba, H.; Yamamoto, M.; Yamaue, H.; Fujii, T. Resection for recurrent pancreatic cancer in the remnant pancreas after pancreatectomy is clinically promising: Results of a project study for pancreatic surgery by the japanese society of hepato-biliary-pancreatic surgery. Surgery 2018, 164, 1049–1056. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Eriguchi, N.; Aoyagi, S.; Imayama, H.; Okuda, K.; Hara, M.; Fukuda, S.; Tamaie, T.; Kanazawa, N.; Noritomi, T.; Hiraki, M.; et al. Resectable carcinoma of the pancreatic head developing 7 years and 4 months after distal pancreatectomy for carcinoma of the pancreatic tail. J. Hepato-Biliary-Pancreat. Surg. 2000, 7, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Takada, T.; Yasuda, H.; Amano, H.; Yoshida, M. A repeated pancreatectomy in the remnant pancreas 22 months after pylorus-preserving pancreatoduodenectomy for pancreatic adenocarcinoma. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.; Ikeda, H.; Kobayashi, H.; Kogire, M.; Imamura, M. Carcinoma in the remnant pancreas after distal pancreatectomy for carcinoma. Eur. J. Surg. Suppl. 2003, 588, 62–65. [Google Scholar]
- Takamatsu, S.; Ban, D.; Irie, T.; Noguchi, N.; Kudoh, A.; Nakamura, N.; Kawamura, T.; Igari, T.; Teramoto, K.; Arii, S. Resection of a cancer developing in the remnant pancreas after a pancreaticoduodenectomy for pancreas head cancer. J. Gastrointest. Surg. 2005, 9, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, R.; Mancini, C.; Crafa, P.; Passalacqua, R. Pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. J. Pancreas 2006, 7, 473–477. [Google Scholar]
- Miura, F.; Takada, T.; Amano, H.; Yoshida, M.; Isaka, T.; Toyota, N.; Wada, K.; Takagi, K.; Kato, K. Repeated pancreatectomy after pancreatoduodenectomy. J. Gastrointest. Surg. 2007, 11, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Tajima, Y.; Kuroki, T.; Ohno, T.; Furui, J.; Tsuneoka, N.; Adachi, T.; Mishima, T.; Kosaka, T.; Haraguchi, M.; Kanematsu, T. Resectable carcinoma developing in the remnant pancreas 3 years after pylorus-preserving pancreaticoduodenectomy for invasive ductal carcinoma of the pancreas. Pancreas 2008, 36, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Sata, N.; Kasahara, N.; Morishima, K.; Sasanuma, H.; Sakuma, Y.; Shimizu, A.; Hyodo, M.; Yasuda, Y. Remnant pancreatectomy for recurrent or metachronous pancreatic carcinoma detected by fdg-pet: Two case reports. J. Pancreas 2010, 11, 36–40. [Google Scholar]
- Kinoshita, H.; Yamade, N.; Nakai, H.; Sasaya, T.; Matsumura, S.; Kimura, A.; Shima, K. Successful resection of pancreatic carcinoma recurrence in the remnant pancreas after a pancreaticoduodenectomy. Hepatogastroenterology 2011, 58, 1406–1408. [Google Scholar] [CrossRef]
- Lavu, H.; Nowcid, L.J.; Klinge, M.J.; Mahendraraj, K.; Grenda, D.R.; Sauter, P.K.; Rosato, E.L.; Kennedy, E.P.; Yeo, C.J. Reoperative completion pancreatectomy for suspected malignant disease of the pancreas. J. Surg. Res. 2011, 170, 89–95. [Google Scholar] [CrossRef]
- Katz, M.H.; Wang, H.; Balachandran, A.; Bhosale, P.; Crane, C.H.; Wang, X.; Pisters, P.W.; Lee, J.E.; Vauthey, J.N.; Abdalla, E.K.; et al. Effect of neoadjuvant chemoradiation and surgical technique on recurrence of localized pancreatic cancer. J. Gastrointest. Surg. 2012, 16, 68–78. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sato, Y.; Hirukawa, H.; Soeno, M.; Shimoda, T.; Matsuoka, H.; Kobayashi, Y.; Tada, T.; Hatakeyama, K. Total pancreatectomy combined with partial pancreas autotransplantation for recurrent pancreatic cancer: A case report. Transplant. Proc. 2012, 44, 1176–1179. [Google Scholar] [CrossRef]
- Thomas, R.M.; Truty, M.J.; Nogueras-Gonzalez, G.M.; Fleming, J.B.; Vauthey, J.N.; Pisters, P.W.; Lee, J.E.; Rice, D.C.; Hofstetter, W.L.; Wolff, R.A.; et al. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J. Gastrointest. Surg. 2012, 16, 1696–1704. [Google Scholar] [CrossRef]
- Shimoike, N.; Fujikawa, T.; Maekawa, H.; Tanaka, A. Aggressive secondary surgery for local recurrence of pancreatic cancer. BMJ Case Rep 2013, 2013, bcr2013009914. [Google Scholar] [CrossRef] [PubMed]
- Akabori, H.; Shiomi, H.; Naka, S.; Murakami, K.; Murata, S.; Ishida, M.; Kurumi, Y.; Tani, T. Resectable carcinoma developing in the remnant pancreas 7 years and 10 months after distal pancreatectomy for invasive ductal carcinoma of the pancreas: Report of a case. World J. Surg. Oncol. 2014, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Zeh, H.J.; Mock, B.K.; Johnson, P.J.; Dvorchik, I.; Lee, K.; Moser, A.J.; Bartlett, D.L.; Marsh, J.W. Resection of isolated local and metastatic recurrence in periampullary adenocarcinoma. HPB 2014, 16, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Chikamoto, A.; Ohmuraya, M.; Sakata, K.; Miyake, K.; Kuroki, H.; Watanabe, M.; Beppu, T.; Hirota, M.; Baba, H. Pancreatic cancer in the remnant pancreas following primary pancreatic resection. Surg. Today 2014, 44, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Yoshitomi, H.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Furukawa, K.; Takayasiki, T.; Kuboki, S.; Okamura, D.; Suzuki, D.; et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: Is it worthwhile? Surgery 2014, 155, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, H.; Mayama, Y.; Orokawa, T.; Oshiro, N. Laparoscopic total remnant pancreatectomy after laparoscopic pancreaticoduodenectomy. Asian J. Endosc. Surg. 2014, 7, 71–74. [Google Scholar] [CrossRef]
- Balaj, C.; Ayav, A.; Oliver, A.; Jausset, F.; Sellal, C.; Claudon, M.; Laurent, V. Ct imaging of early local recurrence of pancreatic adenocarcinoma following pancreaticoduodenectomy. Abdom. Radiol. 2016, 41, 273–282. [Google Scholar] [CrossRef]
- Hardacre, J.M. Completion pancreaticoduodenectomy for a second primary pancreatic cancer: A case report. Case Rep. Pancreat. Cancer 2016, 2, 50–52. [Google Scholar] [CrossRef]
- Ishida, J.; Toyama, H.; Matsumoto, I.; Asari, S.; Goto, T.; Terai, S.; Nanno, Y.; Yamashita, A.; Mizumoto, T.; Ueda, Y.; et al. Second primary pancreatic ductal carcinoma in the remnant pancreas after pancreatectomy for pancreatic ductal carcinoma: High cumulative incidence rates at 5 years after pancreatectomy. Pancreatology 2016, 16, 615–620. [Google Scholar] [CrossRef]
- Sahakyan, M.A.; Yaqub, S.; Kazaryan, A.M.; Villanger, O.; Berstad, A.E.; Labori, K.J.; Edwin, B.; Røsok, B.I. Laparoscopic completion pancreatectomy for local recurrence in the pancreatic remnant after pancreaticoduodenectomy: Case reports and review of the literature. J. Gastrointest. Cancer 2016, 47, 509–513. [Google Scholar] [CrossRef]
- Suzuki, S.; Furukawa, T.; Oshima, N.; Izumo, W.; Shimizu, K.; Yamamoto, M. Original scientific reports: Clinicopathological findings of remnant pancreatic cancers in survivors following curative resections of pancreatic cancers. World J. Surg. 2016, 40, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, A.; Wu, L.; Si, X.; Li, Y. Second pancreatectomy for recurrent pancreatic ductal adenocarcinoma in the remnant pancreas: A pooled analysis. Pancreatology 2016, 16, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Groot, V.P.; van Santvoort, H.C.; Rombouts, S.J.; Hagendoorn, J.; Borel Rinkes, I.H.; van Vulpen, M.; Herman, J.M.; Wolfgang, C.L.; Besselink, M.G.; Molenaar, I.Q. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and sbrt. HPB 2017, 19, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, H.J. Preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after curative intent surgical resection. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 450–455. [Google Scholar] [CrossRef]
- Nakayama, Y.; Sugimoto, M.; Gotohda, N.; Konishi, M.; Takahashi, S. Efficacy of completion pancreatectomy for recurrence of adenocarcinoma in the remnant pancreas. J. Surg. Res. 2018, 221, 15–23. [Google Scholar] [CrossRef]
- Kim, Y.I.; Song, K.B.; Lee, Y.J.; Park, K.M.; Hwang, D.W.; Lee, J.H.; Shin, S.H.; Kwon, J.W.; Ro, J.S.; Kim, S.C. Management of isolated recurrence after surgery for pancreatic adenocarcinoma. Br. J. Surg. 2019, 106, 898–909. [Google Scholar] [CrossRef]
- Moletta, L.; Serafini, S.; Valmasoni, M.; Pierobon, E.S.; Ponzoni, A.; Sperti, C. Surgery for recurrent pancreatic cancer: Is it effective? Cancers 2019, 11, 991. [Google Scholar] [CrossRef]
- Suzuki, S.; Shimoda, M.; Shimazaki, J.; Maruyama, T.; Nishida, K. Clinical outcome of resected remnant pancreatic cancer after resection of the primary pancreatic cancer. J. Investig. Surg. 2019, 32, 670–678. [Google Scholar] [CrossRef]
- Shima, Y.; Okabayashi, T.; Kozuki, A.; Sumiyoshi, T.; Tokumaru, T.; Saisaka, Y.; Date, K.; Iwata, J. Completion pancreatectomy for recurrent pancreatic cancer in the remnant pancreas: Report of six cases and a review of the literature. Langenbeck Arch. Surg. 2015, 400, 973–978. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Katz, M.H.; Pisters, P.W.; Evans, D.B.; Sun, C.C.; Lee, J.E.; Fleming, J.B.; Vauthey, J.N.; Abdalla, E.K.; Crane, C.H.; Wolff, R.A.; et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J. Am. Coll. Surg. 2008, 206, 833–846, discussion 846–838. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.A.; Neoptolemos, J.P. Understanding metastasis in pancreatic cancer: A call for new clinical approaches. Cell 2012, 148, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Haeno, H.; Gonen, M.; Davis, M.B.; Herman, J.M.; Iacobuzio-Donahue, C.A.; Michor, F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 2012, 148, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Psarelli, E.E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.A.; et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: A secondary analysis of the espac-4 randomized adjuvant chemotherapy trial. JAMA Surg. 2019, 154, 1038–1048. [Google Scholar] [CrossRef]
- Fortner, J.G. Surgical principles for pancreatic cancer: Regional total and subtotal pancreatectomy. Cancer 1981, 47, 1712–1718. [Google Scholar] [CrossRef]
- Choi, M.; Lee, S.J.; Shin, D.M.; Hwang, H.K.; Lee, W.J.; Kang, C.M. Laparoscopic repeated pancreatectomy for isolated local recurrence in remnant pancreas following laparoscopic radical pancreatectomy for pancreatic ductal adenocarcinoma: Two cases report. Ann. Hepatobiliary Pancreat. Surg. 2020, 24, 542–546. [Google Scholar] [CrossRef]
| Author, Year | Study Design | Incidence †, % | n | 1st Operation | Time Interval (Months, Mean) | 2nd Operation | OS (Months, Median) |
|---|---|---|---|---|---|---|---|
| PD/DP/PP | CTP/PP | ||||||
| Eriguchi, 2000 [8] | Case report | NA | 1 | 0/1/0 | 88.0 | 1/0 | 8.0 |
| Takamatsu, 2005 [11] | Case report | NA | 1 | 1/0/0 | 47.0 | 1/0 | 10.0 |
| Dalla Valle, 2006 [12] | Case report | NA | 1 | 1/0/0 | 15.0 | 1/0 | 24.0 |
| Miura, 2007 [13] | Case series, single center | 2.3 | 1 | 1/0/0 | 20.0 | 1/0 | 44.0 |
| Tajima, 2008 [14] | Case report | NA | 1 | 1/0/0 | 37.0 | 1/0 | 38.0 |
| Koizumi, 2010 [15] | Case report | NA | 2 | 1/1/0 | 59.5 | 2/0 | 9.0 |
| Lavu, 2011 [17] | Case series, single center | 1.3 | 3 | 2/1/0 | 68.0 | 3/0 | 8.0 |
| Shimoike, 2013 [21] | Case report | NA | 2 | 1/1/0 | 18.0 | 2/0 | 24.0 |
| Boone, 2013 [23] | Case series, single center | NA | 6 | 6/0/0 | 29.9 | 4/2 | 31.0 |
| Hashimoto, 2014 [24] | Case series, single center | 2.6 | 6 | 3/3 | 29.7 | 6/0 | 15.5 |
| Miyazaki, 2014 [25] | Case series, single center | 5.3 | 9 | 6/3/0 | 32.7 | 8/1 | 28.0 |
| Shima, 2015 [39] | Case series, single center | 3.2 | 6 | 4/2/0 | 28.8 | 5/1 | 27.5 |
| Ishida, 2016 [29] | Case series, single center | 0.8 | 1 | 0/1/0 | 53.0 | 1/0 | 21.0 |
| Sahakyan, 2016 [30] | Case report | 0.3 | 1 | 1/0/0 | 36.0 | 1/0 | 4.0 |
| Suzuki, 2016 [31] | Case series, single center | 1.1 | 9 | 4/4/1 | 64.7 | 9/0 | 15.0 |
| n = 50 | |
|---|---|
| Age | 65.0 ± 7.85 |
| Sex, male | 18 (40.9) |
| Type of 1st OP | |
| PD | 32 (64.0) |
| DP | 17 (34.0) |
| TP | 0 (0.0) |
| PP | 1 (2.0) |
| Combined resection | 11 (22.0) |
| R status, 1st OP | |
| R0 | 43 (91.5) |
| R1 or R2 | 4 (8.5) |
| Adjuvant CTx., 1st OP | 25 (58.1) |
| Time interval | 41.3 ± 29.09 |
| Type of 2nd OP | |
| CTP | 46 (92.0) |
| PP | 4 (10.3) |
| R status, 2nd OP | |
| R0 | 32 (84.2) |
| R1 or R2 | 6 (15.8) |
| 30-day mortality | 0 (0.0) |
| 90-day mortality | 1 (2.0) |
| Adjuvant CTx., 2nd OP | 20 (40.9) |
| Variables | HR | 95% CI | p-Value |
|---|---|---|---|
| Time interval | |||
| ≤24 months | 0.280 | ||
| 24 < months ≤ 60 | 0.460 | 0.152–1.390 | 0.169 |
| >60 months | 1.096 | 0.308–3.907 | 0.887 |
| R1 resection, repeated pancreatectomy | 2.785 | 0.287–27.007 | 0.377 |
| Adjuvant CTx after repeated pancreatectomy | 3.704 | 0.788–17.418 | 0.097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.; Kim, N.W.; Hwang, H.K.; Lee, W.J.; Kang, C.M. Repeated Pancreatectomy for Isolated Local Recurrence in the Remnant Pancreas Following Radical Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Pooled Analysis. J. Clin. Med. 2020, 9, 3945. https://doi.org/10.3390/jcm9123945
Choi M, Kim NW, Hwang HK, Lee WJ, Kang CM. Repeated Pancreatectomy for Isolated Local Recurrence in the Remnant Pancreas Following Radical Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Pooled Analysis. Journal of Clinical Medicine. 2020; 9(12):3945. https://doi.org/10.3390/jcm9123945
Chicago/Turabian StyleChoi, Munseok, Na Won Kim, Ho Kyoung Hwang, Woo Jung Lee, and Chang Moo Kang. 2020. "Repeated Pancreatectomy for Isolated Local Recurrence in the Remnant Pancreas Following Radical Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Pooled Analysis" Journal of Clinical Medicine 9, no. 12: 3945. https://doi.org/10.3390/jcm9123945
APA StyleChoi, M., Kim, N. W., Hwang, H. K., Lee, W. J., & Kang, C. M. (2020). Repeated Pancreatectomy for Isolated Local Recurrence in the Remnant Pancreas Following Radical Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Pooled Analysis. Journal of Clinical Medicine, 9(12), 3945. https://doi.org/10.3390/jcm9123945





